Abstract
Background:
The aim of this study was to identify white matter structural networks of amnestic mild cognitive impairment (aMCI) dichotomized by β amyloid (Aβ) status and compare them using network-based statistics (NBS).
Methods:
Patients underwent whole-brain diffusion-weighted magnetic resonance imaging, detailed neuropsychological test and [18F]-Florbetaben amyloid positron emission tomography. We performed the NBS analysis to compare the whole-brain white matter structural networks extracted from diffusion tensor images.
Results:
One hundred sixteen participants (Aβ− cognitively normal [CN], n = 35; Aβ− aMCI, n = 42; Aβ+ aMCI, n = 39) were included. There was no subnetwork showing significant difference between Aβ+ aMCI and Aβ− aMCI. However, by comparing each aMCI group with control group, we found that supplementary motor areas were common hub regions. Intriguingly, Aβ+ aMCI showed reduced connectivity mainly in the medial frontal regions, while Aβ− aMCI showed somewhat uniform disruption when compared to CN.
Conclusion:
Structural network analysis using network-based approach in aMCI may shed light on further understanding of white matter disruption in the prodromal stage of Alzheimer’s disease.
Keywords: Alzheimer’s disease, mild cognitive impairment, β amyloid peptide, neural network
Introduction
Alzheimer’s disease (AD) is a devastating and progressive neurodegenerative disease, which is the most common cause of dementia in the aging process. It gradually deprives patients of their memories and other cognitive abilities, including visuospatial, language, and executive functions. There is growing evidence that suggests clinical impairment occurs sequentially, not simultaneously, which supporting pathogenesis of AD. 1 Accumulation of β amyloid peptide (Aβ) and neurofibrillary tangle, which are pathologic hallmarks of AD, spread incrementally between interconnected subregions of brain consequently causes disruption or disconnection. Therefore, AD might be considered as a “disconnection syndrome,” represented with abnormal brain networks. 2 Mild cognitive impairment (MCI) has been regarded as a pathologic stage, not a mere manifestation of normal aging process. 3 Annual conversion rate to dementia is about 10% to 15% in MCI, whereas that of the normal elderly population is about 1% to 4%. 4,5 Especially, amnestic MCI (aMCI) has been recognized as a clinical diagnosis characterized by a memory deficiency not interfering with activities of daily living. 6 At another point, to figure out the characteristics of aMCI is important because memory impairment is most commonly found in patients with MCI who subsequently progress to a diagnosis of AD dementia. 7 Recently, diagnostic criteria harboring the concept of biomarkers that directly reflect the pathologic hallmark of AD had been proposed. 8 β Amyloid-positive aMCI, which is “MCI due to AD,” is the concept of clinical syndrome on the basis of underlying pathologic etiology. A thorough understanding about MCI due to AD stage would be necessary to grasp in the context of disease continuum.
The human brain forms a large-scale network composed of interconnected subregions that coordinate activities of the brain. Recent advances in neuroimaging techniques enable the research for the human brain networks from topological point of view. 9,10 White matter structural connectivity can be modeled as a network or graph. Network-based statistics (NBS) is a method to control the family-wise error rate when mass univariate testing is performed at every connection comprising the graph. 11 There has already been a plethora of studies covering topics about structural or functional disruption of connectivity in the prodromal AD stage. 12 –16 However, defining a target group only based on the clinical diagnosis may include MCI due to non-AD pathology. Therefore, for further understanding of the transitional stage evolving into AD dementia, we focused aMCI stage stratified based on Aβ pathology.
We hypothesized that white matter structural connectivity based on the concept of “network” may show difference between patients with Aβ+ aMCI, Aβ− aMCI, and Aβ− cognitively normal (CN) participants. To identify associated structural alterations, we sought to find out white matter structural changes using diffusion tensor imaging (DTI) analysis. Diffusion tensor imaging is a commonly used magnetic resonance imaging (MRI) modality to detect structural integrity of the white matter. 17 –19 To evaluate white matter structural changes in the early stage of AD spectrum, DTI may serve as a sensitive tool.
