Abstract
Frontotemporal dementia (FTD) is a heterogeneous disorder featuring language impairment, personality changes, and executive defects, often due to the frontotemporal lobar degeneration (FTLD). Both FTD and FTLD are often associated with olfactory impairment, early biomarker for neurodegeneration, which can be evaluated with different techniques, among which low-cost olfactory tests are widely used. Therefore, we conducted a review of the literature focusing on papers published between January 1, 2007, and June 12, 2017, investigating the usefulness of olfactory testing in FTD/FTLD. A general decrease in the olfactory identification ability was seen in most of the articles and, taken together with a preserved odor discrimination, reveals a higher order impairment, possibly linked to cognitive decrease or language impairments, and not to a specific deficit of the olfactory system. This evidence could represent a useful add-on to the current literature, increasing the diagnostic value of olfactory assessment, particularly in cases where differential diagnosis is difficult.
Keywords: cognition, dementia, neurodegeneration, olfaction, psychophysics
Introduction
Smell is 1 of the 5 human senses and, until a few decades ago, it was considered a minor sense when compared to sight, hearing, or touch. Not long ago, a link between olfactory loss and neurodegeneration was observed, with many neurodegenerative conditions that are frequently associated with olfactory decrease or complete anosmia. 1 According to Hawkes, 1 these conditions include idiopathic Parkinson’s disease (PD), Guam PD–dementia complex, Alzheimer’s disease (AD), Lewy body disease, familial PD, multiple system atrophy, drug-induced PD, and X-linked dystonia–parkinsonism.
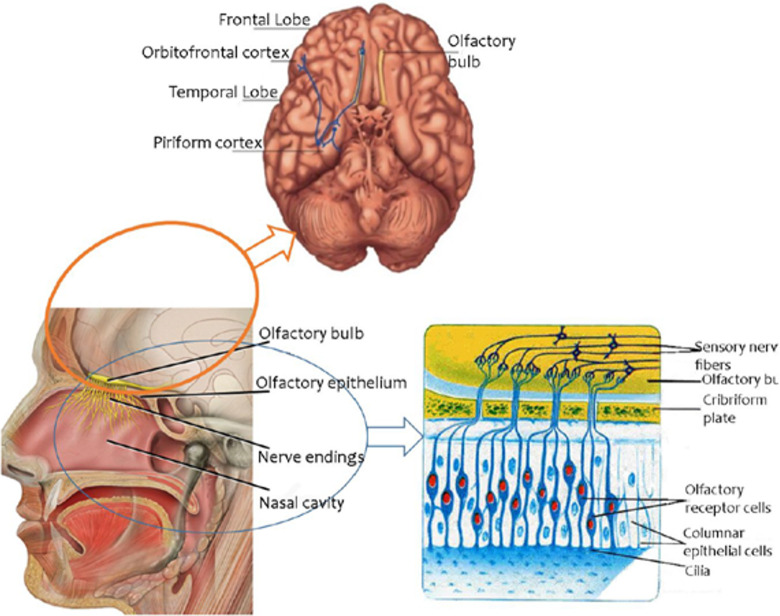
Olfactory processing involves many structures of the brain (Figure 1), in particular the frontal and temporal lobes. 2 -4 From the olfactory epithelium of the nasal cavity, the olfactory pathway arrives at the olfactory bulb (OB), which in humans is located on the ventral aspect of the frontal lobe.
Figure 1.
The olfactory system.
Then, the sensory input is transmitted to the olfactory cortex, where it is received by basal frontal and medial temporal structures, including the piriform cortex, the prepiriform and the entorhinal areas, the amygdala, and the uncus. From the olfactory cortex, the stimulus is conducted to the orbitofrontal cortex, at least partly through the mediodorsal thalamic nucleus. Furthermore, projections from the olfactory cortex are also directed to the hippocampus and to the limbic system, as well as to the associative areas of the neocortex.
Since the number of structures involved in olfactory processing is high, it is easy to understand the complex role of the olfactory system. Commercial kits for olfactory assessment are composed of subtests for odor threshold (or odor detection), odor discrimination, and odor identification. Starting from peripheral tasks, the odor threshold is defined as the smallest concentration (absolute threshold) of a given substance (usually 2-phenylethanol or n-butanol) detected by a participant. This task relies on the correct functioning of peripheral structures, ending up in the OB.
Odor discrimination deals with the ability to distinguish different odors or to judge whether 2 (or more than 2) stimuli are similar or different. Usually, an odor discrimination deficit is reported when the medial orbitofrontal cortex, getting inputs from the piriform cortex, is damaged. Nevertheless, it is considered a marker for the operation of cortical structures, together with odor identification. Odor identification, the most commonly used olfactory test, describes the ability to identify an odor based on a multiple response, forced-choice test. It also relies on the operation of frontal and temporal lobes, integrated with the limbic system and other associative areas. 5
Throughout the years, several studies have used these tests to investigate human olfactory function mainly in AD and PD, which are the most common neurodegenerative disorders in the general population. However, a handful of studies exists, dealing with olfaction in “less common” conditions, including frontotemporal dementia (FTD) or frontotemporal lobar degeneration (FTLD). 6 -16
Frontotemporal dementia is a disorder of behavior and cognition, manifested with remarkable personality changes, collapse in social and executive functions, 17 with a massive evidence of frontal lobes atrophy, prefrontal dysfunction, 18 as well as an involvement of the anterior temporal cortex. Executive functions, compromised in FTD, are most likely to be associated with a deficit in odor identification, whereas odor detection can be impaired due to orbitofrontal atrophy. However, a role for amyloid beta (Aβ) deposition, which is massively present in FTD 19 —and is known to be concerned with key olfactory structures 20 —could be hypothesized in the link between olfaction and FTD. On the other hand, focusing on the behavioral variant of FTD (bvFTD), the involvement of brain structures depends on the stage of the disorder, with frontal cortex and paralimbic areas affected in early stages, 21,22 and parietal and posterior cortex becoming impaired later on. 23 On the other hand, other similar conditions, including the variants of primary progressive aphasia (PPA), display changes on the left side of the brain. In particular, patients with primary nonfluent aphasia (PNFA) experience early deficits in the insula and frontal lobes, 24 followed by impairments in the frontal, superior temporal, and anterior parietal lobes. 25 Conversely, the anterior temporal lobe and limbic system are affected early in semantic dementia (SD), 26 with temporal lobes, insula, and ventromedial frontal areas later involved in the disorder. 27
To date, the literature is scant with articles about the olfactory assessment in FTD, without any review conducted on this topic. Therefore, it could be useful to investigate this topic to help clarifying neurosensorial changes associated with such disorders.
