Abstract
Cholinesterase inhibitors (ChEIs), represented by donepezil, rivastigmine, and galantamine, used to be the only approved class of drugs for the treatment of Alzheimer’s disease. After the approval of memantine by the Food and Drug Administration (FDA), N-methyl-d-aspartic acid (NMDA) receptor antagonists have been recognized by authorities and broadly used in the treatment of Alzheimer’s disease. Along with complementary mechanisms of action, NMDA antagonists and ChEIs differ not only in therapeutic effects but also in adverse reactions, which is an important consideration in clinical drug use. And the number of patients using NMDA antagonists and ChEIs concomitantly has increased, making the matter more complicated. Here we used the FDA Adverse Event Reporting System for statistical analysis , in order to compare the adverse events of memantine and ChEIs. In general, the clinical evidence confirmed the safety advantages of memantine over ChEIs, reiterating the precautions of clinical drug use and the future direction of antidementia drug development.
Keywords: Alzheimer’s disease, cholinesterase inhibitors, NMDA receptor antagonists, FDA Adverse Event Reporting System (FAERS)
Introduction
Alzheimer’s disease (AD) is a progressive, debilitating neurological disorder frequently causing dementia in older populations. One in 10 people over the age of 65 will experience some form of dementia, whereas one-third of those over 85 years is affected. 1 Alzheimer’s disease accounts for approximately 70% of all dementia cases and comprises an estimated 26.6 million people worldwide as of 2006. 2 With 5 million new cases occurring each year, the prevalence of patients with AD is reported to quadruple by 2050. 3 Basic research (eg, genetic association study) 4 indicated that AD may arise from the pathogenic aggregation of specific proteins (amyloid-beta and tau), which results in neuronal dysfunction and cell death. 5 However, a unique challenge of AD is that these pathological changes are thought to occur 10 to 20 years before behavioral symptoms can be detected. The onset and progression of AD-related dementia strongly correlates with increased age. Initial symptoms (eg, memory loss, confusion/disorientation, and loss of reasoning ability) progress from mild cognitive impairment to dementia that disables people from functioning independently in daily life. Such disability results in increasing demand for home care and/or institutionalization of patients with AD. Most AD cases require a high level of care such as that provided in nursing homes. 6 The cost of care for patients with AD and other dementias has been recently estimated at US$172 billion annually in the United States alone 5 and the worldwide estimated societal costs of US$315 billion. 1 The aging global population presents a large and growing challenge to manage AD, identify better interventions, and adequately care for patients with AD.
Despite the huge global challenge AD presents, there are currently no pharmacological interventions that are effective in delaying its onset or progression. However, five drugs have gained Food and Drug Administration (FDA) approval for the relief of AD symptoms by regulating brain neurotransmitter levels. These approved drugs consist of two classes: cholinesterase inhibitors (ChEIs: donepezil, rivastigmine, and galantamine) and the later approved N-methyl-d-aspartic acid (NMDA) receptor antagonist memantine, which is recommended only for moderate to severe AD. 7 Cholinesterase inhibitors function to inhibit the enzymatic hydrolysis of acetylcholine, a neurotransmitter involved in synaptic communication, thus sustaining cholinergic neuronal signal transduction. Glutamate is another common neurotransmitter and NMDA receptor antagonists modulate glutamate signal transmission, blocking excessive calcium uptake thought to be responsible for neuron deterioration. 8 Drugs from these two classes function by distinct modes of action, yet have proven effective in supporting normal nerve signal transduction and temporarily relieving symptoms of AD.
In addition to their pharmacological activities, these drugs are also associated with adverse reactions that can influence clinical decisions regarding drug choice for specific patients with AD. Depending on patient characteristics such as age, disease progression, and other risk factors, the optimal treatment strategy can be more clearly determined when adverse reactions are clearly understood. Due to the distinct modes of action of ChEIs and NMDA receptor inhibitors, adverse reactions may also display nonoverlapping or opposite characteristics that need to be understood for best clinical outcomes. In general, ChEIs produce more frequent adverse effects than NMDA receptor inhibitors despite sharing some adverse events in common. 9 The NMDA receptor inhibitor memantine has recently been used in combination therapy with donepezil and other ChEIs. In fact, the FDA recently (2014) approved Namzaric, a fixed-dose combination of donepezil and memantine, for treatment of moderate to severe AD. Some reports suggest such combination treatments may result in the mitigation of certain competing adverse effects of donepezil by memantine. 10,11 However, more complete analysis and comparison of AD drugs and their adverse drug reactions are necessary for optimal clinical safety and care.