Methods and Materials
Participants
From February 2015 to July 2016, patients were enrolled among those who visited the Dementia & Memory clinic of Asan Medical Center. Registration was completed after patients or their proxy signed the informed consent. All enrolled participants underwent 3 T structural brain MRI, detailed neuropsychological battery and [18F]-Florbetaben amyloid positron emission tomography (PET). Based on the predefined scoring system (regional cortical tracer binding and brain amyloid plaque load [BAPL]), 2 nuclear medicine physicians (M.Oh. and J.S.K.) and 2 neurologists (J.H.L. and J.E.K.) reviewed PET scans. Final judgment for scoring was made after consensus was attained between reviewers. Brain amyloid plaque load 1 was classified as “Aβ negative,” while BAPL 2 and 3 were “Aβ positive.” Current status of cognitive function in each participant was determined based on detailed history taking and neuropsychological battery profiles. Complete blood count, electrolyte level, chemical battery, vitamin B12, folate, VDRL, HIV serology, and thyroid function tests were checked to rule out medical illness which can be a cause of cognitive decline. From the venous blood, genomic DNA was extracted to identify genotype of apolipoprotein epsilon. Magnetic resonance imaging scans showing prominent cerebral white matter hyperintensities (Fazekas scale 2 or 3), 20 multiple lacunes (≥5), large-sized old stroke lesions, and multiple microbleeds were excluded for final analysis in terms of the probability of mixed dementia. We also checked other structural lesions including chronic subdural hemorrhage, hydrocephalus, or brain tumor those may cause progressive cognitive decline. We excluded patients with Parkinsonism or major psychiatric problem except for depression based on the detailed history taking and neurologic examination. The study protocol was approved by the institutional review board of Asan Medical Center.
Mild cognitive impairment patients
The patients with MCI were identified according to the diagnostic criteria of Petersen et al. 4 Amnestic MCI subtype was defined as below the 16th percentile (−1 standard deviation; SD) score of age and education level adjusted norms on the all tasks for assessing verbal or visual memory. All patients who diagnosed as single- or multiple-domain aMCI without impairment in the activities of daily living were included. Furthermore, all patients with aMCI were subdivided into 2 groups based on Aβ positivity according to the amyloid PET scan.
Control participants
In common with aMCI as a target group, we set up a control group for a comparison. Participants were classified as CN group when all subdomains of full neuropsychological battery revealed scores above −1SD of the demographically matched mean score. Therefore, CN group also included patients with proven subjective cognitive decline. We only included participants with Aβ− CN confirmed by the amyloid PET scan.
Neuropsychological Assessments
Seoul Neuropsychological Screening Battery (SNSB) was conducted as a formal cognitive function test for all participants. The SNSB is a comprehensive and detailed neuropsychological battery including tests to assess attention (forward and backward digit span), language (confrontational naming, comprehension, reading, repetition, and writing), calculation, praxis (buccofacial/ideomotor), visuospatial function (Rey Complex Figure Test; RCFT), verbal memory (Seoul Verbal Learning Test; SVLT immediate/ delayed recall and recognition), visual memory (RCFT immediate/delayed recall and recognition), and frontal/executive function (contrasting program, Go-No go, semantic/phonemic fluency, and stroop test). Also, Korean Mini-Mental State Examination (K-MMSE), Clinical Dementia Rating (CDR), Global Deterioration Scale (GDS), Neuropsychiatric Inventory (NPI), Korean Dementia Screening Questionnaire (K-DSQ), and Geriatric Depression Scale (GDepS) were conducted. Among all the subdomains, 14 items showing normally distributed pattern were presented with z score.
Neuroimaging Analysis
We performed the NBS analysis to compare the difference of whole-brain white matter structural networks extracted from DTI between groups, resulting in significantly different subnetworks between groups. After identifying subnetworks, we investigated their characteristics in terms of hub nodes.
