Abstract
Objective:
To investigate the feasibility and effectiveness of Sonas, a group intervention involving multisensory stimulation, reminiscence, and light physical activity.
Methods:
A total of 39 participants with moderate to severe dementia were randomized to receive either 14 sessions of Sonas or treatment as usual. Measures such as quality of life (QoL), communication, depression, anxiety, and behavioral disturbance were administered at baseline and follow-up.
Results:
No statistically significant results were found. However, participant attendance to sessions was good (mean = 12.4 sessions of 14 offered).
Conclusions:
Sonas sessions did not lead to improvements in QoL and behavioral and psychological symptoms of dementia.
Keywords: dementia, quality of life, intervention, multisensory, stimulation, Sonas
Introduction
The Problem
People with dementia can experience symptoms such as depression, anxiety, and aggression, often called behavioral and psychological symptoms of dementia (BPSD). 1 They can also experience poor quality of life (QoL). 2 Evidence suggests there is a link between QoL and BPSD. 3 -6 Given this, interventions targeted toward improving QoL may also lead to the alleviation of BPSD.
The Care Home Environment and the Role of Activities
There is a high level of inactivity in care homes for people with dementia. 7,8 Indeed, it has been found that the most common unmet need for individuals with dementia who were residing in care homes is related to the provision of stimulating daytime activities. 9 This may be in part because residents are dependent on staff to engage in activities, 10 and finding appropriate activities is challenging for staff members who may not have had the opportunity to develop specialized skills in this area.
Humans have an innate biological drive to engage in meaningful activities, as they are essential to survival and health. 11 Activities can provide a sense of self-worth, maintain identity, promote mastery, and encourage social connections. 12 Within care homes for older people with and without dementia, increased activity participation, communication, and social connectedness are related to both QoL and BPSD. 13 -16
There is accumulating evidence to suggest that activities involving physical activity, music therapy, aromatherapy, massage, and reminiscence may benefit individuals experiencing BPSD. 17,18,19
Sonas
Sonas apc (Sonas, an Irish word meaning well-being, joy and contentment, and apc, which stands for activating potential for communication) is an intervention developed in the 1990s by Sister Mary Threadgold, a Speech and Language Therapist. Sister Mary originally supported people with learning disabilities. This work enabled her to recognize the importance of music and touch in facilitating interactions with people who found verbal communication difficult. Through observation, Sister Mary noted the lack of stimulation in care home environments for individuals with dementia. Given individuals with learning disabilities and those diagnosed with dementia experience cognitive impairment, she posited that the approach she had been adopting in her work with people with learning disabilities could also be useful for individuals with dementia. She suggested a structured group approach to provide participants with increased opportunity for social interaction. The main aim of Sonas is therefore to enhance communication and thereby improve QoL. 20
Sonas sessions involve multisensory stimulation (eg, the opportunity to listen to music, smell pleasant/interesting fragrant objects, taste food, and receive a massage), reminiscence activities (listening to proverbs and poems and looking at interesting household/personal items), and physical activities (gentle exercises which group members can do while sitting or standing). Although sessions are structured using a prerecorded tape (see Table 1 for the order of activities), group facilitators are encouraged to use their knowledge of the group members to adapt the materials used in each session. For instance, the Sonas manual suggests using materials such as fob watches, hand cream, perfume, seasonal foods, and flowers. During the opening and closing songs, the main group facilitators greet each group member by name, shaking their hand, and ensuring they make eye contact. Group members are invited to sing and/or dance whenever music is played.
Table 1.