In a series of previously published postmarketing data analyses, the safety issues of various drugs are assessed using adverse event reports submitted to the FDA. This data source, referred to as the FDA Adverse Event Reporting System (FAERS), is a computerized information database of voluntary reports of adverse events generated by health professionals, consumers, and other sources. From 2004 to 2014, the continuous operation of the FAERS has resulted in an enormous data collection, which supports not only the FDA’s postmarketing safety surveillance program for all approved drug products but also clinical research worldwide. 12 –14 An analysis on FAERS reports once required a long time to download and preprocess the raw data. Fortunately, the openFDA initiative 15 launched in June 2014 provides an official application programming interface to access the raw data of adverse event reports in a structured format. In the present study, therefore, we were able to efficiently explore the toxicological differences between ChEIs and memantine by comparing the reporting rates of various types of adverse events that accompany them. In addition, the adverse events associated with the concomitant use of ChEIs and memantine are also examined. To our knowledge, this is the first effort to comprehensively evaluate the pharmacovigilance data of drugs for AD by exploring the FDA clinical data resource. The results generally suggest the safety advantages of memantine over ChEIs.
Materials and Methods
Drug Label Information
A drug label (also referred to as package insert) is a document approved by the FDA on the basis of clinical trials and provided along with a prescription medication that presents additional information about that drug product. 16 We searched DailyMed, the official provider of FDA drug label information, for the side effects marked by the FDA regarding donepezil (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abfaa250-8ab6-4f4b-b4a1-f9eecf9c2fcd), rivastigmine (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4e775022-4b71-4716-bc9b-f374edaf9edb), galantamine (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02b0bed7-96d4-429a-aab0-72c37e0efdaa), and memantine (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9f27baf-aa2a-443a-9ef5-e002d23407ba). We focused on 10 types of major safety risks that were referred to in the section on warnings and precautions, so as to make comparisons between ChEIs and memantine.
The FAERS Data Analysis
The original reports restored in the FAERS database were queried from OpenFDA platform following the official tutorial (https://open.fda.gov/api/reference/), which covered the period from the first quarter of 2004 through the second quarter of 2014. Prior to analysis, all drug names were unified into generic names as shown in Table 1. As for the neurological reactions, generalized convulsions and extrapyramidal symptoms were focused on herein. For the cardiovascular reaction, bradycardia was highlighted. Gastrointestinal bleeding, diarrhea, nausea, and vomiting collectively represented the gastrointestinal reactions. Obstructive pulmonary disease was focused on for pulmonary reaction. Stevens-Johnson syndrome was examined as a form of severe hypersensitivity. Additionally, drug-associated deaths were also queried. Genitourinary reactions were not analyzed due to relatively low number of reports.
Table 1.
The Terms of Drugs and Adverse Reactions Used to Query Reports of Adverse Events From FAERS.
| A. Query of drugs | |
| Drug | FAERS term |
| Memantine | “MEMANTINE HYDROCHLORIDE” |
| Donepezil | “DONEPEZIL” and “DONEPEZIL HYDROCHLORIDE” |
| Rivastigmine | “RIVASTIGMINE” and “RIVASTIGMINE TARTRATE” |
| Galantamine | “GALANTAMINE” and “GALANTAMINE HYDROBROMIDE” |
| B. Query of adverse reactions | |
| Adverse reaction | FAERS term |
| Generalized convulsions | “CONVULSION” |
| Extrapyramidal symptoms | “EXTRAPYRAMIDAL DISORDER” |
| Bradycardia | “BRADYCARDIA” |
| Gastrointestinal bleeding | “GASTROINTESTINAL HAEMORRHAGE” |
| Diarrhea | “DIARRHOEA” |
| Nausea | “NAUSEA” |
| Vomiting | “VOMITING” |
| Death | “DEATH” |
| Obstructive pulmonary disease | “CHRONIC OBSTRUCTIVE PULMONARY DISEASE” |
| Stevens-Johnson syndrome | “STEVENS-JOHNSON SYNDROME” |
Abbreviations: FDA, Food and Drug Administration; FAERS, FDA Adverse Event Reporting System.