Magnetic resonance imaging acquisition protocols
We acquired standardized 3D T1, T2, Fluid Attenuated Inversion Recovery (FLAIR), Susceptibility-Weighted Images (SWI), resting state functional MRI (fMRI), and DTI from all participants using the same 3 T MRI scanner (Philips 3T Achieva; Philips Healthcare, Eindhoven, The Netherlands). The DTI was performed with a single-shot, spin-echo, echo-planar, diffusion-weighted sequence. A series of axial diffusion-weighted images with a diffusion-sensitizing gradient (b value = 1000 s/mm2) along 32 directions was obtained, as well as axial images without diffusion weighting (b value = 0). Other diffusion parameters were as follows: repetition time = 10 965.7 ms, echo time = 70.0 ms, flip angle = 90°, matrix size = 240 × 240 pixels, field of view = 230 mm × 230 mm, number of excitations = 1, and slice thickness = 2 mm with no interslice gap. All T1-weighted MRI was performed in the sagittal plane with a 3D T1 turbo field echo sequence with the following parameters: slice thickness = 1.2 mm, repetition time = 6.76 ms, echo time = 3.11 ms, flip angle = 9°, matrix size = 244 × 244 pixels, field of view = 270 mm × 270 mm, and number of excitations = 1 with no interslice gap.
Diffusion tensor image preprocessing and network construction
First, DTI was constructed from corresponding eddy-current-corrected diffusion-weighted images using ordinary least square estimation in FMRIB Software Library. 21 We then performed whole-brain deterministic tractography using the Fiber Assignment by Continuous Tracking algorithm. 22 Fiber tracking was started at the 8 random points of the seed voxels with a fractional anisotropy (FA) >0.3 and ended at the voxels with FA <0.2 or a tract turning-angle <45° using Diffusion Toolkit. 23 We constructed whole-brain white matter structural networks from the tractographies on 90 regions of interest (ROIs) defined in automated anatomical labeling. 24 For defining the ROIs on diffusion space, we coregistered diffusion-weighted images to corresponding T1-weighted images, followed by nonlinear registration of the T1-weighted images to the MNI152 standard image. We then counted the number of streamlines connected between all pairs of ROIs using the UCLA Multimodal Connectivity Package (http://ccn.ucla.edu/wiki/index.php) for the structural networks.
Statistical Analysis
The statistical analyses were performed using SPSS (version 21.0, IBM Corp, Armonk, New York). For the normally distributed data, 1-way analysis of variance and paired t test were used. In case of data showing non-normal distribution, Kruskal-Wallis test was used for continuous variables while χ2 test was used for ordinal scales. Categorical variables were represented as proportions and continuous variables as mean (SD). Statistical significance was set up at P value <.05 but in case of multiple comparisons, post hoc P value was adjusted by Bonferroni correction method (statistical significance under .05/number of testing).
For comparing structural networks between groups, we employed the NBS for multiple comparison correction. Specifically, we performed 2-sample t test edge-by-edge and computed the size of the subnetworks whose edges had bigger weights than user-defined initial threshold. We then randomly assigned all participants into 1 of 2 groups maintaining each size of groups N-1 times, and computed maximum sizes of subnetworks whose edges also had bigger weights than the threshold, resulting an empirical null-distribution of maximum sizes. Then, we assigned the P value of subnetwork with a fraction of the occurrence whose sizes were larger than the size of the subnetwork of the original assignment. In this study, we used 2.35 and 10 000 as the initial threshold and the number of permutation (N), respectively.
We then extracted hub regions of the identified subnetworks, whose degrees were larger than the 2SD away from the mean. The hub regions represent the influential brain regions compared with other regions in the network.
Results
Demographic and Clinical Characteristics
A total of 116 participants (Aβ− CN, n = 35; Aβ− aMCI, n = 42; Aβ+ aMCI, n = 39) were included for analysis. Detailed demographic and clinical characteristics are presented in Table 1. Both aMCI groups were older than control group.
Table 1.