Order of Activities Within a Sonas Session.
| Activity | Example |
|---|---|
| Signature tune | Instrumental music |
| Greeting song | Group facilitators sing: “Hello … How are you? It’s so nice to see you, we welcome you” to each group member, shaking their hand, and using their name |
| Exercises to music | Stretching and circling hands |
| Stimulation of the sense of smell | Flowers, soap, and baking spices |
| Singing to music | “Deep in the Heart of Texas” |
| Relaxing music with stimulation of taste/touch | Instrumental music with shoulder massage and offer of cake |
| Lively music with dancing and percussion instruments | Lively instrumental music, clapping, and use of tambourine encouraged |
| Proverbs and poetry | Daffodils by William Wordsworth |
| Group members’ contributions (pause CD here) | Anything group members may enjoy sharing/participating in, for example, blowing bubbles and throwing a bean bag |
| Singing to music | “Catch a Falling Star” |
| Closing song | Instrumental music |
| Signature tune | “We will meet again and I know where and when, yes I know we will meet again some sunny day” (as with greeting song, Sonas training encourages facilitators to shake hands with each group member individually and sing this using each person’s name) |
The Sonas manual states that all sessions should be facilitated by 2 staff, at least one of whom is trained in the Sonas approach. Sonas training consists of 2 full day workshops separated by approximately 1 month to give trainees the opportunity to practice and discuss experiences during the second day. Individuals are not required to have any formal qualifications to attend these workshops. Further detail on the Sonas approach is available at http://www.sonasapc.ie.
To date, over 6200 members of health care staff from a variety of disciplines have been trained to deliver the Sonas program in both Ireland and the United Kingdom. Anecdotal evidence suggests that Sonas can lead to improvements in awareness, mood, memory, and communication. 20-21
One unpublished study conducted in Ireland examined the effects of weekly Sonas sessions delivered over a 3-month period. The authors used the Mini-Mental State Examination (MMSE), Confusion Symptoms Checklist, the Adaptive Behavior Scale, the Holden Communication Scale, and 2 observational scales (1 devised by the authors and 1 based on a checklist examining social skills in individuals with speech and language difficulties) to assess potential change. It was concluded that participants displayed a significant increase in purposeful activity, social interaction, verbal communication, and independent functioning, but no significant improvements in affect, interaction, or cognition were reported. Problems with this research included the lack of a control group, an absence of blinding, data mining, substantial missing data, inclusion of participants without dementia, and the fact that some Sonas sessions were facilitated by staff not trained in the Sonas approach.
A second unpublished study, also conducted in Ireland, investigated the impact of twice weekly Sonas sessions delivered over a 6-month period. They attempted to overcome some of the methodological weaknesses in the original study by recruiting a larger participant group, using blinded researchers and including a control group. The authors used the MMSE, a depression rating scale, the Baumgarten Dementia Rating Scale, the Blessed-Roth Scale, and the Holden Communication Scale. This second study reported significant improvements in activities of daily living, behavior, cognition, and communication for the experimental group but not the control group. There were also problems with this study such as including people without dementia. In addition, there were statistical flaws such as multiple comparisons with no statistical adjustment for this and no direct statistical comparison between the experimental and the control groups. The outcome measures used did not have established reliability and validity and lastly the method of randomization was unclear.
Given its widespread use and the importance of establishing quality psychosocial interventions for people with moderate to severe dementia, there is the need for a methodologically sound evaluation of Sonas for this population.
The aims of this pilot randomized controlled trial (RCT) were to:
investigate whether Sonas improves depression, anxiety, behavioral disturbance, communication, and QoL for residents with dementia;
investigate feasibility (ie, participant attendance and staff reports of implementing the intervention) by gathering information relevant to the practicalities of conducting future, larger trials examining the effectiveness of Sonas.
Methods
Participants
Participants were people with dementia aged 65 years or older. They were recruited from 4 care homes in the United Kingdom. All care homes were privately owned and located near London. They cared for approximately 40 to 71 residents. Eligible participants met the following criteria:
diagnosis of dementia according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria (APA, 2000);
moderate to severe cognitive impairment as classified by pre-trial MMSE scores of 0 to 17 22 ; the MMSE was conducted by the principal researcher;
no serious health problems that could impact on their attendance;
no exposure to Sonas approach in the past 3 months;
functionally able to attend a group (ie, sufficient mobility, able to maintain some concentration, and remain in a 45- to 60-minute session, minimal challenging behavior that would be unlikely to cause disruption);
English speaking.