For each type of adverse reaction, memantine alone served as the reference drug, which was compared with individual ChEIs or the combination of memantine and ChEIs. In each comparison, a two-by-two contingency table was used as the framework for analysis. For example, when donepezil was compared with memantine for nausea events, the numbers of nausea and nonnausea reports that co-occurred with donepezil were defined as n11 and n10, respectively. On the other hand, the nausea and nonnausea reports that co-occurred with memantine were defined as n01 and n00, respectively. Then, the nausea reporting odds ratio was calculated as (n11 × n00) / (n10 × n01). And the statistical significance of the difference in the reporting rate of nausea was determined by two-tailed Fisher’s exact test.
Results
The Drug Label Information of ChEIs and Memantine
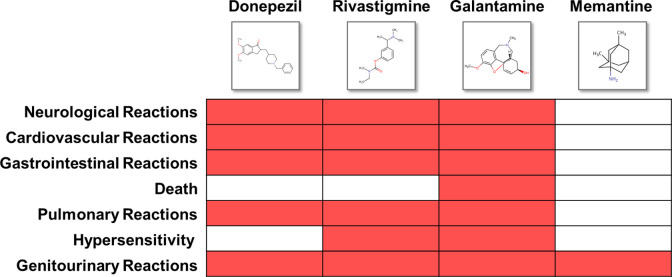
We queried drug labels of ChEIs and memantine from DailyMed 17 so as to extract information (see Materials and Methods) on adverse reactions. As shown in drug labels (Figure 1), fewer safety concerns were reported in the clinical trials of memantine. In contrast, all the ChEIs show warnings for neurological, cardiovascular, gastrointestinal, and pulmonary reactions. There were also patients experiencing hypersensitivity when administered with rivastigmine 18 or galantamine. 19 In particular, galantamine was observed to be associated with deaths in patients with mild cognitive impairment in two randomized, placebo controlled trials. 19 However, some recent studies showed different results than drug label information. For instance, ChEI use was observed to be related to a decreased risk of myocardial infarction and death. 20 In addition, patients with AD who responded to ChEIs and received higher doses have shown longer survival time. 21 To clarify the inconsistency between drug labels and clinical data, we further queried the reports associated with ChEIs and memantine via OpenFDA platform and report our findings with regard to the list of adverse reactions mentioned in the drug labels (Table 1).
Figure 1.
The adverse reactions referred to in Food and Drug Administration (FDA)-approved drug labels.
The Difference in Reporting of Adverse Events Between ChEIs and Memantine Use
We retrieved 3825 adverse events that accompanied memantine use (but not ChEIs), among which the number of reports for individual side effects was counted. On the other hand, the adverse events related to donepezil, rivastigmine, and galantamine (but not memantine) were also downloaded (with 7646, 6517, and 1172 reports, respectively). For each of the 10 major side effects of relevance, memantine was used as the reference drug, so the reporting rate of each ChEI could be compared with that of memantine to calculate the odds ratio and significance level (see Materials and Methods). As suggested by the results (Table 2), all members of ChEIs exhibited significantly stronger signals (P value < .001 in most comparisons) of extrapyramidal symptoms, bradycardia, nausea, and vomiting compared to memantine. In addition, as compared to memantine, some ChEIs (but not all) were more frequently reported for a variety of other adverse reactions, such as generalized convulsions, gastrointestinal bleeding, diarrhea, death, and obstructive pulmonary disease. While memantine was less reported for side effects in most comparisons, on the other hand, Stevens-Johnson syndrome proved to be the only side effect that exhibited no obvious difference in reporting rate between memantine and ChEIs. It was also observed that rivastigmine use was even less concurrent with generalized convulsions than memantine (odds ratio = 0.64, P value < .01). But it is still clear that FAERS data indicate the overall safety advantage of memantine over ChEIs.
Table 2.
The Comparison Between Adverse Events Co-Occurring With Memantine and ChEIs.