Demographic and Clinical Characteristics.a
| Group | (a) CN | (b) Aβ− aMCI | (c) Aβ+ aMCI | P Valueb |
|---|---|---|---|---|
| N | 35 | 42 | 39 | |
| Agec | 67.7 (6.5) | 72.5 (8.9) | 73.5 (7.9) | a<b, c |
| Onset agec | 64.7 (6.8) | 70.2 (9.2) | 70.9 (7.9) | a<b, c |
| Sex, M:F (%) | 8:27 (77.1) | 20:22 (52.4) | 18:21 (53.8) | NSd |
| Disease duration (years)c | 3.0 (2.6) | 2.3 (1.7) | 2.6 (1.5) | NS |
| Education (years)c | 10.7 (4.8) | 9.6 (5.3) | 11.1 (5.5) | NS |
| Handedness (R/L/Both) | 33/1/1 | 42/0/0 | 38/1/0 | NS |
| HTN (%) | 20 (57.1) | 26 (61.9) | 21 (53.8) | NS |
| DM (%) | 5 (14.3) | 12 (28.6) | 6 (15.4) | NS |
| HL (%) | 19 (54.3) | 18 (42.9) | 14 (35.9) | NS |
| Alcohol (%) | 8 (24.2) | 19 (45.2) | 14 (35.9) | NS |
| Smoking (%) | 2 (5.9) | 3 (7.1) | 2 (5.1) | NS |
| BMIc | 25.1 (3.6) | 24.8 (3.4) | 23.6 (3.4) | NS |
| ApoE4 frequency (%)e | 9 (28.1) | 10 (28.6) | 19 (59.4) | a, b<cf |
Abbreviations: aMCI, amnestic mild cognitive impairment; Aβ, β amyloid; ApoE, apolipoprotein epsilon; BMI, body mass index; CN, cognitively normal; DM, Diabetes Mellitus; HL, Hyperlipidemia; HTN, Hypertension.
a N = 116.
b P value for post hoc analysis was adjusted with Bonferroni correction for multiple comparisons.
c Values are presented as mean (standard deviation).
d Nonsignificant P value.
e Available N = 99.
f Statistically significant difference between Aβ+ aMCI and Aβ− aMCI.
It is notable that the frequency of the APOE4 allele was significantly greater in Aβ+ aMCI (59.4%) than Aβ− aMCI (28.6%; P = .002) and CN (28.1%; P = .002). Other demographic factors including sex distribution, disease duration, educational level, handedness, vascular risk factors, and body mass index did not show significant differences between groups.
Neuropsychological Assessments
Detailed neuropsychological profiles of global scales and subdomain scores are presented in Table 2. Global scale scores including K-MMSE, GDS, CDR global score, CDR sum of boxes, and K-DSQ were not different between the 2 aMCI groups but scored lower than CN. The NPI and GDepS scores showed no statistical significance in the between-group comparison. There was no difference in all the subdomain scores including attention, visuospatial function, verbal memory, visual memory, and frontal/executive performances between 2 aMCI groups.
Table 2.