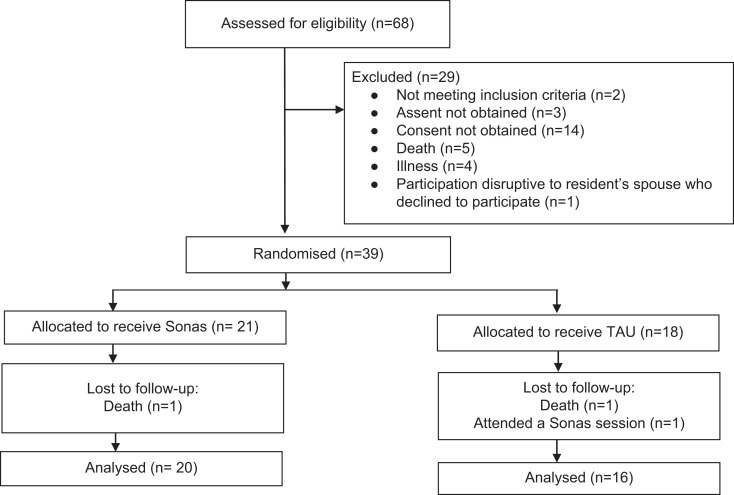
Sixty-eight care home residents were assessed for eligibility for the study; however, only 39 participants were randomized. For further details regarding the recruitment process see Figure 1.
Figure 1.
Flowchart showing patient’s progression through study.
Procedure
The managers of 9 care homes supporting people with dementia were contacted via telephone by the Chief Executive of Sonas apc. These homes either employed staff who had already been trained in the Sonas approach or were run by managers interested in having staff attend Sonas training. Four homes agreed to participate. For the remaining 5 homes, time constraints seemed to be the main prohibiting factor for participation in the study. Some of these 5 homes had also already been implementing the Sonas approach for some time, and given the size of these homes, it would have been difficult to recruit residents who had not already been exposed to the intervention. The managers and Sonas-trained staff from the 4 homes discussed the inclusion criteria with the researchers and identified care home residents who could potentially take part. Consent procedures for care home residents followed the Mental Capacity Act (2005). The principal researcher CH (who had received training on the Mental Capacity Act) met with all identified care home residents to explain the study and assess their capacity to consent. After a capacity assessment, all identified care home residents were not considered to have capacity to consent to the study. Informed consent was therefore obtained from family members, nominated contact persons, or general practitioners where a relative or named contact person was not available. As the outcome measures used in the study involved interviewing staff about the residents, consent was also obtained from all staff members involved in the assessments. The study was approved by the South East Research Ethics Committee.
Randomization
A total of 39 people with dementia were randomly assigned to the experimental group or treatment as usual (TAU) group by simple randomization. A computer program entitled “Random Allocation Software” (accessed from http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html) was used. Randomization was conducted separately at each site. Participants in the experimental group were invited to attend 14 Sonas sessions over a 7- to 8-week period. Sessions lasted for approximately 45 minutes. Participants in the control group continued to engage in their usual activities.
Assessment
Alongside data on attendance to Sonas sessions, the following assessments were conducted by the principal researcher (CH) who was blinded to treatment allocation. CH interviewed care home staff who knew the participants. Staff were requested to retain information that could affect blinding. Staff based their responses on observations they had made of participants over the required period. Assessments were administered 1 to 2 weeks before and 1 week after the intervention.