| Reported | Not Reported | Odds Ratio (95% CI)a | P Valueb | ||
|---|---|---|---|---|---|
| Generalized convulsions | Memantine | 109 | 3716 | – | – |
| Donepezil | 293 | 7353 | 1.36 (1.09-1.70) | 7.03E-03c | |
| Rivastigmine | 120 | 6397 | 0.64 (0.49-0.83) | 1.09E-03c | |
| Galantamine | 38 | 1134 | 1.14 (0.79-1.66) | 4.90E-01 | |
| Extrapyramidal symptoms | Memantine | 3 | 3822 | – | – |
| Donepezil | 77 | 7569 | 12.96 (4.09-41.10) | 1.72E-10d | |
| Rivastigmine | 44 | 6473 | 8.66 (2.69-27.91) | 2.18E-06d | |
| Galantamine | 9 | 1163 | 9.86 (2.66-36.48) | 2.28E-04d | |
| Bradycardia | Memantine | 12 | 3813 | – | – |
| Donepezil | 313 | 7333 | 13.56 (7.61-24.17) | 1.15E-40d | |
| Rivastigmine | 161 | 6356 | 8.05 (4.47-14.49) | 3.21E-20d | |
| Galantamine | 30 | 1142 | 8.35 (4.26-16.36) | 5.19E-11d | |
| Gastrointestinal bleeding | Memantine | 20 | 3805 | – | – |
| Donepezil | 139 | 7507 | 3.52 (2.20-5.64) | 2.49E-09d | |
| Rivastigmine | 47 | 6470 | 1.38 (0.82-2.34) | 2.54E-01 | |
| Galantamine | 13 | 1159 | 2.13 (1.06-4.30) | 3.84E-02e | |
| Diarrhea | Memantine | 105 | 3720 | – | – |
| Donepezil | 313 | 7333 | 1.51 (1.21-1.89) | 2.56E-04d | |
| Rivastigmine | 255 | 6262 | 1.44 (1.15-1.82) | 1.82E-03c | |
| Galantamine | 39 | 1133 | 1.22 (0.84-1.77) | 3.18E-01 | |
| Nausea | Memantine | 122 | 3703 | – | – |
| Donepezil | 379 | 7267 | 1.58 (1.29-1.95) | 9.72E-06d | |
| Rivastigmine | 378 | 6139 | 1.87 (1.52-2.30) | 9.81E-10d | |
| Galantamine | 57 | 1115 | 1.55 (1.13-2.14) | 9.00E-03c | |
| Vomiting | Memantine | 83 | 3742 | – | – |
| Donepezil | 334 | 7312 | 2.06 (1.61-2.63) | 8.75E-10d | |
| Rivastigmine | 463 | 6054 | 3.45 (2.72-4.37) | 1.07E-30d | |
| Galantamine | 50 | 1122 | 2.01 (1.41-2.87) | 2.43E-04d | |
| Death | Memantine | 77 | 3748 | – | – |
| Donepezil | 198 | 7448 | 1.29 (0.99-1.69) | 6.04E-02 | |
| Rivastigmine | 841 | 5676 | 7.21 (5.69-9.14) | 1.16E-95d | |
| Galantamine | 33 | 1139 | 1.41 (0.93-2.13) | 1.11E-01 | |
| Obstructive pulmonary disease | Memantine | 15 | 3810 | – | – |
| Donepezil | 61 | 7585 | 2.04 (1.16-3.60) | 1.03E-02e | |
| Rivastigmine | 25 | 6492 | 0.98 (0.52-1.86) | 1.00E+00 | |
| Galantamine | 10 | 1162 | 2.19 (0.98-4.88) | 5.89E-02 | |
| Stevens-Johnson syndrome | Memantine | 8 | 3817 | – | – |
| Donepezil | 18 | 7628 | 1.13 (0.49-2.59) | 8.39E-01 | |
| Rivastigmine | 9 | 6508 | 0.66 (0.25-1.71) | 4.53E-01 | |
| Galantamine | 2 | 1170 | 0.82 (0.17-3.85) | 1.00E+00 |
Abbreviations: ChEI, cholinesterase inhibitor; CI, confidence interval.
aOdds ratios were calculated for the ChEIs events relative to the memantine ones.
bSignificance level of 2-tailed Fisher’s exact test: cP < .01; dP < .001; eP < .05.
Concomitant Use of ChEIs and Memantine
Earlier we showed the adverse events co-occurred with either memantine or ChEIs, but not both. More recently, as ChEIs and memantine have been increasingly used concomitantly in clinical practice, the adverse reactions of the patients using them as a combination therapy attracted our further attention. It has already been found in our data that as a class of drugs, all ChEIs showed significantly higher risks of extrapyramidal disorder, bradycardia, nausea, and vomiting. Therefore, we tried to find the reporting rates of these four side effects when memantine was used in combination with one of the ChEIs. According to the results (Table 3), concomitant use could generally neutralize the adverse effects of ChEIs, since the reporting frequencies of extrapyramidal symptoms, nausea, and vomiting among the patients using memantine and ChEIs in combination were no longer significantly higher than the patients using memantine alone. As an exception, bradycardia was still more frequently associated with ChEI–memantine combination therapy (odds ratio = 4.45, P value < .001), suggesting the notable risk for ChEI-associated cardiac events.
