Abstract
Patients diagnosed with Alzheimer’s disease (AD) tend to underestimate their pain degree as disease progresses. Their caregivers are the most important source of information by providing regular pain evaluation. Our objectives were to compare pain intensity and affective pain between patients with AD and cognitively normal individuals (N) and to evaluate differences in pain perception between their caregivers. We evaluated pain scores of 121 patients with chronic osteoarticular pain, 60 AD, and 61 N using the colored pain scale/faces pain scale and the caregiver’s perception. Data were analyzed using one and two-tailed paired t tests (P < .05). We found that the AD group reported less pain intensity and that their pain was less perceived by their caregiver. This study also points to the need of, when evaluating patients with ADalways measure their pain degree using appropriate scales, instead of relying only on the caregiver.
Keywords: Alzheimer, pain, scales, caregiver
Introduction
Progressive dementia encompasses a variety of diseases, with Alzheimer’s disease (AD) being the most prevalent. 1 This disease leads to a deterioration of intellectual faculties, such as memory, concentration, and judgment, resulting from an organic disease or a disorder of the brain. It is sometimes accompanied by emotional disturbance and personality changes. Its course is gradual and results in significant impairment of social and occupational functioning. 2
Overall, older people are more likely to have painful pathology due to the increased incidence of chronic medical conditions, particularly of rheumatologic osteoporosis, osteoarthritis, and oncology fields; pain represents 25% to 50% of the complaints in outpatient and 45% to 80% of the complaints in institutionalized patients. 3 Insufficient use of analgesics for treating nursing home residents with pain was frequently reported, especially in those with a low cognitive status. 4 Several epidemiological studies have shown that in many cases pain in the elderly patients is not recognized and therefore not treated, 5 which can alter their quality of life, increasing phenomena such as depression, aggression, social withdrawal, and decreased function.
There are several studies pointing out that in AD, perception and processing of pain are not decreased, in contrary to what was usually accepted. 6,7 The motor and cognitive impairment in patients with AD is accompanied by a reduction in the ability to communicate, which makes it difficult to detect pain in these patients. By failing to obtain adequate pain treatment, structural and irreversible changes may occur in the structures of the central nervous system involved in the transmission/modulation of nociceptive information, which accounts to chronic pain installation. 8 Curiously, the 2 components of pain response are differentially affected in patients with AD. 7,9 Although the sensory-discriminative component is preserved, pain tolerance, associated with the affective-emotional aspect, largely increases.
These apparent discrepancies appear to have a neurobiological explanation since the somatosensory cortex and thalamic nuclei involved in sensory-discriminating component of pain response appear to be preserved in AD while the neuronal loss was detected in the prefrontal and limbic structures, with obvious implications for affective-emotional pain-related reactions. 3 Although psychophysical and neuroimaging techniques have evolved over the recent years in order to unravel the structural and functional changes in the brain of patients with AD, it remains challenging interpreting the relationship between these tools and pain reports. 10
The evaluation of pain, with no specific test or tracers and large individual variability, is always complex. To worsen matters, in the case of dementias, one-third of the patients are in later stages of the disease therefore unable to complete a proper evaluation. 11 According to the 2002 “American Geriatrics Society Panel on persistent pain in older persons,” 12 the assessment of pain is extremely important in patients having dementia and should be performed using validated scales. There is evidence that the administration of pain questionnaires can be reliable in people with mild and moderate cognitive impairment, 13 although there are fears about the expressive and receptive language abilities that deteriorate during the course of AD. 14 As children less than 7 years old also have problems with language, use of visual analog scales (VASs) developed for them can be reliably administered in early/moderate patients with AD. 14,15 A variety of instruments are available to measure pain intensity. Psychometric evaluation of pain intensity scales suggests that variations in the numeric rating scales, verbal descriptor scale, faces pain scales (FPSs), and VAS are appropriate for use with older adults. As mentioned earlier, a prerequisite for selecting an appropriate pain measurement scale involves determining the individual’s ability to learn and understand the directions for completing the tool. 16
Frequently, the health professional relies on the family caregiver testimony when evaluating patients with AD. However, the accuracy of this testimony may be impacted by behavioral changes of the patient and be in potential disagreement with that of the patients themselves. 17 -19 In the case of AD, studies comparing self-report and family caregiver pain perception are scarce. However, many actuation protocols in the field of pain are based on caregiver report. Moreover, accurate pain and comfort assessment relieves patient and caregiver-associated stress. 20
The objectives of this study were (1) to compare the pain intensity and to a lesser degree the affective component of pain in both patients with AD and N and (2) to analyze the difference in pain perception between patients and their family caregivers.