The Rating Anxiety in Dementia (RAID) Scale 23 measures anxiety symptoms categorized as worry, apprehension and vigilance, motor tension, autonomic hypersensitivity, phobias, and panic attacks. Scores range from 0 to 54, with higher scores indicating greater anxiety. A score of 11 or more suggests significant clinical anxiety. The scale has moderate to good test–retest and interrater reliability and good content validity. 23
The Cornell Scale for Depression in Dementia 24 (CSDD) examines depressive symptoms categorized as mood, behavioral disturbance, physical signs, cyclic functions, and ideational disturbances. Scores range from 0 to 38. Higher scores indicate increased levels of depression. The CSDD has good internal and interrater reliability and high concurrent validity. 25
The Neuropsychiatric Inventory Questionnaire 25 (NPI-Q) assesses mood and behavioral disturbances including delusions, hallucinations, dysphoria, agitation/aggression, euphoria, disinhibition, and apathy. Within each domain, the respondent rates the severity of the behavioral disturbance (ranging from 1-3) and the distress they experience when supporting the resident with the disturbance (ranging from 0-5). From these ratings, 3 scores can be calculated: a total distress score, a total severity score, and an overall score (the sum of frequency × severity ratings for each domain). Higher scores indicate greater disturbance. The NPI-Q has adequate test–retest reliability and convergent validity. 26
The Quality of Life-Alzheimer’s Disease (QoL-AD) Scale 27 assesses QoL. It covers areas such as physical health, energy, mood, friends, fun, self, and life as a whole. Where possible, the scale obtains separate ratings of the patient’s QoL from the patient and the caregiver. In the current study, only caregiver ratings were used. Scores range from 13 to 52, with higher scores indicating better QoL. It has good content, concurrent, and construct validity and excellent interrater reliability and internal consistency. 28
The Holden Communication Scale 29 examines social behavior and communication. It includes items assessing conversation, awareness, humor, and responsiveness. Scores range from 0 to 48. Higher scores suggest more difficulties. Although no formal psychometric properties have been reported for this scale, it has been used in other published trials examining interventions for dementia
Intervention
As Sonas was developed in Ireland during the 1990s, the researchers suggested that a consensus conference was held to discuss adaptations to the program to make it more suitable for individuals from the United Kingdom. However, the Sonas organization emphasized their wish for Sonas to be evaluated without it being changed.
Staff facilitating Sonas sessions were asked to conduct sessions in a designated room away from other residents not participating in Sonas sessions, although some care home residents who were not part of the study were also invited to Sonas sessions. Two staff members in each care home had attended Sonas training and were asked to adhere to guidance of having 2 staff members facilitating sessions, at least 1 of whom was Sonas trained. Sonas sessions were conducted at various times of day.
Sonas sessions were planned to be facilitated twice per week for 7 weeks. Sessions were facilitated at different times and days each week due to staff availability. Sonas sessions lasted between 45 minutes and 1 hour.
Analysis
Because of the absence of adequate prior research investigating Sonas, the likely effect size of the intervention could not be accurately estimated. Despite this, assuming equal group sizes, power calculations were carried out using the “G*Power 3” computer program, 30 setting α at .05 (5%) and desired power at .80 (80%). This showed that if a sample size of 50 participants was obtained, the study would be adequate to detect an effect size of .81 or above (large effect). Given the possibility of attrition and/or recruitment difficulties, by setting α at .1 (which would still indicate trends in outcome data) and desired power at .80, it was found that a sample size of 40 would be sufficient to detect an effect size of .80 or above. However, as the study was a pilot, it was considered that it would still be of value even if no statistically significant results were found, as it would provide useful information about the feasibility of the intervention and research methods. Effect size calculation in the current study could also be used to determine the likelihood of significant effects if a larger sample was recruited in future. The use of a pilot study to determine the viability of both an intervention, and further research into its effectiveness, is consistent with the MRC Complex Interventions Guidance. 31 Data were analyzed using SPSS 17.0 for Windows. Descriptive statistics are reported. An independent measures t test was used to analyze the difference in pre- and postscore changes between groups for all outcome measures. Effect size r was also used.
Results
Recruitment of Participants
The recruitment of residents is shown in Figure 1.