Table 3.
The Comparison Between Adverse Events Co-Occurring With Memantine Alone and Concomitant Use of Memantine and ChEIs.
| Reported | Not Reported | Odds Ratio (95% CI) | P Valueb | ||
|---|---|---|---|---|---|
| Extrapyramidal Disorder | Memantine | 3 | 3822 | 2.86 (0.71-11.44) | 1.74E-01 |
| Memantine + ChEIsa | 6 | 2674 | |||
| Bradycardia | Memantine | 12 | 3813 | 4.45 (2.32-8.55) | 1.30E-06b |
| Memantine + ChEIs | 37 | 2643 | |||
| Nausea | Memantine | 122 | 3703 | 1.26 (0.97-1.64) | 8.76E-02 |
| Memantine + ChEIs | 107 | 2573 | |||
| Vomiting | Memantine | 83 | 3742 | 1.14 (0.82-1.58) | 4.49E-01 |
| Memantine + ChEIs | 66 | 2614 |
Abbreviations: ChEI, cholinesterase inhibitor; CI, confidence interval.
aPatients using memantine in combination with either donepezil, rivastigmine, or galantamine were included.
bSignificance level of 2-tailed Fisher’s exact test: P < .001.
Discussion
Pharmaceutical interventions bring desirable benefits to modify disease progression and/or relieve symptoms while unfortunately also producing undesired side effects and adverse reactions in patients. Certain side effects may be more tolerable for patients with different disease profiles or accompanying host factors than others (eg, nausea should be more tolerable than Stevens-Johnson syndrome for most patients). 22 Furthermore, the two classes of AD drugs, ChEIs and NMDA receptor antagonists, function by different pharmacologic mechanisms and exhibit generally distinct adverse events. 23 Even among ChEIs, different drugs have differing target protein specificities and thus produce different adverse effects. 24 Most common AEs among ChEIs are dizziness, headaches, nausea, vomiting, diarrhea, abdominal pain, and fatigue. Donepezil is generally regarded as having the lowest frequency of adverse events and rivastigmine having the most. 25 Whereas reports of NMDA receptor antagonist (memantine) adverse events are generally more rare but include dizziness, headache, hypertension, somnolence, and constipation. 9 Thus, in order to accurately estimate the benefit/risk ratio and optimize drug selection for best clinical outcomes, it is crucial to characterize and report adverse events thoroughly. This is especially true for patients with AD where varying host factors inherent in advancing age (eg, decreasing metabolic capacity, polypharmacy, comorbidities, and different quality of life goals) play an increasing role in choosing the best pharmaceutical intervention. 3,26 Furthermore, as AD is a large and growing problem with increasing burden on health professionals and care providers, even modest advances in drug safety and tolerability will have significant impact on society. 1 That is a strong incentive for us to explore the FAERS data to illuminate the toxicological differences between ChEIs and NMDA receptor antagonists.
Despite the widely recognized value of FAERS data, there are some limitations inherent to its spontaneous nature. 27 For example, most cases are manually submitted to FAERS, so the same drug name or adverse reaction may be described by variant synonyms, resulting in difficulty of analysis. Fortunately, OpenFDA platform has already annotated all drug names with generic names and adverse reactions with Medical Dictionary for Regulatory Activities terms, which enabled us to efficiently unify variant synonyms in the raw data. Another concern is that all samples in FAERS are drug users affected by certain drug side effects, with no credible randomized control group. Typically, besides the patients affected by the adverse reaction of interest, the patients reporting other adverse reactions are used as controls, even though they are not really sampled from the unaffected population. 28 Therefore, we adopt an alternative strategy by making a direct comparison between ChEIs and memantine reports. With memantine-related reports serving as the control subjects of ChEIs’ adverse events, the problem caused by the lack of control is at least partly avoided.