Methods
A literature systematic review, covering articles from January 1, 2007, to June 12, 2017, was conducted in PubMed and Science Direct. The search strategy was as follows: (“smell”[MeSH Terms] OR “smell”[All Fields] OR “olfaction”[All Fields]) AND (“pick disease of the brain”[MeSH Terms] OR (“pick”[All Fields] AND “disease”[All Fields] AND “brain”[All Fields]) OR “pick disease of the brain”[All Fields] OR (“frontotemporal”[All Fields] AND “dementia”[All Fields]) OR “frontotemporal dementia”[All Fields] OR “frontotemporal dementia”[MeSH Terms] OR (“frontotemporal”[All Fields] AND “dementia”[All Fields])).
The search was limited to articles published in peer-reviewed journals in English language. The obtained results were sorted by relevance, and the most significant works using olfactory testing in FTD or FTLD in humans were selected.
Given the considerable heterogeneity in the study designs and sample characteristics, the main features of study populations and protocols were summarized, and the study outcomes were reported using descriptive statistics without conducting any meta-analyses.
We will first present the results of the literature review and then critically discuss the possible implications of olfactory impairment in FTD or FTLD in light of the most recent findings.
Results
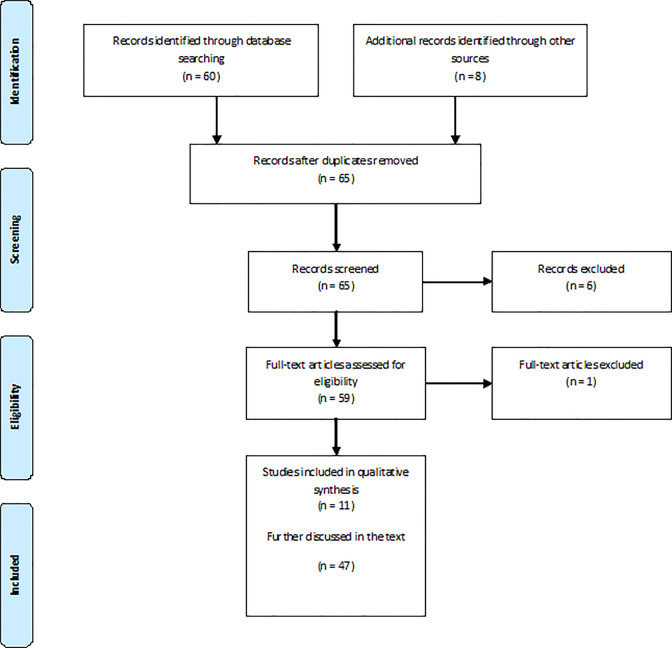
This systematic review of the current literature, whose details are shown in Figure 2, revealed 11 articles dealing with olfactory assessment in FTD or FTLD in the period taken into consideration (Table 1).
Figure 2.
Study selection.
Table 1.
Studies Directly Dealing With olfaction in FTD/FTLDa.
| Study | N (Case/Controls) | Olfactory Test | Design | Findings |
|---|---|---|---|---|
| Luzzi et al 6 | 60 (40/20) | SPSB | Cross-sectional study, 14 AD (71 ± 8 years, 7 M, 7 F), 11 FTD (64 ± 7 years, 8 M, 3 F), 8 SD (68 ± 6 years, 5 M, 3 F), 7 CBD (64 ± 7 years, 4 M, 3 F) early stages, 20 Con (age 65 ± 7 years, 10 M, 10 F). SPSB battery | Odor discrimination impaired in AD (AD 11 ± 2, FTD 14 ± 2, SD 14 ± 1, CBD 14 ± 1, Con 14 ± 3; P < .0001), odor naming (AD 1 ± 1, FTD 3 ± 2, SD 0 ± 0, CBD 5 ± 2, Con 10 ± 2; P < .0001) and odor–picture matching (AD 6 ± 3, FTD 9 ± 2, SD 6 ± 3, CBD 9 ± 3, Con 15 ± 1; P < .0001) impaired for AD, FTD, SD, CBD, picture naming (AD 14 ± 1, FTD 13 ± 3, SD 3 ± 4, CBD 14 ± 1, Con 15 ± 1; P < .0001) and word–picture matching (AD 15 ± 2, FTD 15 ± 2, SD 9 ± 3, CBD 15 ± 1, Con 16 ± 0; P < .0001) impaired for SD |
| Rami et al 7 | 7 (3/4) | UPSIT | Cross-sectional study, 3 FTLD (mean age 70 years, range 66-72 years, 3 M), 4 Con (mean age 69.5 years, range 69-70 years, 4 M). UPSIT for odor identification, odor discrimination based on UPSIT odors | Intact odor discrimination (FTLD 12.3 ± 1.5, Con 16 ± 1.8 right; FTLD 16.7 ± 1.5, Con 16 ± 2.9 left), 2/3 showed odor identification deficit in verbal UPSIT (FTLD 15.3 ± 3.2, Con 29.5 ± 1.9) |
| McLaughlin and Westervelt 8 | 42 (28/14) | B-SIT | Cross-sectional study, 14 FTD (6 behavioral subtypes, 8 nonfluent aphasia), 14 probable AD, 14 Con. BSIT for olfactory evaluation | FTD showed odor identification impairment (FTD 7 ± 3, AD 7.8 ± 2.4, Con 10.4 ± 0.9; P < .001), somewhat correlated with the severity of the disease |
| Pardini et al 9 | 61 (47/14) | UPSIT | Cross-sectional study, 25 CBS (12 M, 13 F), 22 FTD (12 M, 10 F), 14 Con. UPSIT for olfactory identification evaluation | FTD (60.3 ± 8.3a) more severely impaired than CBS (62 ± 9a). Among CBS: 4 anosmic, 4 with severe hyposmia, 4 with moderate hyposmia, 5 with mild hyposmia, 8 normosmic. Among FTD: 10 anosmic, 4 with severe hyposmia, 4 with moderate hyposmia, 3 with mild hyposmia, 1 normosmic |
| Piwnica-Worms et al 10 | 10 (4/6) | Modified UPSIT | Cross-sectional study, 3 SD (2 M, 1 F, mean age 59 years, age range 55-63 years), 1 LPA Con (1 M, 56 years old), 6 healthy Con (4 M, 2 F, mean age 61.5 years, age range 52-67 years). Modified UPSIT with visual/verbal indicators for olfactory assessment. Further tasks for flavor perception, flavor combination congruence, flavor identification, pleasantness of flavor combinations | Patients olfactory score: 20.2 ± 6.7, Con: 31.8 ± 2.6. Of 4 patients, 3 (2 SD, 1 LPA) were under the normal range for odor identification (Con performed normally); LPA poorly performed on flavor perception; all patients impaired in flavor combination congruence and flavor identification; LPA, but not SD, showed incongruence on flavor pleasantness |