Detailed Neuropsychological Profiles.a
| Group | (a) CN | (b) Aβ− aMCI | (c) Aβ+ aMCI | P Valueb |
|---|---|---|---|---|
| Global scores | ||||
| K-MMSE | 28.4 (1.8) | 24.9 (3.3) | 23.5 (3.1) | b, c<a |
| GDS [min, max] | 2.0 (0.3) [1,3] | 3.1 (0.4) [2,4] | 3.2 (0.5) [2,4] | a<b, c |
| CDR global score | 0.1 (0.2) | 0.5 (0.1) | 0.5 (0.1) | a<b, c |
| CDR-SB | 0.1 (0.3) | 1.7 (1.1) | 2.2 (1.1) | a<b, c |
| K-DSQ | 4.0 (2.9) | 8.1 (5.8) | 9.5 (3.6) | a<b, c |
| NPI (/144) | 4.6 (8.4) | 8.1 (13.6) | 6.7 (7.4) | NS |
| GDepS (/30) | 13.1 (8.4) | 13.1 (7.2) | 12.3 (7.0) | NS |
| Subdomain scores | ||||
| DS-F | 0.5 (1.2) | −0.2 (1.0) | −0.2 (0.9) | b, c<a |
| DS-B | 0.0 (1.7) | −0.3 (1.1) | −0.0 (1.0) | NS |
| K-BNT | 0.6 (0.8) | −0.9 (1.2) | −1.0 (1.4) | b, c<a |
| RCFT copy | 0.5 (0.6) | −1.0 (1.5) | −0.9 (2.4) | b, c<a |
| SVLT recall, imm | 0.6 (0.9) | −1.4 (0.8) | −1.5 (1.2) | b, c<a |
| SVLT recall, del | 0.4 (0.9) | −1.5 (0.9) | −1.7 (0.8) | b, c<a |
| SVLT, rec | 0.3 (1.0) | −1.4 (1.0) | −1.6 (1.1) | b, c<a |
| RCFT recall, imm | 0.4 (0.8) | −1.0 (0.8) | −1.2 (0.1) | b, c<a |
| RCFT recall, del | 0.3 (0.7) | −1.2 (0.9) | −1.3 (0.9) | b, c<a |
| RCFT, rec | 0.1 (1.5) | −1.0 (1.6) | −1.2 (2.3) | b, c<a |
| COWAT, Animal | 0.6 (1.0) | −0.8 (1.1) | −0.7 (1.0) | b, c<a |
| COWAT, Supermarket | 0.5 (1.0) | −0.6 (1.1) | −0.7 (0.8) | b, c<a |
| COWAT, Phonemic | 0.2 (0.9) | −0.6 (1.0) | −0.5 (0.9) | b, c<a |
| K-CWST-CR | 0.1 (1.0) | −1.1 (1.3) | −1.1 (1.0) | b, c<a |
Abbreviations: aMCI, amnestic mild cognitive impairment; Aβ, β amyloid; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating-Sum of boxes; CN, cognitively normal; COWAT; Controlled Oral Word Association Test; DS-B, Digit span-backward; DS-F, Digit span-forward; del, delayed; GDepS, Geriatric Depression Scale; GDS, Global Deterioration Scale; imm, immediate; K-BNT, Korean version of the Boston Naming Test; K-CWST-CR, Korean Color Word Stroop Test-Color reading; K-DSQ, Korean Dementia Screening Questionnaire; K-MMSE, Korean Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; RCFT, Rey Complex Figure Test; rec, recognition; SVLT, Seoul Verbal Learning Test.
a Z scores are presented as mean (standard deviation).
b P value for post hoc analysis was adjusted with Bonferroni correction for multiple comparisons.
Whole-Brain Mapping of Structural Networks
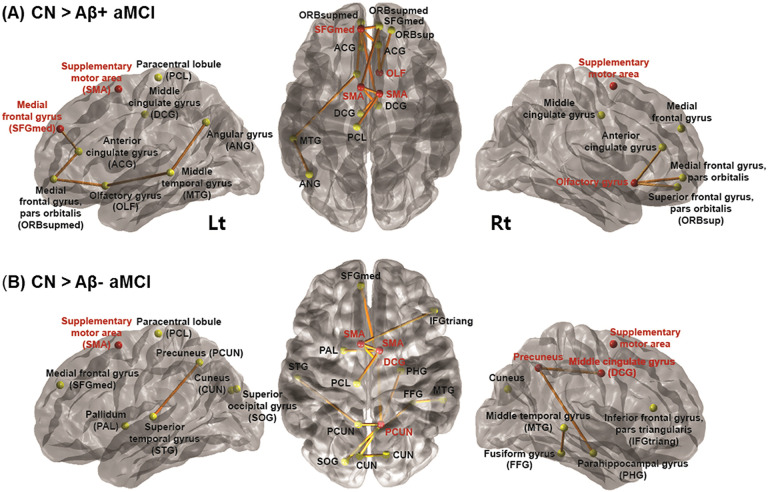
First, we sought to identify subnetworks whose edge weights decreased in Aβ+ aMCI when compared with Aβ− aMCI. However, there was no subnetwork showing significance, thus we additionally characterized a subnetwork between CN and each of aMCI groups. Identified subnetworks (P = .014 for Aβ− aMCI vs CN and P = .007 for Aβ+ aMCI vs CN) showed reduced connectivity involving hub regions known as parts of sensorimotor network (SMN) in aMCI groups compared to CN. Supplementary motor areas (SMA) in both hemispheres, reported as parts of SMN, were common hub regions in Aβ− aMCI versus CN as well as Aβ+ aMCI versus CN. In addition, right middle cingulate gyrus and right precuneus were extracted as hub regions in Aβ− aMCI; right olfactory cortex and left medial frontal gyrus were extracted as hub regions in Aβ+ aMCI.