Our current analysis using the FAERS data clearly suggest that memantine, as the first approved NMDA receptor antagonist, exhibits significant safety advantage over ChEIs. In comparison to all three ChEIs, the proportions of extrapyramidal symptoms, bradycardia, nausea, and vomiting are obviously lower among memantine reports. Extrapyramidal symptoms are a variety of drug-induced side effects characterized by movement disorders, such as tardive dyskinesia, Parkinsonism, akathisia, and so on. 29 The onset of extrapyramidal symptoms is associated with cholinesterase inhibition in many AD cases. 30 Bradycardia, also known as bradyarrhythmia, refers to an aberrantly slow heart rate. A greater risk of bradycardia has been found in patients with dementia taking ChEIs than in those not taking these drugs. 31 Because these two adverse reactions can seriously impair the tolerability and safety of ChEIs, 32 medication is often discontinued in some patients with AD. 33 In that case, memantine may be considered as a safer alternative drug for moderate AD, with less neurological and cardiovascular concerns. On the other hand, generalized convulsions, gastrointestinal bleeding, diarrhea, death, and obstructive pulmonary disease are frequently reported with some, but not all, ChEIs (as shown in Table 2). For patients affected by these side effects, other less risky ChEIs and memantine may both be considered as candidate substitutes.
Due to the limited number of patients involved and the short duration of the clinical trials, we believe the drug label information should be reassessed and confirmed with postmarket pharmacovigilance data collected from the general population, after a drug is used over a longer time span and by large numbers of people who have a wide variety of medical conditions. Most of the above results consolidate the information in FDA-approved drug labels. However, FAERS data also indicate some signals that are inconsistent with the label information. For instance, convulsions, as a type of neurological reaction, is warned only in the labels of ChEIs but not memantine. However, only donepezil is significantly more reported for convulsions as compared to memantine, while the difference between galantamine and memantine is not evident (P value = .49). And surprisingly, rivastigmine co-occurs with convulsions even less than memantine. Another example is hypersensitivity mentioned in the labels of rivastigmine and galantamine. But Steven-Johnson syndrome, as a form of severe hypersensitivity, does not account for a high proportion of the ChEI-related events. More importantly, death events are only referred to in the label of galantamine but not detected in the FAERS data. In contrast, a very strong signal of death events (as shown in Table 2, P value = 1.16E-95) is observed in rivastigmine-associated reports, which is not mentioned in the FDA label. Such inconsistencies between drug labels and postmarketing data require further clinical validations, and perhaps reconsideration about the label information of ChEIs. But it should be noted that, although the FDA’s adverse events data were used for this study, the results and conclusions of this article were solely those of the authors and should not represent the opinions of FDA.
The link between stated adverse reactions and drugs used by a patient is subject to the reporter’s assessment and may not represent a cause-and-effect relationship. Therefore, besides analysis on pharmacovigilance data, we are acutely aware that several future extensions will be important to carry forward the current results and better understand the underlying toxicological mechanisms of AD drugs. First, it has been suggested that various side effects may result from uncharacterized drug off-target effects, that is, the unexpected interactions between drug molecules and some theoretically irrelevant target proteins. Although experimental validation of drug off-targets remains expensive and time-consuming, some in silico methods, such as chemical–protein interactome, 34 –36 have provided cost-effective alternative solutions. So we urge to further explore the off-targets specifically interacting with ChEIs or NMDA receptor antagonists in subsequent research. Second, as off-target binding can trigger profound downstream signals, some previous studies have successfully demonstrated that drug toxicity could be efficiently predicted and explained with genomic expression, 37,38 microRNA, 39 and metabolomics data. 40 Therefore, multi-omics data would further expand the dimensions of information for understanding the toxicological differences between ChEIs and NMDA receptor antagonists. Third, several prior studies (eg, a recent research published by FDA) 41 showed that adverse drug reactions are closely associated with high drug dose. However, dosage information is not clearly recorded in most FAERS reports and therefore not analyzed in the present study. So we suggest that the dosage factor should be examined in other clinical data of patients with AD so as to further optimize the regimens. Fourth, samples were divided by only drug use and adverse event occurrence in the most FAERS-based studies so far, without considering other factors. So in the subsequent research, we would like to further explore the demographic and clinical characteristics (eg, age, sex, AD severity, comorbidities, etc), which might profoundly influence the presence of side effects. Fifth, besides oral administration, AD drugs (eg, rivastigmine) can also be used as transdermal patch, which showed favorable safety profile. 42 However, the route of administration and dose information were not clear in most FAERS reports. So we suggest that other clinical data with route information should be examined to better confirm the advantage of transdermal patch in drug safety.