| Omar et al 11 | 42 (25/17) | UPSIT | Cross-sectional study, 25 FTLD (12 bvFTD, 8 svPPA, 5 nfvPPA; 18 M, 7 F, age 65.2 ± 7.3 years), 17 Con (8 M, 9 F, age 66.2 ± 8.1 years). Flavor stimuli through JellyBelly candies, UPSIT for odor identification assessment, brain MR image acquisition through Siemens Trio TIM 3 T scanner | Abnormal eating behaviors in 50% of bvFTD, 63% of svPPA, and 40% of nfvPPA. Olfactory symptoms in 33% of bvFTD, while 8% of bvFTD and 13% of svPPA had altered flavor processing. Alterations of both eating behavior and chemosensory function in 12% of the patients overall. bvFTD (16.6 ± 8.4), svPPA (17.5 ± 6.6), and nfvPPA (26.2 ± 6) performed significantly worse (P < .05) than Con (34.7 ± 3). Flavor identification positively associated with gray matter volume in left anterior temporal lobe (entorhinal cortex, hippocampus, parahippocampal gyrus, temporal pole) |
| Heyanka et al 12 | 59 (21/38) | AST | Cross-sectional study, 21 FTD (age 72.86 ± 5.17 years), 38 MDD (age 71.58 ± 7.03 years). AST for odor identification | FTD group (6 ± 3.2) impaired (P < .001) compared to MDD group (10.6 ± 3.3). Olfactory impairment able to distinguish the 2 groups |
| Magerova et al 13 | 45 (30/15) | MHST | Cross-sectional study, 9 bvFTD (3 M, 6 F, age 63.11 ± 9.16 years), 7 PNFA (1 M, 6 F, age 61.71 ± 8.18 years), 6 SD (4 M, 2 F, age 66.33 ± 16.05 years), 8 PSP (4 M, 4 F, age 65.63 ± 10.56 years), 15 Con (4 M, 11 F, age 66.93 ± 11.57 years). MHST for odor identification, picture identification test when needed | All patient groups (bvFTD 10.7 ± 2.8, PNFA 9.7 ± 5.1, SD 10.2 ± 3.8, PSP 11 ± 3.2) outperformed (P < .05) by Con (14.8 ± 2.1) on MHST. No difference between groups of patients. Absence of correlation with neuropsychological measures |
| Körtvélyessy et al 14 | 68 (43/25) | Odor screening test derived from the Sniffin’ Sticks | 27 AD (9 M, 18 F, age 72.7 ± 6.9 years), 16 FTD (5 M, 11 F, age 68.4 ± 7.7 years), 25 Con (age 62.5 ± 9.1 years). 12-item odor screening test. CSF levels of Aβ42, T-τ, and P-τ181P determined using single-parameter ELISA kits. Genetic testing performed for the known progranulin gene mutations in patients with FTD and AD with a low CSF-PGRN level | PGRN levels correlated with olfaction across all patients. No olfactory difference between AD (7.1 ± 2.9) and FTD (6.3 ± 2.7) |
| Orasji et al 15 | 20 (9/11) | B-SIT + odor naming task | Cross-sectional study, 9 bvFTD (8 M, 1 F, age 73.1 ± 10.3 years), 11 Con (6 M, 5 F, age 71.6 ± 6.1 years). BSIT for odor identification, SPSB for odor naming, odor discrimination, and odor association | Reduced odor association in bvFTD (bvFTD 8 ± 3.6 vs Con 11.8 ± 2.6, P = .014), no significant difference in odor identification (bvFTD 2.9 ± 3.6 vs Con 4.7 ± 3.3, P = .243), naming (bvFTD 6.7 ± 2.8 vs Con 8 ± 2.6, P = .288) and discrimination (bvFTD 11.1 ± 3.1 vs Con 11.8 ± 2.9, P = .603) |
| Pilotto et al 16 | 58 (28/30) | Modified Sniffin’ Sticks | Cross-sectional study, 11 ALS-N (5 M, 6 F, age 64.3 ± 10.1 years), 17 ALS-FTD (8 M, 9 F, age 71.4 ± 7.9 years), 30 age-matched Con. Modified version of the Sniffin’ Sticks test for odor verbal and visual identification and discrimination | Olfactory function significantly different (P < .001 for discrimination and identification with verbal presentation right side; P = .001 for identification with verbal presentation left side; P = .002 for identification with visual presentation) between spectrum patients with ALS-FTD and ALS-N Con, as well as between overall ALS and Con (P < .001 for discrimination, identification with visual presentation and identification with verbal presentation left side; P = .001 for identification with verbal presentation right side) inversely correlated with behavioral and cognitive performance |
Abbreviations: AD, Alzheimer’s disease; ALS-FTD, amyotrophic lateral sclerosis frontotemporal dementia spectrum; ALS-N, amyotrophic lateral sclerosis normal cognition; AST, Alberta smell test; B-SIT, brief smell identification test; bvFTD, behavioral variant frontotemporal dementia; CBD, corticobasal degeneration; CBS, corticobasal syndrome; Con, controls; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; F, female(s); FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration; LPA, logopenic variant of primary progressive aphasia; M, male(s); MDD, major depressive disorder; MHST, Motol Hospital smell test; MR, magnetic resonance; nfvPPA, nonfluent variant primary progressive aphasia; PGRN, progranulin; PNFA, primary nonfluent aphasia; PSP, progressive supranuclear palsy; SD, semantic dementia; SPSB, odor perception and semantics battery; svPPA, semantic variant primary progressive aphasia; UPSIT, University of Pennsylvania Smell Identification Test.
aData expressed in percentiles.