The Participant Group
The mean age of the participants was 86.6 (standard deviation [SD] = 6.7, range 70-99), the mean number of years spent in the care home was 2.5 (SD = 2.1, range = .1-8.7), and the mean MMSE score was 4.9 (SD = 5.2, range = 0-17). The majority of the participant group was female (86.1%), caucasian (97.2%), and placed within the severe dementia range (80.6%) as opposed to the moderate range. Participants had been diagnosed with Alzheimer’s disease (33.3%), vascular dementia (13.9%), Lewy body dementia (2.8%), mixed dementia (2.8%), and 47.2% of participants had an unspecified diagnosis. Baseline scores are reported in Table 2.
Table 2.
Baseline Scores.a
| Measure | M | SD | Range |
|---|---|---|---|
| CSDD | 5.3 | 4.0 | 0-16 |
| RAID | 6.8 | 5.7 | 0-21 |
| NPI-Q (overall) | 14.0 | 15.1 | 0-51 |
| NPI-Q (distress) | 7.1 | 6.2 | 0-23 |
| NPI-Q (severity) | 8.5 | 5.9 | 1-26 |
| QoL-AD (carer version) | 33 | 5 | 24-41 |
| Holden | 19.0 | 8 | 5-40 |
Abbreviations: CSDD, Cornell Scale for Depression in Dementia; RAID, Rating Anxiety in Dementia Scale; NPI-Q, Neuropsychiatric Inventory Questionnaire; QoL-AD, Quality of Life in Alzheimer’s Disease Scale; Holden = Holden Communication Scale; SD, standard deviation.
an = 36.
Implementation of Sonas Program and Research Protocol
The mean number of Sonas sessions attended was 12.4 (of 14) with a range from 8 to 14. Between 2 and 6 participants attended each Sonas session. The main reasons for resident nonattendance were physical illness, agitation, and a lack of interest. At times, some residents were disruptive during sessions.
As mentioned earlier, the Sonas manual stipulates that all sessions should be facilitated by 2 members of staff, at least one of whom is Sonas trained. Resources for this were arranged—2 members of staff in each home had attended the Sonas training and session schedules were preagreed by the researcher and care homes. Despite this, in 2 of the homes, some sessions were facilitated by only 1 Sonas-trained staff member, sometimes alone and sometimes with the support of another non-Sonas trained member of staff. The main reason cited by staff for this was that sessions could be run on different days and at different times to fit the schedule of the main staff member, and the supporting staff member was not available at short notice. In 1 home, 1 member of staff took unplanned leave for 1 week. This absence meant there was 1 week when the group sessions were not run. The intervention period in this home was therefore extended to 8 weeks instead of 7 weeks.
Evaluation of Effects of Sonas Sessions
Data from 3 participants were excluded, as 1 participant was randomly allocated to the TAU condition but mistakenly attended 1 Sonas session, and 2 participants (1 in the TAU group and 1 in the Sonas group) died before the follow-up data were collected; see Figure 1 for further details. There were no statistically significant findings for any of the outcome measures. Using r as a measure of effect size, it was found that the difference between the change scores for the CSDD yielded a small effect in favor of the TAU group; see Table 3 for further details.
Table 3.