Methods
Patients and their caregivers were informed about the aim and procedure of this study and gave their informed consent. A local medical ethical committee approved the study.
Patients
The sample consisted of 138 patients with chronic osteoarticular pain (selected from a Portuguese hospital database), 75 of them with the diagnosis of AD (AD group) and 63 without any known cognitive impairment (N group). Patients who had a history of cerebral injury, transient ischemic attack, neoplasm, epilepsy, disturbances of consciousness or focal brain disorders, or were incapable of communication were excluded from participation. After exclusion criteria, the final sample consisted of a total of 121 patients, 60 in the AD group and 61 in the N group. In the AD group, 72% of the individuals were female, and the mean age was 72.8 (standard deviation [SD] 6.8) years old. The mean time of diagnosis and follow-up in neurology consultation was of 14.8 months. In the N group, the individuals had similar population characteristics: 69% female and an average age of 68.8 (SD 7.2) years old. They did not have any known cognitive impairment and were subjected to the same evaluation. The AD and the N groups were also compared for education using 5 categories (Table 1): elementary school not finished, elementary school, lower secondary school, higher secondary school, and higher vocational training for 18+/university. Scoring these 5 categories 1 to 5 from the lowest to the highest academic level, the mean values for AD and N groups were 1.6 and 2.1, respectively.
Table 1.
Demographic Characteristics of the Controls (N Group) and Patients With AD (AD Group).
| Controls | Patients With AD | |
|---|---|---|
| Sex | 69% female; 31% male | 72% female; 28% male |
| Age | 68.8 (SD 7.2) | 72.8 (SD 6.8) |
| Educational level (% of the patients) | Elementary school not finished: 24.5; elementary school: 54.09; lower secondary school: 13.11; higher secondary school: 4.91; higher vocational training for 18+/university: 3.27; (average: 2.1) | Elementary school not finished: 50; elementary school: 43.33; lower secondary school: 5; higher secondary school: 1.67; higher vocational training for 18+/university: 0; (average: 1.6) |
Abbreviations: AD, Alzheimer’s disease; SD, standard deviation; N, cognitively normal individuals.
All patients with AD met the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for the clinical diagnosis of probable AD. 21 Mini-Mental State Examination (MMSE) in patients with AD was obtained in a previous psychological consultation (comprehensive evaluation of cognitive, behavioral, and emotional functioning performed using standardized tests and procedures such as MMSE, Mini-cog, and mood valuation). They presented MMSE values of 19.4 (SD 2.1), meaning mild to moderate cognitive impairment.
The patients in the AD group were selected based on the following criteria:
moderate AD with signs of moderate cognitive impairment (difficulty in concentrating, decreased memory of recent events, and difficulties in managing finances, or traveling alone to new locations; some patients in these stages have trouble in completing complex tasks efficiently or accurately and may be in denial about their symptoms) assessed at Neurology and Psychology consults (ie, stages 4-5 of the Global Deterioration Scale) 22 ;
previous complaints of osteoarticular pain due to arthrosis/arthritis for more than 3 months, ability to perform simple tasks (meaning these patients still remember significant details about themselves and their family and require no assistance with eating or using the toilet), and recognize patterns.
Patients with AD were compared to individuals without any known neurologic disease but with a similar history of osteoarticular pain.
Characteristics of Painful Conditions
A prerequisite for participation in the survey was that the participants had complaints of chronic osteoarticular pain at rest for more than 3 months before the diagnosis of AD, determined by review of the medical records. These painful conditions included arthritis/arthrosis, status post recent fractures (in the last year) or miscellaneous conditions (tendinitis and neuropathy), and the number of painful conditions did not differ between the AD and the N groups (χ2 = 0.5, df = 2, P > .05). Other comorbidities such as congestive heart failure, pulmonary disease, diabetes mellitus, chronic renal disease, hypertension, and tumors were also evaluated and their incidence did not differ between the groups.
Pain Evaluation
There is evidence that the patient’s self-report is an accurate and reliable evidence of the existence of pain and its intensity 16,23 using appropriate scales, such as VAS.