Olfactory Testing in FTD/FTLD
As reported above, olfactory testing is the simplest method for assessing olfactory function in FTD/FTLD and neurodegenerative diseases (Table 2). This approach includes the assessment of olfactory identification ability, the odor discrimination evaluation, and the odor threshold calculation.
Table 2.
Studies Employing Olfactory Testing in FTD/FTLD.
| Type of Olfactory Assessment | Number of Studies | Findings (for FTD/FTLD) |
|---|---|---|
| Odor discrimination test | 4 | 25% decreased olfaction
16
75% normal olfaction 6,7,15 |
| Odor naming test/odor identification test | 11 | 81.8% decreased olfaction6,7,8,9a,10,11,12,13,16
18.2% normal olfaction14b,15 |
| Odor/picture matching test | 1 | 100% decreased olfaction
6
0% normal olfaction |
Abbreviations: AD, Alzheimer’s disease; CBS, corticobasal syndrome; FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration.
aCompared to CBS.
bCompared to AD.
Studies Dealing With Odor Identification Assessment
As happens in other neurological 28,29 and neurodegenerative conditions, 30 odor identification testing, relying on higher order cortical processing, is the most commonly employed method for olfactory assessment, thanks to its reliability, 31 easy administration, 32 and adaptability to cognitively impaired patients. 33
Specifically, this particular assessment was performed in 11 studies dealing with FTD/FTLD, with a clear evidence toward decrease in olfactory identification abilities in such patients compared to controls.
Works Employing the University of Pennsylvania Smell Identification Test
The University of Pennsylvania Smell Identification Test (UPSIT) 34 was used as the preferred method for olfactory identification assessment in most studies. It consists of 40 scratch-and-sniff booklets containing 40 different odors, which should be identified by the participant evaluated through multiple-choice questions with 4 possible options. The score is calculated, based on the sum of correct responses. The UPSIT (Sensonics Inc, Haddon Heights, New Jersey) is one of the most common test for olfactory evaluation, the other being the Sniffin’ Sticks (Burghart Messtechnik, Wedel, Germany); therefore, it is well accepted by the scientific community for its high reliability and easy administration. 34 However, the relatively high number of odors contained in the UPSIT could account for a reduced ability to maintain attention throughout the test administration or an increased olfactory fatigue. 35
Lower UPSIT scores were seen in 2 of 3 patients with FTLD in the study by Rami and colleagues 7 —1 of which (but also the nonhyposmic patient) had language impairment—while Pardini et al 9 found that 21 of 22 (12 males and 10 females) patients with FTD were hyposmic (3 mildly hyposmic, 4 moderately hyposmic, and 4 severely hyposmic) or anosmic (10 patients). This result displayed a slightly higher severity of odor impairment in patients with FTD when compared to patients with corticobasal syndrome (12 males and 13 females) and controls (14 patients). Magnetic resonance imaging data acquired in patients with FTD revealed a volume loss in the right superior and midfrontal gyri and bilaterally in the caudate. In addition, correlations with normalized UPSIT revealed a volume effect in the right midfrontal gyrus. Further significant correlations with olfactory function were seen for the Wechsler Memory Scale general score (associative memory) and the Boston naming test score, among patients with FTD.
In another study, UPSIT revealed an olfactory impairment in the majority of 25 patients with FTLD, demonstrated after adjusting for potentially relevant cognitive (executive or semantic) variables, with abnormal eating behaviors seen in 50% of patients with bvFTD, 63% of semantic variant of PPA (svPPA), and 40% of nonfluent variant of PPA. Focusing on the subgroups of FTLD, the patients with bvFTD and svPPA showed a significantly worse performance than controls. 11
Other Odor Identification Tests
Three tests similar to the UPSIT were also employed in the olfactory evaluation of FTD/FTLD. The Alberta smell test (AST) 36 was employed in 1 research. 12 This test was demonstrated to be equivalent to the UPSIT, however, without having normative data published as happens with other frequently used testing methods.
This study revealed a poorer identification in 21 patients with FTD (age 72.86 ± 5.17 years) compared to 38 patients with major depression disorder (age 71.58 ± 7.03 years), without being apparently dependent on the cognitive profile of a patient, calculated through the Mini-Mental State Examination (MMSE). 12
Similarly, 45 patients with several variants of FTLD (bvFTD, PNFA, SD, progressive supranuclear palsy [PSP]) exhibited impaired olfactory identification ability, assessed through the Motol Hospital smell test (MHST), 37 compared to controls (n = 15). 13 The 18-item MHST was developed as a shorter method to assess odor identification ability and displayed a good correlation with the UPSIT. 37 Similar to the AST, the MHST does not have normative data published and has a good diagnostic value just for the Czech population, since the odors employed for the test were typical everyday compounds encountered by such population. There was no significant difference between cohorts and the subgroups of FTLD, with the identification ability uncorrelated to neuropsychological results. The neuropsychological assessment was performed through the MMSE, the auditory verbal learning test, the free and cued selective reminding test, trail making tests A and B, the digit span, the controlled oral word association test, the Rey-Osterrieth complex figure, and the Boston naming test.
The brief smell identification test (B-SIT), a reduced version of the UPSIT, was used in a preliminary study in 14 patients with FTD, 14 with probable AD, and 14 controls. 8 This method was seen to be well suited for assessing odor identification deficits in older adults, but still requires some fine-tuning mainly concerning the difficulty of some items. 38
In the study mentioned, patients scored lower than controls on B-SIT, in particular those with FTD, with a slight correlation between the olfactory performance and the severity of the disease, assessed by the clinical dementia rating, and with the average cognitive state of each group, assessed by the MMSE. In the patients with FTD, no difference was found between the nonfluent aphasia subtype and the behavioral subtype.