Due to the large demand and limited pharmaceutical options currently approved for management of AD, an increased number of drugs are expected in the drug development pipeline and general market. 2 Persistent knowledge gaps regarding the mechanisms of action and clinical efficacies for AD pharmacotherapies will also drive the development of novel pharmacological interventions. 3 Thus, understanding of safety risks and tolerability of current drugs are essential for refining the effectiveness of future therapies. The key factors that frame our understanding of current drugs and their adverse effects relate to their (1) recommended stages for clinical use, (2) symptomatic versus disease modifying status, (3) physiological context, and (4) impact of cotreatments. First, clinical trials and pharmacovigilance data indicate that memantine is better tolerated with fewer indications of adverse effects than ChEIs, 43,44 suggesting the bright prospects of drug development around NMDA receptors, especially for mild to moderate AD. Secondly, the interplay between cholinergic and glutamatergic neuron transmission and amyloid-beta pathophysiology is complex with respect to ChEI and NMDA receptor antagonist pharmacology in merely slowing the decline in AD symptoms 8 versus modifying disease onset and progression. Thirdly, AD drug mechanism of action and target protein specificity play a complex role with regard to primary pharmacological role in the central nervous system (primary target) versus their relationship to off-target effects. 45 Lastly, the recent addition of Namzaric, a fixed dose formulation of donepezil and memantine, to the list of FDA-approved AD drugs was based upon several clinical reports highlighting their distinct and complementary mechanisms of action, the lack of pharmacokinetic interaction, and improved clinical outcomes of cotreatment of the 2 drugs compared to monotherapies. 10,26,43 The successful approval of such combination therapy provides a potential pattern for future AD drug formulations. In the present study, the FAERS data have implied the potential capacity of memantine to neutralize the toxicity of ChEIs. Therefore, further evidence in assessments of drug safety and tolerability may be a key research object for combination therapies in AD.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China, the Natural Science Foundation of Jiangsu Province, Major University Science Research Program of Jiangsu Province, the Scientific Research Foundation for the Talents by Xuzhou Medical College, and Qing Lan Project of Jiangsu Province.
Footnotes
Authors’ Note: Xiaodong Shi and Xiaotian Lin contributed equally to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81300930 to XDS, 81471101 and 81273489 to CG), the Natural Science Foundation of Jiangsu Province (BK20130232 to XDS and BK2012582 to CG), Major University Science Research Program of Jiangsu Province (12KJA180008 to CG), the Scientific Research Foundation for the Talents by Xuzhou Medical College (D2012005 to XDS), and Qing Lan Project of Jiangsu Province (XDS and CG).
References
- 1. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. [DOI] [PubMed] [Google Scholar]
- 3. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. [DOI] [PubMed] [Google Scholar]
- 4. Bian L, Yang JD, Guo TW, et al. Association study of the A2 M and LRP1 Genes with Alzheimer disease in the Han Chinese. Biol Psychiatry. 2005;58(9):731–737. [DOI] [PubMed] [Google Scholar]
- 5. Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3(77):77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen CJ, Inker J. Strengthening the dementia care triad: identifying knowledge gaps and linking to resources. Am J Alzheimers Dis Other Demen. 2015;30(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchi C, Lucca U, Tettamanti M, et al. Cholinesterase inhibitor use in Alzheimer’s disease: the EPIFARM-Elderly Project. Pharmac oepidemiol Drug Saf. 2011;20(5):497–505. [DOI] [PubMed] [Google Scholar]
- 8. Danysz W, Parsons CG. Alzheimer’s disease, beta-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br J Pharmacol. 2012;167(2):324–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones RW. A review comparing the safety and tolerability of memantine with the acetylcholinesterase inhibitors. Int J Geriatr Psychiatry. 2010;25(6):547–553. [DOI] [PubMed] [Google Scholar]
- 10. McKeage K. Memantine: a review of its use in moderate to severe Alzheimer’s disease. CNS Drugs. 2009;23(10):881–897. [DOI] [PubMed] [Google Scholar]
- 11. Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I; Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. [DOI] [PubMed] [Google Scholar]
- 12. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36(2):288–295. [DOI] [PubMed] [Google Scholar]
- 13. Yang L, Wang K, Chen J, et al. Exploring off-targets and off-systems for adverse drug reactions via chemical-protein interactome–clozapine-induced agranulocytosis as a case study. Plos Comput Biol. 2011;7(3):e1002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512–518. [DOI] [PubMed] [Google Scholar]
- 15. https://open.fda.gov/. Accessed January 2015.
- 16. Yang L, Wang K, He L. Drug label indexing and mining for drug repurposing. Natural Science. 2013;2013(6):6. [Google Scholar]
- 17. de Leon J. Highlights of drug package inserts and the website DailyMed: the need for further improvement in package inserts to help busy prescribers. J Clin Psychopharmacol. 2011;31(3):263–265. [DOI] [PubMed] [Google Scholar]
- 18. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4e775022-4b71-4716-bc9b-f374edaf9edb. Accessed March 2015.