It is worthy of notice that Aβ+ aMCI showed reduced connectivity mainly in the medial frontal regions, while Aβ− aMCI showed somewhat uniform disruption when compared to CN (Figure 1).
Figure 1.
Subnetworks identified by the network-based statistics (NBS) analysis. From left to right, the lateral view of the left hemisphere, transverse view of the both hemispheres and the lateral view of the right hemisphere, respectively. A, Subnetwork including bilateral supplementary motor areas (SMA), left medial frontal gyrus and right olfactory gyrus as hub nodes demonstrated reduced connectivity in Aβ+ aMCI compared to CN. B, Subnetwork including bilateral SMA, right middle cingulate gyrus and right precuneus demonstrated reduced connectivity in Aβ− aMCI compared to CN (Red circles represent hub regions that were most affected by the white matter disruption; yellow circles, non-hub regions; thickness of edges represents how significantly 2 groups are different). aMCI indicates amnestic mild cognitive impairment; Aβ, β amyloid.
Discussion
Here, we reported whether there is significant difference between CN participants and aMCI based on Aβ status in terms of structural connectivity using NBS. Recently, numerous researches have been reported focusing on the brain connectivity, a key element to understand structural and functional aspects of the human brain. 12,25 Brain connectivity analyses propose substantial promise for understanding patterns about breakdown of network integration in the neurodegenerative disease. 15,16,26 In AD, it is believed that pathomechanism is triggered by the accumulation of Aβ peptide, hyperphosphorylation of τ protein and neurofibrillary tangle formation. 27,28 As disease progresses, gray matter undergoes widespread neuronal loss, revealed as diffuse cortical and hippocampal atrophy. As neurons located in the gray matter are destroyed, myelin degeneration and axonal loss in neural fiber also happens, eventually leading to decreased white matter volume. 19,29 There have been enormous attempts to explain structural and functional disruption with cognitive decline in the patients with AD, from a “network” perspective. Whole-brain white matter networks constructed by using DTI are novel surrogates of structural brain connectivity. 17 When compared to CN elderly people, it is reported that patients with Alzheimer’s dementia show impaired global connectivity of brain networks. 30,31 On the other hand, there has been rather inconsistent results about MCI, probably due to heterogeneity of study protocol, especially for inclusion criteria and participant selection. Although some authors found no difference between MCI and normal control, in terms of white matter integrity and functional connectivity, 32 others proved difference according to the method of network construction. 33 We compared white matter structural networks between the aMCI groups stratified by the brain Aβ status. But above all, well-characterized target group is a prerequisite of the study aimed at prodromal AD stage. Owing to the lack of neuropathologic markers, identifying aMCI solely based on clinical diagnostic criteria can demonstrate mixed results of aMCI due to AD or non-AD. Clinical features and disease severity of Aβ+ aMCI can be very similar to Aβ− aMCI although they are not identical phenocopies. However, to differentiate them in the clinical setting is hardly possible without an information about neuropathologic biomarkers. Hence, we dichotomized patients with aMCI based on brain Aβ status using visual assessments of amyloid PET scan. Normal control group was also defined based on combination of Aβ status and clinical criteria. By doing this, we were able to further understand Aβ+ aMCI as a transitional stage evolving into AD. Although we did not find significant subnetwork in comparison between the 