The B-SIT, together with the odor naming task, was also employed in a Dutch cross-sectional study. 15 However, contrary to the findings of other studies, the 9 patients with bvFTD performed similarly to the 11 controls, even though their odor association ability was clearly impaired. In addition, no correlations were found between olfaction and neurocognitive variables, including those assessed through the MMSE, the frontal assessment battery, and a visual semantic association test.
In contrast to the tests mentioned above, an odor perception and semantics battery (SPSB) was used by Luzzi and colleagues 6 in one of the first studies investigating this topic. The SPSB was specifically developed by the authors and customized on the target population of this study; however, its reliability and related normative data are not available, making difficult to make comparisons with the general population. This work confirmed a lower identification ability in patients compared to controls, and in particular—in order of severity—in patients with SD, AD, FTD, and CBD, all of them evaluated in an early phase of the disorder. Furthermore, such patients were also impaired in the odor/picture matching test. Concerning the cognitive domain, a mild-to-moderate deficit was seen in all groups, with specific deficits for each group, among which those in executive function in FTD.
A 12-item odor screening test derived from the Sniffin’ Sticks test 31 was employed by Körtvélyessy and colleagues 14 in 16 patients with FTD (5 males, 11 females, age 68.4 ± 7.7 years), 27 patients with AD (9 males, 18 females, age 72.7 ± 6.9 years), and 25 controls (age 62.5 ± 9.1 years). In this study, employing a slightly modified version of one of the best known and reliable olfactory testing methods around the world, olfaction was not different among the groups of patients, despite patients with FTD displayed an important deficit in language-dependent neurocognitive tests, including naming and categorical tasks as well as in cerebrospinal fluid-progranulin (PGRN) levels. The authors did not specify that eventual correlations between olfaction and neurocognitive domains were retrieved.
More recently, Pilotto and colleagues 16 used a modified version of the reliable Sniffin’ Sticks test for the assessment of odor verbal and visual identification in 2 groups of patients with amyotrophic lateral sclerosis (ALS), one with normal cognition (ALS-N) and the other one with FTD spectrum. Olfactory identification was decreased in patients with ALS-FTD compared to controls, inversely correlated with behavioral and cognitive performances, including those calculated through the MMSE, the trail making tests A and B, the phonemic and semantic fluency test, short story, the Rey-Osterrieth complex figure test, the Token test, as well as the frontal assessment battery.
Studies Evaluating Odor Discrimination
Contrary to odor identification, olfactory discrimination tests, also relying on cortical functioning—especially concerning the primary olfactory cortex—are not extensively used in scientific literature, even though they provide useful information about the abovementioned portion of the cortical olfactory pathway.
In this literature review, we found 4 studies using odor discrimination tests, all of which are composed of odors using either the UPSIT or the Sniffin’ Sticks test.
The work of Luzzi and colleagues 6 failed to find a significant difference between patients with FTD and other neurodegenerative conditions (AD, SD, and CBD), as well as with controls in terms of odor discrimination, and a similar outcome was found by Rami et al, 7 who used the odors of UPSIT to build up an olfactory discrimination test for patients with FTLD. In addition, Orasji and colleagues 15 found a similar result in a group of 9 patients with bvFTD compared to 11 controls.
Conversely, Pilotto and colleagues 16 found a significant olfactory impairment in patients with ALS-FTD and controls.
Studies on Other FTLD-Like Syndromes
A further study was included, using the UPSIT, modified with visual/verbal indicators, and performed in patients with SD and the logopenic variant of PPA (LPA). 10 This study displayed deficits in 3 of 4 patients studied, in particular in 2 of 3 patients with SD and in the only patient with LPA, which also experienced deficits in flavor identification. Given the low number of patients, a correlation was not established between olfactory ability and cognitive domains studied, including nonverbal intelligence.
Discussion
This literature review focuses in patients with FTD/FTLD and their olfactory ability. This early marker of neurodegeneration can be evaluated using several methods, among which the psychophysical olfactory tests are frequently employed and reliable. Such methods allow the odor ability assessment through low-cost kits containing odors either encapsulated within scratch-and-sniff booklets, as in the case of UPSIT, 34 or contained within felt tip pens (Sniffin’ Sticks) 31 or bottles.
Common test kits assess the personal ability in some typical tasks performed by the olfactory system, including odor threshold, discrimination, and identification.
Olfactory Involvement in FTD/FTLD
Sensory processing circuits in the olfactory system have a large involvement of cortical chemosensory regions, and specifically of temporal and frontal lobes. 8,13,39 Evidences arising from this work displayed no particular impairments on the odor discrimination task, but deficits are more likely to occur in odor identification/odor naming tasks, 9,12 relying on higher order, more complex cortical processes, and on intact semantic abilities. 6 In addition, the disruption of specific associative areas, mostly temporal lobe and amygdala, involved in olfactory processing could contribute to olfactory identification deficits evidenced in some studies. 15 Despite a significant overlap between brain areas involved in olfactory discrimination and identification tasks, some minor but relevant differences were seen, in particular concerning the need for higher verbal skills required to successfully carry out odor identification. 40 Such abilities are probably correlated with gray matter thickness of a large area, extending from the right insular cortex up to the lateral temporal lobe. 40 In addition, defects in the salience network (anterior insula, anterior cingulate cortex, amygdala, ventral striatum, and medial thalamus), massively impaired in bvFTD 41 and concerned with complex olfactory tasks, 42 could contribute to the decreased ability to identify odors, which is typical of this condition.
Further explanations are possible for this phenomenon, somewhat strengthening the abovementioned hypothesis. It is argued that an odor identification deficit could reflect a specific impairment of olfactory knowledge or an impairment of cross-modal matching or verbal labeling. 7 Indeed, the presence of mild odor-semantic association impairments could contribute to the olfactory deficits observed in patients with FTD, 15 due to the involvement of the temporal neocortex. In fact, Pardini et al 9 found a significant olfactory impairment in patients with FTD and a correlation between olfactory loss and midfrontal gyrus atrophy, suggesting a likely involvement of memory in properly performing olfactory identification. Furthermore, maxima of gray matter volume loss were located in areas involved in higher order olfactory information processing 43 rather than in the primary olfactory areas (eg, entorhinal and piriform cortices), suggesting a cognitive effect prior to the olfactory deficit and the need for neuropsychological evaluation together with the olfactory testing. In addition, Magerova and colleagues 13 speculated that the odor identification deficit seen in the various subgroups of FTD could reflect an early pathological involvement in key structures of the olfactory system, in particular frontal cortical areas in bvFTD and temporal limbic areas in SD. They excluded the hypothesis of cognitive failure in the population evaluated, supporting possible changes in olfactory structures, since there was no presence of olfactory impairment across all FTLD subgroups and no correlation between odor identification and neuropsychological findings. It is worth noting, however, that the lack of correlation between olfaction and cognitive domains seen in most of the studies included in this review could be due to the extreme heterogeneity of the cognitive assessment performed in each study, as specified in the description of the single studies previously mentioned.