- 19. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02b0bed7-96d4-429a-aab0-72c37e0efdaa. Accessed March 2015.
- 20. Nordstrom P, Religa D, Wimo A, Winblad B, Eriksdotter M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer’s disease. Eur Heart J. 2013;34(33):2585–2591. [DOI] [PubMed] [Google Scholar]
- 21. Wattmo C, Londos E, Minthon L. Response to cholinesterase inhibitors affects lifespan in Alzheimer’s disease. BMC Neurol. 2014;14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laroche ML, Perault-Pochat MC, Ingrand I, et al. Adverse drug reactions in patients with Alzheimer’s disease and related dementia in France: a national multicentre cross-sectional study. Pharmacoepidemiol Drug Saf. 2013;22(9):952–960. [DOI] [PubMed] [Google Scholar]
- 23. Jiang J, Jiang H. Efficacy and adverse effects of memantine treatment for Alzheimer’s disease from randomized controlled trials. Neurol Sci. 2015;36(9):1633–1641. [DOI] [PubMed] [Google Scholar]
- 24. Parsons CG, Danysz W, Dekundy A, Pulte I. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez OL, Becker JT, Wahed AS, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. [DOI] [PubMed] [Google Scholar]
- 28. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf. 2005;28(3):191–208. [DOI] [PubMed] [Google Scholar]
- 30. Heinze M, Andreae D, Grohmann R. Rivastigmin and impaired motor function. Pharmacopsychiatry. 2002;35(2):79–80. [DOI] [PubMed] [Google Scholar]
- 31. Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs new England healthcare system. J Am Geriatr Soc. 2009;57(11):1997–2003. [DOI] [PubMed] [Google Scholar]
- 32. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;(127):45–63. [PubMed] [Google Scholar]
- 33. Frankfort SV, Appels BA, de Boer A, et al. Discontinuation of rivastigmine in routine clinical practice. Int J Geriatr Psychiatry. 2005;20(12):1167–1171. [DOI] [PubMed] [Google Scholar]
- 34. Luo H, Chen J, Shi L, et al. DRAR-CPI: a server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011;39(Web Server issue):w492–w498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang L, Chen J, Shi L, Hudock MP, Wang K, He L. Identifying unexpected therapeutic targets via chemical-protein interactome. PLoS One. 2010;5(3):e9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang L, Wang KJ, Wang LS, et al. Chemical-protein interactome and its application in off-target identification. Interdiscip Sci. 2011;3(1):22–30. [DOI] [PubMed] [Google Scholar]
- 37. Wang K, Weng Z, Sun L, Sun J, Zhou SF, He L. Systematic drug safety evaluation based on public genomic expression (Connectivity Map) data: myocardial and infectious adverse reactions as application cases. Biochem Biophys Res Commun. 2015;457(3):249–255. [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Sun J, Zhou S, et al. Prediction of drug-target interactions for drug repositioning only based on genomic expression similarity. Plos Comput Biol. 2013;9(11):e1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Z, Qin T, Wang K, et al. Integrated microRNA, mRNA, and protein expression profiling reveals microRNA regulatory networks in rat kidney treated with a carcinogenic dose of aristolochic acid. BMC Genomics. 2015;16:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun L, Li J, Zhou K, et al. Metabolomic analysis reveals metabolic disturbance in the cortex and hippocampus of subchronic MK-801 treated rats. PLoS One. 2013;8(4):e60598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weng Z, Wang K, Li H, Shi Q. A comprehensive study of the association between drug hepatotoxicity and daily dose, liver metabolism, and lipophilicity using 975 oral medications. Oncotarget. 2015;6(19):17031–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grossberg G, Sadowsky C, Frostl H, et al. Safety and tolerability of the rivastigmine patch: results of a 28-week open-label extension. Alzheimer Dis Assoc Disord. 2009;23(2):158–164. [DOI] [PubMed] [Google Scholar]
- 43. Kavirajan H. Memantine: a comprehensive review of safety and efficacy. Expert Opin Drug Saf. 2009;8(1):89–109. [DOI] [PubMed] [Google Scholar]
- 44. Tampi RR, van Dyck CH. Memantine: efficacy and safety in mild-to-severe Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res. 2013;36(4):375–399. [DOI] [PubMed] [Google Scholar]