The colored analog scale (CAS; Figure 1) was initially developed to assess the intensity of pain in children in a nonverbal manner. Selecting the appropriate scale position takes place by sliding a horizontal marker from the bottom (no pain) to the top (maximum pain). The patient’s score is the numerical value on the back of the scale, which matches the selected scale position. 24
Figure 1.

The Colored Pain Scale
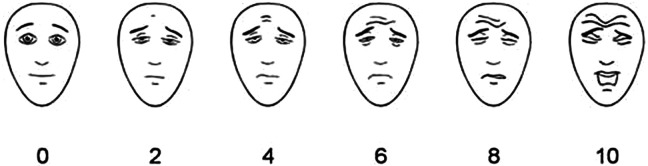
The FPS (Figure 2) primarily measures the intensity of pain and, to a lesser extent, its affective components. This scale consists of 6 design faces lined with a neutral face, and the other 5 corresponding to a series of progressively distressed facial expressions/increasing pain sensations. The faces are ranked 0 to 10, from left to right. Individuals can set their sensations as “no pain” (score 0) to “most severe pain” (score 10). 25
Figure 2.
The Faces Pain Scale
Psychometric evaluations of the FPS suggest that it is an authentic and a valid alternative for measuring pain intensity in cognitively intact elders and elders with mild to moderate impairment. 25 Preliminary evaluation of the FPS with 39 cognitively intact and impaired African American older adults suggests that the FPS may actually measure a broader pain construct that includes affective and sensory components, 26,27 The FPS is also advantageous for older adults with limited education, low literacy levels, or dyslexia. 16
Administration of scales
Evaluation of patients took place once in the morning at a quiet hospital office. During evaluation, patients omitted their usual analgesic medication. Family caregivers (people living with the patients or caring for them on a daily basis) completed the tools at the same time but in a different setting to avoid knowing of each other’s results.
First, we analyzed whether patients understood the concept of the scale. In the case of FPS, they were asked to indicate which face showed most pain and which face showed least pain. For the CAS, they were asked to indicate at what level the marker should be positioned when a person had the most severe pain (top of the scale) or no pain at all (bottom of the scale). They were then asked to paint on the CAS where the marker should be to match their own pain and on the FPS to paint the face which best reflected their pain. The CAS was also shown to the family caregiver who was asked to indicate which level of pain intensity would be appropriate for describing their relative’s degree of pain.
Data Analysis
The SPSS-PC program was used for statistical analyses, including 1-tailed and 2-tailed paired t tests for comparing pain intensity. The significance level was accepted for a P < .05. The normality of data distribution was analyzed using the Kolmogorov-Smirnov test (P > .05).
Results
Data were collected and analyzed for the:
Patients with AD and N report on pain using CAS and FPS
Family caregivers report on pain of patients with AD (FAD) or N (FN) using CAS and FPS
The SPSS-PC program was used for statistical analyses, including 1-tailed and 2-tailed paired t tests for comparing pain intensity. The significance level was accepted for a P < .05.
Colored Analog Scale/FPS
Report on pain intensity (Figure 3):
Figure 3.
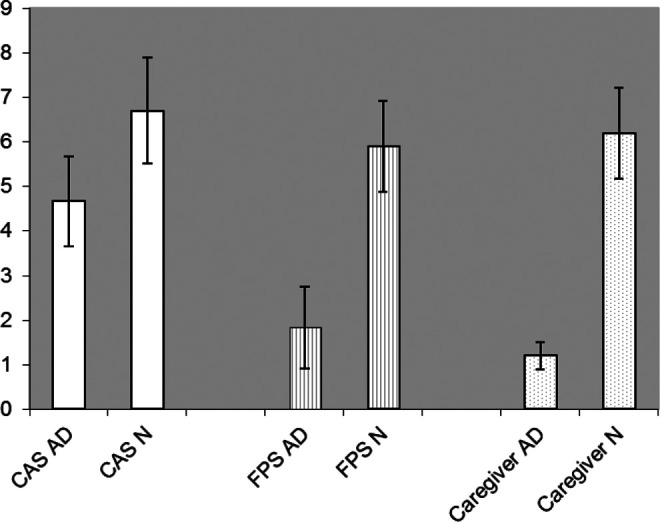
Difference in pain report between AD group and N group.