Conversely, as odor identification is a very complex process with respect, for example, to pictures/words identification; since it requires a larger amount of executive control, Luzzi and colleagues 6 speculated about its correlation to general measures of executive functions in FTD. It is worth noting that none of the works published to date has investigated odor threshold; therefore, the peripheral section of the olfactory pathway was not explored in studies dealing with olfactory tests in FTD.
Neurophysiological Bases of Olfactory Impairment in FTD/FTLD
Olfactory tyrosine hydroxylase immunoreactive periglomerular neurons have found to be increased in FTD, compared to controls and, together with the disruption of the mitral cell layer, the disorganization of glomeruli and the ectopic glomeruli found in the OB of patients could account for the olfactory deficits observed.
Since their unbalance was found in all those conditions, such modification could be caused by common features to the 3 diseases, 44 including the early degeneration of cholinergic, noradrenergic, and serotonergic systems. 45,46 In particular, cholinergic and noradrenergic centrifugal inputs to the OB inhibit the mitral cell layer, while serotonergic input could activate periglomerular neurons to release γ-aminobutyric acid. A deficit in the centrifugal olfactory input due to the early degeneration of such systems could cause an inhibitory imbalance possibly compensated by the increased number of dopaminergic periglomerular neurons. 47
Otherwise, the typical deposits of such pathologies reduce the synaptic efficiency of the OB and modify the structures of the olfactory epithelium and mitral cells. 48,49 Such modifications could be compensated by an increase in dopaminergic periglomerular neurons modulating the olfactory output.
τ-Protein deposits have been found in the OB of patients, as also happened in other neurodegenerative conditions (AD and PD). However, in FTD, they appeared as less developed than in AD, while β-amyloid deposits were quite rare in the OB of patients with FTD and PD compared to patients with AD.
Finally, PGRN, one of the key growth factors expressed in the human brain (in neurons and microglia, in particular), was found to be deficient in FTD, similarly to what happens in AD, and correlated with frontal lobe dysfunction and with olfactory deficits. 14 The PGRN is secreted in an activity-dependent manner 50 ; thus, a PGRN defect might be the result of synaptic loss due to frontal neurodegeneration. 51 A lack of PGRN may allow Aβ accumulation; therefore, the disruption of key structures for olfactory processing, including temporal areas and amygdala, causes a deficit in the related tasks. 15
Olfactory Function in FTLD-Like Syndromes
Of particular interest is the case of PNFA, featuring an olfactory identification impairment, 13 but normally having an intact olfactory pathway. In such cases, the insula and the dorsal frontal lobes, largely involved in the pathologic process, are thought to be key structures for olfactory processing, 52 even though no consensus about this argument has been reached. 53
The case of PSP is also of interest. In fact, Magerova and colleagues 13 found olfactory impairments in this condition, while previous studies were conflicting, some of them suggesting similar evidence, 54 and others failing to find a clear deficit in this population. 55 This fact could reflect a different degree of impairment in cortical structures of such patients, with frontal and temporal cortical gray matter loss associated with cognitive deficits in this disease, 56 supporting a correlation between olfaction and cognition in PSP. 13
On the other hand, the olfactory deficits in patients with SD are possibly associated with a massive involvement of temporal limbic areas and probably rely on a semantic disorder as the basis for their olfactory impairment, therefore highlighting the pivotal role of temporal lobes in olfactory memory.
In ALS-FTD, the olfactory identification deficits could be due to specific impairments in olfactory knowledge, reflecting a TAR DNA-binding protein 43 (TDP-43) pathology of both frontotemporal and insular associative areas. The occurrence of defective olfactory perception, linked to the functioning of OB, is more unlikely to be present in this condition 7 and could be helpful in identifying patients with ALS featuring more diffuse cortical involvement. 16
Interestingly, in all those disorders, olfactory deficits were correlated with the severity of the pathology 8 and the involvement of temporal lobes. 57
Link Between Smell and Taste Processing in FTD/FTLD
Being odor and flavor processing strictly related to each other, 11 the olfactory deficit experienced in the abovementioned conditions could be correlated with the impaired attitude toward feeding, which is typical of some neurodegenerative conditions. Such conditions include not only FTD but also SD and LPA. 10 A further evidence for this link is given by the presence of semantic deficits also in flavor processing, as found, for example, in FTLD and SD. 11
Summarizing those findings, perceptual and semantic chemosensory mechanisms are thought to interact and might contribute differentially to chemosensory function in different FTLD syndromes. 11
Conclusions
The existing literature about olfactory symptoms in FTD/FTLD suggests the presence of olfactory identification deficits in these conditions, somewhat correlated with the severity of the pathology. The mechanisms underlining this association are still unknown, even though an early degeneration of cholinergic, noradrenergic, and serotonergic systems is hypothesized to have a role in this process, as well as the deficit of PGRN, likely to be protective against Aβ deposition and toxicity. In addition, since the odor discrimination ability appears to be intact in most cases, it is hypothesized that the relevant impairment could be associated to a deficit in verbal skills, more largely required in the olfactory identification tasks, justifying the involvement of gray matter thickness in the olfactory processing, especially in areas spanning from the right insular cortex to the lateral temporal lobe.