Descriptive Statistics and Analysis of Difference Scores Using Independent Measures t Test.a
| Variable Assessed | Test Used | Baseline Scores, Mean (SD) | Follow-Up Scores, Mean (SD) | Change + Improve − Decline | Difference in Mean Change Scores | Between Group Differences (Independent t Test) | Effect Size (r) | |
|---|---|---|---|---|---|---|---|---|
| t | P | |||||||
| Depression | CSDD | T 5.10 (3.34) C 5.44 (4.82) | 4.75 (3.35) 4.38 (3.72) | +.35 +1.06 | .71 | .700 | .489 | .119 |
| Anxiety | RAID | T 7.30 (6.51) C 6.06 (4.63) | 7.05 (5.84) 5.75 (5.88) | +.25 +.31 | .06 | .039 | .969 | .007 |
| Behavior (overall score) | NPI-Q | T 17.20 (14.42) C 10.06 (15.34) | 14.68 (16.38) 9.31 (13.26) | +2.52 +.75 | 1.77 | .413 | .682 | .071 |
| Behavior (severity) | NPI-Q | T 9.40 (5.21) C 7.38 (6.64) | 8.25 (6.09) 7.06 (4.91) | +1.15 +.32 | .83 | −.467 | .643 | .080 |
| Behavior (distress) | NPI-Q | T 8.71 (5.91) C 5.17 (6.20) | 7.72 (7.31) 4.38 (5.77) | +.99 +.79 | .2 | −.102 | .919 | .018 |
| Quality of life (carer version) | QoL-AD | T 31.70 (4.57) C 33.78 (6.21) | 32.26 (4.64) 32.91 (7.37) | +.56 −.87 | 1.43 | .726 | .498 | .124 |
| Communication | Holden | T 21.80 (8.51) C 15.44 (5.81) | 22.05 (9.39) 17.31 (5.68) | −.25 −1.87 | 1.62 | −.938 | .355 | .159 |
Abbreviations: T, treatment group, C, control group; CSDD, Cornell Scale for Depression in Dementia; RAID, Rating Anxiety in Dementia Scale; NPI-Q, Neuropsychiatric Inventory Questionnaire; QoL-AD, Quality of Life in Alzheimer’s Disease Scale; Holden, Holden Communication Scale; SD, standard deviation.
a P < .05.
Discussion
This is the first well-designed evaluation of Sonas using an RCT. No significant differences were found between the Sonas and the TAU groups for any of the outcome measures between baseline and follow-up. Notably, the effect sizes were so small (<.20) that there was no evidence to even suggest that a very large sample might produce a significant result indicating any worthwhile benefit. At times, Sonas-trained staff facilitated sessions without another member of staff to support them, citing scheduling difficulties and the frequency of sessions as a reason for this. The intensity of the intervention and staff resources required may therefore make it difficult to implement consistently in care homes. Sonas was developed in Ireland. Much of the program therefore incorporates Irish poetry and music which participants in the United Kingdom are unlikely to be familiar with. Research suggests that music selection for dementia interventions is important; although standardized music can lead to reductions in agitation, when given a choice, participants are likely to pick music in keeping with their cultural context. 32 Furthermore, music that is not carefully selected can increase behavioral disturbance. 33 This may go some way in explaining the lack of significant findings.
Limitations
It was difficult to rate some items on the RAID and Cornell. For instance, 1 question on the scale asks whether a person with dementia has been worried about their physical health. Given the limited capacity for residents to verbalize their concerns, staff reported being uncertain about how to rate this and other similar items. Although staff could make a judgment on the basis of a resident’s nonverbal behavior, this has limited accuracy. It should also be noted that although the researcher administering the assessments was blind to randomization, care home staff members were not. This may have biased the results. Furthermore, no stipulation was placed on how much time staff members spent with a resident—this did mean that at times staff found it difficult to recall specific information which could help them provide accurate ratings.
No measure of adherence to the Sonas intervention was used. This may have limited the likelihood of obtaining significant treatment effects. Indeed, some Sonas sessions were shorter than planned due to limitations in participants’ attention.
Some studies investigating interventions for BPSD have only included participants who display a minimum level of the variable under interest (eg, agitation) and/or the intervention is conducted when the variables under study are at their highest. However, no such inclusion criterion was set for the current study. This decision was made for several reasons. First, there is a high prevalence of BPSD among people placed in long-term care homes. 34 Furthermore, while some level of behavioral disturbance could be tolerated in a group setting, high levels may cause disruption to other residents in the session. Having no minimum cutoff point meant that the average baseline scores for many of the areas examined were low. Therefore, floor effects may have been an issue. However, some studies investigating the impact of interventions on BPSD have not set minimum cutoff points but have found significant treatment effects. Furthermore, no significant treatment effect was found for the QoL-AD, which would not have been at such a risk of floor or ceiling effects given the baseline scores (see Table 2).
Finally, evidence suggests that, for individuals with dementia, comorbid depression can fluctuate considerably and dissipate as the disease progresses. 35 Although the range of dementia in the current study was moderate to severe, the mean MMSE score was low (4.9); therefore, the changes in CSDD may reflect normal variation in this population.