Regarding CAS, we found a statistically significant difference (P < .05) between the AD and the N groups: 4.67 (SD 1.01) versus 6.7 (SD 1.19), respectively (Table 2).
Regarding FPS, we found a statistically significant difference (P < .05) between the AD and the N groups: 1.83 (SD 0.92) versus 5.9 (SD 1.02), respectively (Table 3).
The family caregivers were asked about the degree of pain of his or her relative using CAS. The results differ (P < .05) between FAD and FN scores: 1.2 (SD 0.3) versus 6.2 (SD 1.12), respectively (Table 2).
Table 2.
Difference in Pain Intensity Report Between the AD and N Groups Using CAS, and Family Caregiver’s Pain Report.
| Controls | Patients With AD | Student t Test | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | |
| CAS | 6.7 | 1.19 | 4.67 | 1.01 | <.05 |
| Family caregiver report | 6.2 | 1.12 | 1.2 | 0.3 | <.05 |
Abbreviations: AD, Alzheimer’s disease; CAS, colored analog scale; SD, standard deviation.
Table 3.
Difference in Pain Intensity Report Between the AD and N Groups Using FPS.
| Controls | Patients With AD | Student t Test | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | |
| FPS | 5.9 | 1.02 | 1.83 | 0.92 | <.05 |
Abbreviations: AD, Alzheimer’s disease; SD, standard deviation; FPS, faces pain scale.
Discussion and Conclusions
In this study, we evaluated the pain intensity report in patients with mild to moderate AD using 2 self-report scales: CAS and FPS. Self-report is a valuable tool to assess pain intensity because it is simple to use in the daily setting and permits to evaluate the variation of pain in the same individual during time. 16 It was also shown that elders without cognitive impairment and those in early stages of disease can report their pain degree in a reliable way. 23 However, studies also point to patients with AD reporting less pain as their disease progresses. 3,9,16
In our study, both in the case of CAS and FPS, patients with AD reported significantly less pain in comparison with normal individuals. Furthermore, the discrepancy between FPS and CAS was higher in the AD group than in the N group. These results suggest that even in mild to moderate cases of the disease, pain perception can be affected, with patients with dementia reporting less pain. Also, the discrepancy between FPS and CAS could also suggest that the affective component of pain in AD is compromised relatively to normal individuals, and/or that patients with AD do not value their complaints even in mild/moderate stages of the disease.
We also found that, in contrast to other caregiver pain reports, 19 the family caregiver considered the pain degree of their relative substantially lower in the case of patients with AD. This is important since in most cases they are the primary source of information regarding the well-being of patients with AD, and health professionals rely on the caregiver information to add or alter prescribed drugs such as painkillers. The FN group result matches the CAS N and FPS N results, which is self-consistent and a sign that the method is robust.
As mentioned earlier, most researchers agree that in patients with dementia, particularly of the Alzheimer type, sensitivity to pain is observed, although the emotional component, dependent on higher brain structures, is reduced or even abolished. This means that patients with AD present less pain complaints, which appear to decrease as the disease progresses. Our study supports that in patients with prior osteoarticular pathology, with and without AD, the AD group reported less subjective pain, and that their pain was not as perceived by their family caregiver as in normal individuals.
This study also points to the need of, when evaluating patients with AD, always measure their pain level using appropriate scales instead of solely depending on the information provided by their primary care provider.
Limitations
The most significant limitations of this study are:
Data are not a demonstration that there is less pain in patients with AD because only specific brain areas are affected, which could be evaluated measuring responses to determined pain stimuli;
Pain reports were for a point in time; assessment on various days would be valuable, as pain report is dependent on several physical and psychological factors that can vary over days;
Our patients were in a mild/moderate stage of the disease. We do not know how their behavior and their caregiver perception would change with the evolution of disease.