2 aMCI groups, each comparison with CN demonstrated some difference in terms of extracted hub regions and distribution of subnetwork. Regardless of Aβ status, all aMCI groups showed reduced connectivity surrounding bilateral SMA as hub nodes. The SMA is a part of SMN, known as one of the resting-state functional brain networks. Traditionally, the human SMA had been often regarded as a pure motor area, 34 but it has been well known for its diverse functions up to recently. Several studies about SMA using various brain mapping strategies including fMRI or PET have revealed that SMA is involved in task sequencing and complexity as well as motor control and initiation. 35,36 In addition, one study reported functional heterogeneity of the SMA using fMRI during multiple tasks for assessing motor, sensory, word generation, comprehension, and working memory. 37 Authors reported that SMA is involved in a variety of cognitive tasks including memory function along with that topology appeared to have a relation with engaged function. Intriguingly, we found that Aβ+ aMCI showed decreased connectivity mainly in the medial frontal regions indicative of early white matter structural change with a suspected vulnerability to Aβ pathology. Medial surface of the superior frontal gyrus, forming a part of the dorsomedial prefrontal cortex is reported as one of the key regions in semantic system. 38 Medial frontal regions are located adjacent to motivation and sustained attention networks, raising a likelihood of candidate for retrieval role. Previous study proposed an interesting hypothesis that the specific linguistic deficit affecting fluent semantic retrieval may be due to dysfunction of medial frontal cortex combined with SMA damage. In addition, if we compare functional connectivity during cognitive tasks between groups, more reliable interpretation about the function of hub regions will be possible.
There are some limitations in this study. First, this is a cross-sectional study without information for longitudinal changes. If we get follow-up data of brain MRI in our participants, more comprehensive analysis would be possible from the perspective of disease continuum. Second, we used visual rating method to judge Aβ pathology status based on amyloid PET scan result. Contrary to quantitative analysis, this method might consequently cause misclassification of early AD with undetectable level of amyloid plaque into Aβ− aMCI. However, even allowing for the sensitivity of amyloid PET, it might be reasonable to interpret Aβ− aMCI as “MCI with suspected non-Alzheimer’s pathology (SNAP)” in context. Because, although we didn’t assess conventional markers such as increased CSF τ, medial temporal lobe atrophy on MRI, or hypometabolism on fluorodeoxyglucose PET supporting evidence of neurodegeneration, we found network-based structural change of white matter in the Aβ− aMCI group when compared to CN group. Follow-up brain imaging with clinical observation in Aβ− aMCI group would be also interesting to figure out this phenocopy.
Our study has strength in that we classified participants using clinical diagnostic criteria combined with Aβ positivity, enabling to further understand prodromal AD more clearly. Our structural connectivity analysis using network-based approach in aMCI may shed light on further understanding of changes in the interconnected brain regions. White matter structural disruption in the preclinical or prodromal stage of AD may serve as a potential role to identify candidates at risk of macrostructural change and consequently providing timely therapeutic options such as cognitive training and disease-modifying therapy.
Footnotes
Authors’ Note: J.E.K. and S-.W.K. contributed equally to this article as co-first authors.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIP) (No. 2016R1A2B4014398).