As mentioned previously, it was not possible to conduct a consensus conference prior to the current study. Before any further research examining Sonas is conducted, it is recommended that a conference is held and inclusion of other, more relevant music, poetry, and prose within the Sonas program (which may increase meaningfulness for the target audience) should be considered. The focus of the current study was to examine whether Sonas leads to changes in QoL, communication and BPSD which can be observed outside sessions. Analysis of within session changes using a measure such as Dementia Care Mapping would enhance the results. Indeed, as with other multisensory interventions, 36 it may be that benefit only occurs during sessions. In the current study, the intervention under examination was group based. Although group interventions provide the opportunity for social interaction, if 1 group member displays disturbed behavior this can be disruptive for others. Future research could evaluate the effectiveness of Sonas Individual Multisensory Sessions (SIMS). The SIMS implements similar interventions to those used in the group Sonas sessions but on a one-to-one basis. The individualized nature of SIMS may provide more opportunity to support individuals with BPSD who may find attending a group difficult.
Conclusion
The results of this study do not suggest that Sonas has any therapeutic benefit. Nevertheless, Sonas continues to be widely used and remains popular with dementia residents and staff, particularly in Ireland. Adaptations to the approach should be considered, particularly in relation to the applicability of the intervention to its recipients and the feasibility of implementation,
Footnotes
Authors’ Note: This manuscript contains original unpublished work and is not being submitted for publication elsewhere at the same time.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Clara Kalu, University College London (Sponsor reviewed ethics application only. They were not involved in any other part of the project). The corresponding author received a sum of money to cover travel expenses and pay staff participants for their time.
References
- 1. Finkel S. Introduction to behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. 2000;15 (suppl 1):S2–S4. [DOI] [PubMed] [Google Scholar]
- 2. Lakey L, Chandaria K, Quince C, Kane M, Saunders T. Dementia 2012: A National Challenge. Alzheimer’s Society 2012. [Google Scholar]
- 3. Banerjee S, Smith SC, Lamping D L, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatry. 2006;77 (2):146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. González-Salvador T, Lyketsos CG, Baker A, et al. Quality of life in dementia patients in long-term care. Int J Geriatr Psychiatry. 2000;15 (2):181–189. [DOI] [PubMed] [Google Scholar]
- 5. Hoe J, Hancock G, Livingston G, Orrell M. Quality of life of people with dementia in residential care homes. Br J Psychiatry. 2006;188 (5):460–464. [DOI] [PubMed] [Google Scholar]
- 6. Hoe J, Katona C, Orrell M, Livingston G. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry. 2007;22 (10):1031–1036. [DOI] [PubMed] [Google Scholar]
- 7. Burgio LD, Scilley K, Hardin JM, et al. Studying disruptive vocalization and contextual factors in the nursing home using computer-assisted real-time observation. J Gerontol. 1994;49 (5):P230–P239. [DOI] [PubMed] [Google Scholar]
- 8. Challis D, Mozley CG, Sutcliffe C, et al. Dependency in older people recently admitted to care homes. Age Ageing. 2000;29 (3):255–260. [DOI] [PubMed] [Google Scholar]
- 9. Hancock GA, Woods B, Challis D, Orrell M. The needs of older people with dementia in residential care. Int J Geriatr Psychiatry. 2006;21 (1):43–49. [DOI] [PubMed] [Google Scholar]
- 10. Holthe T, Thorsen K, Josephsson S. Occupational patterns of people with dementia in residential care: an ethnographic study. Scand J Occup Ther. 2007;14 (2):96–107. [DOI] [PubMed] [Google Scholar]
- 11. Wilcock AA. Occupation and health: are they one and the same? J Occup Sci. 2007;14 (1):3–8. [Google Scholar]