For that, we suggest a study including groups from different stages of the disease or following the same patients during an enlarged period of time.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Cacabelos R, Martínez R, Fernández Novoa L, et al. Genomics of dementia: APOE- and CYP2D6-related pharmacogenetics. Int J Alzheimers Dis. 2012;2012:518901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Edition). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3. Scherder E, Eggermont L, Plooij B. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer disease. Gerontology. 2008;54(1):50–58. [DOI] [PubMed] [Google Scholar]
- 4. Takai Y, Yamamoto Mitani N, Okamoto Y, Koyama K, Honda A. Literature review of pain prevalence among older residents of nursing homes. Pain Manag Nurs. 2010;11(4):209–223. [DOI] [PubMed] [Google Scholar]
- 5. Frampton M. Experience assessment and management of pain in people with dementia. Age Ageing. 2003;32(3):248–251. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt R, Bach M, Dal-Bianco P, et al. Dementia and pain. Neuropsychiatr. 2010;24(1):1–13. [PubMed] [Google Scholar]
- 7. Benedetti F, Vighetti S, Ricco C, Lagna E, Bergamasco B, Pinessi L. Pain threshold and tolerance in Alzheimer's disease. Pain. 1999;80(1-2):377–382. [DOI] [PubMed] [Google Scholar]
- 8. Borsook D. Neurological diseases and pain. Brain. 2012;135(pt 2):320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farrell MJ, Katz B, Helme RD. The impact of dementia on the pain experience. Pain. 1996;67(1):7–15. [DOI] [PubMed] [Google Scholar]
- 10. Monroe TB, Gore JC, Chen LM, Mion LC, Cowan RL. Pain in people with Alzheimer disease: potential applications for psychophysical and neurophysiological research, J Geriatr Psychiatry Neurol. 2012;25(4):240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krulewitch H, London MR, Skakel VJ, Lundstedt GJ, Thomason H, Brummel-Smith K. Assessment of pain in cognitively impaired older adults: a comparison of pain assessment tools and their use by nonprofessional caregivers. J Am Geriatr Soc. 2000;48(12):1607–1611. [DOI] [PubMed] [Google Scholar]
- 12. American Geriatrics Society Panel on persistent pain in older persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:205–524. [Google Scholar]
- 13. Rastogi R, Meek BD. Management of chronic pain in elderly, frail patients: finding a suitable, personalized method of control. Clin Interv Aging. 2013:8;37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martine A, Fedio P. Word production and comprehension in Alzheimer’s disease: the breakdown of semantic knowledge. Brain Lang. 1983;19(1):124–141. [DOI] [PubMed] [Google Scholar]
- 15. Scherder E, Bouma A. Visual analogue scales for pain assessment in Alzheimer’s disease. Gerontology. 2000;46(1):47–53. [DOI] [PubMed] [Google Scholar]
- 16. Herr K, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med. 2001;17(3):457–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karp JF, Shega JW, Morone NE, Weiner DK. Advances in understanding the mechanisms and management of persistent pain in older adults. Br J Anesth. 2008;101(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miaskowski C, Zimmer EF, Barrett KM, Dibble SL, Wallhagen M. Differences in patients' and family caregivers' perceptions of the pain experience influence patient and caregiver outcomes. Pain. 1997;72(1-2):217–226. [DOI] [PubMed] [Google Scholar]
- 19. Arons AM, Krabbe PF, Schölzel Dorenbos CJ, van der Wilt GJ, Rikkert MG. Quality of life in dementia: a study on proxy bias. BMC Med Res Methodol. 2013;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim J, Zebrack B. Caring for family members with chronic physical illness: a critical review of caregiver literature. Health Qual Life Outcomes. 2004;2:50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 22. Reisberg B, Ferris SH, De Leon MJ, Crook T. The global deterioration scale for assessment of primary dementia. Am J Psychiatry. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 23. Pautex S, Gold G. Assessing pain intensity in older adults. Geriatr Aging. 2006;9(6):399–402. [Google Scholar]
- 24. McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children’s pain: an initial validation study. Pain. 1996;64(3):435–443. [DOI] [PubMed] [Google Scholar]
- 25. Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. Faces pain scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation of ratio scale properties. Pain. 1990;41(2):139–150. [DOI] [PubMed] [Google Scholar]
- 26. Taylor LJ, Herr K. Use of Faces Pain Scale by minority elders: Psychometric properties. American Geriatrics Society/American Federation for Aging Research 2000 Annual Scientific Meeting; Nashville: [Google Scholar]
- 27. Goodenough B, van Dongen K, Brouwer N, Abu-Saad HH, Champion GD. A comparison of the faces pain scale and the facial affective scale for children's estimates of the intensity and unpleasantness of needle pain during blood sampling. Eur J Pain. 1999;3(4):301–315. [DOI] [PubMed] [Google Scholar]