However, a few limitations are present. First, the number of articles published to date in this specific topic is relatively low, making their interpretation particularly tricky. Second, the irreproducibility in olfactory tests in successive assessments (in particular in odor identification tests) appears to be accentuated in neurodegenerative disease 58 and should be carefully taken into account when assessing olfactory ability in such populations. Therefore, longitudinal assessments of olfactory function should be useful to overcome, at least partially, this limitation. Third, it would be important to include in the studies the genetic assessment of the patients, since it has been observed that genetic factors can influence olfactory ability. 58 Fourth, since in most cases self-made olfactory tests are employed, without much literature support by normative data, olfactory characterization in these cohorts should be performed by validated, internationally recognized olfactory testing methods (ie, UPSIT, Sniffin’ Sticks), in order to avoid methodological confusion and to get able to compare retrieved data with those published in the already existing literature.
Finally, since most of the studies included in this review involve a relatively low number of patients, mainly due to the low prevalence of FTD/FTLD on the general population, further investigation on larger cohorts both in humans and animal models is required, in order to understand the cause of this important deficit, which could have a strong impact on the patients’ lifestyle (eg, food selection, given that olfaction is correlated with flavor processing) and as a clinical investigation method, especially in differential diagnosis. Such studies should be based on a multidisciplinary approach, spanning from cell biology to psychophysics (including the administration of olfactory threshold tests), to clinics, to diagnostic imaging, in order to provide a clear and complete framework of this condition and of the link with the olfactory deficit, and to optimize treatment focusing on the peculiarity of the disease and on the personalization of care.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alessandro Tonacci  http://orcid.org/0000-0001-8335-5541
http://orcid.org/0000-0001-8335-5541
References
- 1. Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18(4):364–372. [DOI] [PubMed] [Google Scholar]
- 2. Jones-Gotman M, Zatorre RJ, Cendes F, et al. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120(pt 10):1845–1856. [DOI] [PubMed] [Google Scholar]
- 3. Shepherd GM. Smell images and the flavour system in the human brain. Nature. 2006;444(7117):316–321. [DOI] [PubMed] [Google Scholar]
- 4. Tonacci A, Baldus G, Corda D, et al. Olfactory non-cancer effects of exposure to ionizing radiation in staff working in the cardiac catheterization laboratory. Int J Cardiol. 2014;171(3):461–463. [DOI] [PubMed] [Google Scholar]
- 5. Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry. 1997;42(8):721–732. [DOI] [PubMed] [Google Scholar]
- 6. Luzzi S, Snowden JS, Neary D, et al. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45(8):1823–1831. [DOI] [PubMed] [Google Scholar]
- 7. Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. J Neurol. 2007;254(4):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLaughlin NCR, Westervelt HJ. Odor identification deficits in frontotemporal dementia: a preliminary study. Arch Clin Neuropsychol. 2008;23(1):119–123. [DOI] [PubMed] [Google Scholar]
- 9. Pardini M, Huey ED, Cavanagh AL, Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66(1):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex. 2010;46(6):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omar R, Mahoney CJ, Buckley AH, Warren JD. Flavour identification in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2013;84(1):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heyanka DJ, Golden CJ, McCue RB, 2nd, Scarisbrick DM, Linck JF, Zlatkin NI. Olfactory deficits in frontotemporal dementia as measured by the Alberta Smell Test. Appl Neuropsychol Adult. 2014;21(3):176–182. [DOI] [PubMed] [Google Scholar]
- 13. Magerova H, Vyhnalek M, Laczo J, et al. Odor identification in frontotemporal lobar degeneration subtypes. Am J Alzheimers Dis Other Demen. 2014;29(8):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Körtvélyessy P, Gukasjan A, Sweeney-Reed CM, Heinze HJ, Thurner L, Bittner DM. Progranulin and amyloid-β levels: relationship to neuropsychology in frontotemporal and Alzheimer’s disease. J Alzheimers Dis. 2015;46(2):375–380. [DOI] [PubMed] [Google Scholar]
- 15. Orasji SS, Mulder JL, de Bruijn SF, Wirtz PW. Olfactory dysfunction in behavioral variant frontotemporal dementia. Olfactory dysfunction in behavioral variant frontotemporal dementia. Clin Neurol Neurosurg. 2016;141:106–110. [DOI] [PubMed] [Google Scholar]
- 16. Pilotto A, Rossi F, Rinaldi F, et al. Exploring olfactory function and its relation with behavioral and cognitive impairment in amyotrophic lateral sclerosis patients: a cross-sectional study. Neurodegener Dis. 2016;16(5-6):411–416. [DOI] [PubMed] [Google Scholar]
- 17. Li P, Zhou YY, Lu D, Wang Y, Zhang HH. Correlated patterns of neuropsychological and behavioral symptoms in frontal variant of Alzheimer disease and behavioral variant frontotemporal dementia: a comparative case study. Neurol Sci. 2016;37(5):797–803. [DOI] [PubMed] [Google Scholar]
- 18. Cerciello M, Isella V, Proserpi A, Papagno C. Assessment of free and cued recall in Alzheimer’s disease and vascular and frontotemporal dementia with 24-item Grober and Buschke test. Neurol Sci. 2017;38(1):115–122. [DOI] [PubMed] [Google Scholar]
- 19. Vergallo A, Carlesi C, Pagni C, et al. A single center study: Aβ42/p-Tau181 CSF ratio to discriminate AD from FTD in clinical setting. Neurol Sci. 2017;38(10):1791–1797. doi: 10.1007/s10072-017-3053-z. [DOI] [PubMed] [Google Scholar]
- 20. Alvarado-Martínez R, Salgado-Puga K, Peña-Ortega F. Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS One. 2013;8(9):e75745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frisoni GB, Laakso MP, Beltramello A, et al. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology. 1999;52(1):91–100. [DOI] [PubMed] [Google Scholar]
- 22. Kril JJ, Halliday GM. Clinicopathological staging of frontotemporal dementia severity: correlation with regional atrophy. Dement Geriatr Cogn Disord. 2004;17(4):311–315. [DOI] [PubMed] [Google Scholar]