- 12. Hocking C. Contribution of occupation to health and well-being. In: Crepeau EB, Cohn ES, Boyt Schell BA, eds. Willard and Spackman’s Occupational Therapy. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:45. [Google Scholar]
- 13. DePoy E, Archer L. The meaning of quality of life to nursing home residents: a naturalistic investigation. Top Geriatr Rehabil. 1992;7 (4):64–74. [Google Scholar]
- 14. Mitchell JM, Kemp BJ. Quality of life in assisted living homes: a multidimensional analysis. J Gerontol Series B Psychol Sci Soc Sci. 2000;55 (2):P117–P127. [DOI] [PubMed] [Google Scholar]
- 15. Mozley CG. Exploring connections between occupation and mental health in care homes for older people. J Occup Sci. 2001;8 (3):14–19. [Google Scholar]
- 16. Zimmerman S, Sloane PD, Williams CS, et al. Dementia care and quality of life in assisted living and nursing homes. The Gerontologist. 2005;45 (suppl 1):133–146. [DOI] [PubMed] [Google Scholar]
- 17. Basu A, Brinson D. The effectiveness of non-pharmacological interventions for behavioural and psychological symptom management for people with dementia in residential care settings. HSAC Report. 2010;3(19). [Google Scholar]
- 18. Kong E, Evans LK, Guevara JP. Nonpharmachological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13 (4):512–520. [DOI] [PubMed] [Google Scholar]
- 19. Van Mierlo LD, Van der Roes HG, Meiland FJM, Droes RM. Personalized dementia care: Proven effectiveness of psychosocial interventions in subgroups. Ageing Res Rev. 2010;9 (2):163–183. [DOI] [PubMed] [Google Scholar]
- 20. Threadgold M. Touching the soul through the senses. J Dement Care.1995;3 (4):18–19. [Google Scholar]
- 21. Black C. Speech and language therapy work in Sonas groups. In: Marshall M, ed. Perspectives on Rehabilitation and Dementia. London: Jessica Kingsley; 2004: 129. [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12 (3):189–198. [DOI] [PubMed] [Google Scholar]
- 23. Shankar KK, Walker M, Frost D, Orrell MW. The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging Ment Health. 1999;3 (1):39–49. [Google Scholar]
- 24. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23 (3):271–284. [DOI] [PubMed] [Google Scholar]
- 25. Cummings JL, Mega M, Gray K. The neuropsychiatric inventory. Neurology. 1994;44 (12):2308–2308. [DOI] [PubMed] [Google Scholar]
- 26. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12 (2):233–239. [DOI] [PubMed] [Google Scholar]
- 27. Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging. 1999;5 (1):21–32. [Google Scholar]
- 28. Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway?: The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord. 2003;17 (4):201–208. [DOI] [PubMed] [Google Scholar]
- 29. Holden U, Woods RT. Positive Approaches to Dementia Care. 3rd ed. New York: Churchill Livingstone; 1995: 200. [Google Scholar]
- 30. Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39 (2):175–191. [DOI] [PubMed] [Google Scholar]
- 31. Craig P, Dieppe P, Macintyre S. Developing and evaluating complex interventions: the new medical research council guidance. BMJ. 2008;337 (7676):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sung HC, Chang AM, Lee WL. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J Clin Nurs. 2010;19 (7-8):1056–1064. [DOI] [PubMed] [Google Scholar]
- 33. Nair BK, Heim C, Krishnan C, D'Este C, Marley J, Attia J. The effect of Baroque music on behavioural disturbances in patients with dementia. Australas J Ageing. 2011;30 (1):11–15. [DOI] [PubMed] [Google Scholar]
- 34. Margallo-Lana M, Swann A, O’Brien J, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry. 2001;16 (1):39–44. [DOI] [PubMed] [Google Scholar]
- 35. Eustace A, Coen R, Walsh C, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17 (10):968–973. [DOI] [PubMed] [Google Scholar]
- 36. Baker R, Bell S, Baker E, et al. A randomized controlled trial of the effects of multisensory stimulation (MSS) for people with dementia. Br J Clin Psychol. 2001;40 (pt 1):81–96. [DOI] [PubMed] [Google Scholar]