ORCID iD: Ji Eun Kim  https://orcid.org/0000-0002-6647-4183
https://orcid.org/0000-0002-6647-4183
References
- 1. Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer’s disease: findings from neuropathological and neuroimaging studies. J Alzheimers Dis. 2014;42(suppl 4):S421–S429. [DOI] [PubMed] [Google Scholar]
- 2. Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011;10(9):829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 4. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 5. Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106(6):403–414. [DOI] [PubMed] [Google Scholar]
- 6. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 7. Maioli F, Coveri M, Pagni P, et al. Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr. 2007;44(suppl 1):233–241. [DOI] [PubMed] [Google Scholar]
- 8. Visser PJ, Vos S, van Rossum I, Scheltens P. Comparison of international working group criteria and national institute on Aging-Alzheimer’s Association criteria for Alzheimer’s disease. Alzheimers Dement. 2012;8(6):560–563. [DOI] [PubMed] [Google Scholar]
- 9. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. [DOI] [PubMed] [Google Scholar]
- 10. Papo D, Buldu JM, Boccaletti S, Bullmore ET. Complex network theory and the brain. Philos Trans R Soc Lond B Biol Sci. 2014;369(1653):pii:20130520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Sanz D, Bruna R, Garces P, et al. Functional connectivity disruption in subjective cognitive decline and mild cognitive impairment: a common pattern of alterations. Front Aging Neurosci. 2017;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rathore S, Habes M, Iftikhar MA, Shacklett A, Davatzikos C. A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. Neuroimage. 2017;155:530–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossini PM, Di Iorio R, Granata G, Miraglia F, Vecchio F. From mild cognitive impairment to Alzheimer’s disease: a new perspective in the “Land” of human brain reactivity and connectivity. J Alzheimers Dis. 2016;53(4):1389–1393. [DOI] [PubMed] [Google Scholar]
- 15. Fischer FU, Wolf D, Scheurich A, Fellgiebel A. Altered whole-brain white matter networks in preclinical Alzheimer’s disease. Neuroimage Clin. 2015;8:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sui X, Zhu M, Cui Y, et al. Functional connectivity hubs could serve as a potential biomarker in Alzheimer’s disease: a reproducible study. Curr Alzheimer Res. 2015;12(10):974–983. [DOI] [PubMed] [Google Scholar]
- 17. Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20(2):209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantarci K, Murray ME, Schwarz CG, et al. White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging. 2017;56:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. [DOI] [PubMed] [Google Scholar]
- 21. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 22. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. [DOI] [PubMed] [Google Scholar]
- 23. Wang R, Benner T, Sorensen A, Wedeen VJ. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007;15. [Google Scholar]
- 24. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 25. Sala-Llonch R, Bartres-Faz D, Junque C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 2015;6:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daianu M, Jahanshad N, Nir TM, et al. Breakdown of brain connectivity between normal aging and Alzheimer’s disease: a structural k-core network analysis. Brain Connect. 2013;3(4):407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. [DOI] [PubMed] [Google Scholar]
- 28. Nisbet RM, Polanco JC, Ittner LM, Gotz J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015;129(2):207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P. Resting-state network dysfunction in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement (Amst). 2017;8:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seo EH, Lee DY, Lee JM, et al. Whole-brain functional networks in cognitively normal, mild cognitive impairment, and Alzheimer’s disease. PLoS One. 2013;8(1):e53922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T. Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Comput Biol. 2010;6(11):e1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phillips DJ, McGlaughlin A, Ruth D, Jager LR, Soldan A. Graph theoretic analysis of structural connectivity across the spectrum of Alzheimer’s disease: the importance of graph creation methods. Neuroimage Clin. 2015;7:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanji J, Shima K. Supplementary motor cortex in organization of movement. Eur Neurol. 1996;36(suppl 1):13–19. [DOI] [PubMed] [Google Scholar]
- 35. Hinds O, Thompson TW, Ghosh S, et al. Roles of default-mode network and supplementary motor area in human vigilance performance: evidence from real-time fMRI. J Neurophysiol. 2013;109(5):1250–1258. [DOI] [PubMed] [Google Scholar]
- 36. Vergani F, Lacerda L, Martino J, et al. White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry. 2014;85(12):1377–1385. [DOI] [PubMed] [Google Scholar]
- 37. Chung GH, Han YM, Jeong SH, Jack CR, Jr. Functional heterogeneity of the supplementary motor area. AJNR Am J Neuroradiol. 2005;26(7):1819–1823. [PMC free article] [PubMed] [Google Scholar]
- 38. Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]