- 23. Schroeter ML, Raczka K, Neumann J, Von Cramon DY. Neural networks in frontotemporal dementia—a meta-analysis. Neurobiol Aging. 2008;29(3):418–426. [DOI] [PubMed] [Google Scholar]
- 24. Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(6):1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol. 1996;39(2):166–173. [DOI] [PubMed] [Google Scholar]
- 26. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tonacci A, Billeci L, Tartarisco G, et al. Olfaction in autism spectrum disorders: a systematic review. Child Neuropsychol. 2017;23(1):1–25. [DOI] [PubMed] [Google Scholar]
- 29. Muratori F, Tonacci A, Billeci L, et al. Olfactory processing in male children with autism: atypical odor threshold and identification. J Autism Dev Disord. 2017;47(10):3243–3251. doi:10.1007/s10803-017-3250-x. Erratum in: J Autism Dev Disord. 2017;47(10):3252. doi: 10.1007/s10803-017-3291-1. [DOI] [PubMed] [Google Scholar]
- 30. Velayudhan L. Smell identification function and Alzheimer’s disease: a selective review. Curr Opin Psychiatry. 2015;28(2):173–179. [DOI] [PubMed] [Google Scholar]
- 31. Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264(3):237–243. [DOI] [PubMed] [Google Scholar]
- 32. Tonacci A, Billeci L, Tartarisco G, et al. A novel application for cognitive evaluation in mountain ultramarathons: olfactory assessment. Wilderness Environ Med. 2016;27(1):131–135. [DOI] [PubMed] [Google Scholar]
- 33. Tonacci A, Chilosi AM, Pioggia G, Morales MA, Cioni G. Effect of olfactory stimulation in agenesis of the corpus callosum: a case report. Brain Impairment. 2015;15(3):216–222. [Google Scholar]
- 34. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 35. Hugh SC, Siu J, Hummel T, et al. Olfactory testing in children using objective tools: comparison of Sniffin’ Sticks and University of Pennsylvania Smell Identification Test (UPSIT). J Otolaryngol Head Neck Surg. 2015;44:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fortin A, Lefebvre MB, Ptito M. Traumatic brain injury and olfactory deficits: the tale of two smell tests! Brain Inj. 2010;24(1):27–33. [DOI] [PubMed] [Google Scholar]
- 37. Magerova H, Vyhnalek M, Laczo J, Bojar M, Hort J. Odor perception testing in early diagnosis of neurodegenerative dementia. Cesk Neurol Neurochir. 2008;71:298–302. [Google Scholar]
- 38. Menon C, Westervelt HJ, Jahn DR, Dressel JA, O’Bryant SE. Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a project FRONTIER study. Clin Neuropsychol. 2013;27(6):946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36:165–181. [DOI] [PubMed] [Google Scholar]
- 40. Frasnelli J, Lundström JN, Boyle JA, Djordjevic J, Zatorre RJ, Jones-Gotman M. Neuroanatomical correlates of olfactory performance. Exp Brain Res. 2010;201(1):1–11. doi:10.1007/s00221-009-1999-7. [DOI] [PubMed] [Google Scholar]
- 41. Agosta F, Galantucci S, Filippi M. Advanced magnetic resonance imaging of neurodegenerative diseases. Neurol Sci. 2017;38(1):41–51. doi:10.1007/s10072-016-2764-x. [DOI] [PubMed] [Google Scholar]
- 42. Stevenson RJ, Mahmut MK, Oaten MJ. The role of attention in the localization of odors to the mouth. Atten Percept Psychophys. 2011;73(1):247–258. [DOI] [PubMed] [Google Scholar]
- 43. Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. [DOI] [PubMed] [Google Scholar]
- 44. Zelaya MV, Pérez-Valderrama E, Martínez de Morentin X, et al. Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer’s disease: identification of common and distinct olfactory targets across Alzheimer-related co-pathologies. Oncotarget. 2015;6(37):39437–39456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirano S, Shinotoh H, Shimada H, et al. Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain. 2010;133(7):2058–2068. [DOI] [PubMed] [Google Scholar]
- 47. Mundiñano IC, Caballero MC, Ordóñez C, et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122(1):61–74. [DOI] [PubMed] [Google Scholar]
- 48. Witt M, Bormann K, Gudziol V, et al. Biopsies of olfactory epithelium in patients with Parkinson’s disease. Mov Disord. 2009;24(6):906–914. [DOI] [PubMed] [Google Scholar]
- 49. Ubeda-Bañon I, Saiz-Sanchez D, de la Rosa-Prieto C, Argandoña-Palacios L, Garcia-Muñozguren S, Martinez-Marcos A. alpha-Synucleinopathy in the human olfactory system in Parkinson’s disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol. 2010;119(6):723–735. [DOI] [PubMed] [Google Scholar]
- 50. Petoukhov E, Fernando S, Mills F, et al. Activity-dependent secretion of progranulin from synapses. J Cell Sci. 2013;126(pt 23):5412–5421. [DOI] [PubMed] [Google Scholar]
- 51. Minami SS, Min SW, Krabbe G, et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse model. Nat Med. 2014;20(10):1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kettenmann B, Hummel C, Stefan H, Kobal G. Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses. 1997;22(5):493–502. [DOI] [PubMed] [Google Scholar]
- 53. Olofsson JK, Rogalski E, Harrison T, Mesulam MM, Gottfried JA. A cortical pathway to olfactory naming: evidence from primary progressive aphasia. Brain. 2013;136(4):1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silveira-Moriyama L, Hughes G, Church A, et al. Hyposmia in progressive supranuclear palsy. Mov Disord. 2010;25(5):570–577. [DOI] [PubMed] [Google Scholar]
- 55. Suzuki M, Hashimoto M, Yoshioka M, Murakami M, Kawasaki K, Urashima M. The odor stick identification test for Japanese differentiates Parkinson’s disease from multiple system atrophy and progressive supra nuclear palsy. BMC Neurol. 2011;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giordano A, Tessitore A, Corbo D, et al. Clinical and cognitive correlations of regional gray matter atrophy in progressive supranuclear palsy. Parkinsonism Relat Disord. 2013;19(6):590–594. [DOI] [PubMed] [Google Scholar]
- 57. Savic I. Brain imaging studies of the functional organization of human olfaction. Neuroscientist. 2002;8(3):204–211. [DOI] [PubMed] [Google Scholar]
- 58. Markopoulou K, Chase BA, Robowski P, et al. Assessment of olfactory function in MAPT-associated neurodegenerative disease reveals odor-identification irreproducibility as a non-disease-specific, general characteristic of olfactory dysfunction. PLoS One. 2016;11(11):e0165112. [DOI] [PMC free article] [PubMed] [Google Scholar]