Abstract
Background:
Some studies report a low suicide risk in general dementia and in Alzheimer’s disease (AD).
Objective:
To evaluate suicidal behavior among patients with semantic dementia (SD), a disorder that impairs semantic knowledge.
Methods:
We reviewed the presence of active suicidal behavior and related factors among 25 patients with SD compared to 111 age-matched patients with early-onset AD.
Results:
In all, 5 (20%) patients with SD had suicidal behavior (2 successfully killed themselves) compared to 1 (0.9%) with AD (P < .001). There was significantly more depression and greater premorbid history of suicidal behavior among the patients with SD compared to those with AD. Among the patients with SD, those with suicidal behavior, compared to those without, had more depression and greater insight into their deficits.
Conclusions:
Patients with SD are at special risk of committing suicide, particularly if they have depression and preserved insight. Possible mechanisms include an impaired sense of semantic competence with increased impulsivity.
Keywords: dementia, semantic dementia, Alzheimer’s disease, suicide, suicidal, depression, primary progressive aphasia
Introduction
Recognizing the risks of suicide is crucial for prevention. Aging is a risk factor for suicidal behavior, and dementia is a major problem associated with aging. 1 The association of suicidal behavior with dementia, however, appears complex. Epidemiological studies suggest that the overall risk of suicide is not greater among those with dementia compared to the general population. 2,3 Recent studies, however, have challenged the view that dementia does not pose a suicide risk and have shown increased suicidal behavior among selective populations with dementia. 4,5 Part of the inconsistency may be that most studies evaluating suicidal behavior in dementia involve generic dementia or Alzheimer’s disease (AD), the commonest form of dementia. Suicidal behavior may be associated with specific non-Alzheimer dementias. 6
One dementia syndrome that may be at particular risk of suicidal behavior is semantic dementia (SD) also known as primary progressive aphasia semantic variant. 7 This disorder results in a loss of semantic knowledge and concepts. 8–10 The SD is a relatively rare neurodegenerative disorder due to frontotemporal lobar degeneration with focal atrophy of the anterior and inferior regions of the temporal lobes. Most cases of SD are due to ubiquitin-positive, tau-negative intraneuronal inclusions that contain the transcriptive reactive 43-Da DNA protein. Clinically, patients with SD may have retained insight into their semantic loss, resulting in a loss of the sense of mental competence, which can lead to demoralization and depression. 11–13 Of added concern is the frequent involvement of the adjacent orbitofrontal region, potentially resulting in poor impulse control with facilitation of suicidal behavior in these patients.
The recognition of the risk of suicide among patients with dementing illnesses is important in order to initiate supportive measures. This study investigates the presence of suicidal behavior among patients with SD compared to age-matched patients with AD. This investigation is possible because of a university-based clinic that receives referrals of patients with this infrequent dementia syndrome. The hypothesis is that patients with SD are at especially high risk of suicide. The study additionally reviews associated features such as depression and insight among these patients and includes case descriptions of the patients with SD who had active suicidal behavior.
Methods
Patients
This study retrospectively reviewed all patients who attended a university clinic dedicated to early-onset dementia from 2000 to 2012. A neuropsychiatric assessment was a routine part of all initial evaluations in this program and available on all patients presenting to this clinic. The major inclusion criteria involved completion of a diagnostic evaluation with the diagnosis of either SD or AD. A total of 25 patients with SD were identified. All the patients were well characterized and met the criteria for SD as well as for primary progressive aphasia semantic variant. 7,8 The evaluation included neuroimaging with magnetic resonance imaging (MRI) and either fluorodeoxyglucose positron emission tomography (PET) or single-photon emission computed tomography (SPECT) scans. In addition, 111 patients with early-onset AD were included as a comparison group. All the patients with AD were well characterized and met recent National Institute on Aging and the Alzheimer’s Association criteria. 14 None of the patients with SD or AD had a familial dementia per family history. This study was approved by the university institutional review board.
Neuropsychiatric Assessment
As part of the initial evaluation, both patients and caregivers were asked whether a checklist of neuropsychiatric symptoms was present either during dementia or before developing dementia. They were asked about the presence of depression, suicidal behavior, delusions, hallucinations, obsessive-compulsive or rigid behavior, apathy and emotional blunting, inappropriate behavior, aggression, changes in eating behavior, and other personality changes. A neuropsychiatric symptom was positive if it was endorsed through self-report or caregiver report and, if endorsed, validated through targeted questions verifying the presence of core features for these conditions. A detailed psychiatric history was obtained for past neuropsychiatric difficulties, and a family psychiatric history was also obtained for known psychiatric disorders among first-degree relatives. In this study, “suicidal behavior” referred to more than just reports of suicidal ideation; “suicidal behavior” referred to suicide attempts or active suicidal behavior (plan to kill themselves)necessitating urgent psychiatric intervention. “Depression” referred to any endorsement by the patient or the caregiver of feelings or symptoms of depression.
Neurocognitive Assessment
The patients with SD were reviewed for cognitive measures related to SD, including the Mini-Mental State Examination (MMSE) score, 15 the categorical word generation (animals/minute), and the mini-Boston Naming Test (mBNT; 15-item or 20-item versions), with percentage of word comprehension score on missed items (semantic anomia). 16,17 Face recognition was evaluated with the face identification test (20- or 24-item versions) in which patients are presented with photographs of famous people, either well-known politicians or entertainers, for naming. 18 The patients had the UCLA insight interview, 19 consisting of interview items for insight graded as follows: normal = awareness of disease/disability with expressed concern; mild =acknowledgement of disease/disability but without expressed concern; and poor = denial of disease/disability.
Neuroimaging Assessment
The patients had a range of structural and functional neuroimaging studies obtained at various institutions. These studies were generally available for review as visual images only, and for 25 patients with SD, we were able to visually rate most of their MRI scans (see Table 1).
Table 1.
Semantic Dementia Group Assessment.
| # | MMSE | Category WPM | %Correct mBNTa | %Correct face recognitionb | Degree insight | LAT atrophy | RAT atrophy |
|---|---|---|---|---|---|---|---|
| 1 | 29 | 9 | 53.3 | 20 | Mild | 2 | 1 |
| 2c | 30 | 9 | 40.0 | NA | Normal | 2 | 1 |
| 3 | 30 | 6 | 33.3 | 0 | Mild | 3 | 2 |
| 4 | 27 | 3 | 33.3 | 5 | Poor | 3 | 3 |
| 5c | 25 | 2 | 33.3 | NA | Normal | 2 | 1 |
| 6c | 30 | 4 | 40.0 | 0 | Normal | 2 | 1 |
| 7 | 30 | 7 | 33.3 | 50 | Poor | 2 | 1 |
| 8 | 22 | 2 | 20.0 | 30 | Poor | 3 | 1 |
| 9 | 27 | 10 | 26.7 | NA | Mild | 2 | 2 |
| 10 | 30 | 10 | 26.7 | 10 | Mild | 2 | 1 |
| 11 | 27 | 0 | 53.3 | 15 | Mild | 2 | 1 |
| 12 | 26 | 11 | 26.7 | 0 | Poor | 3 | 1 |
| 13 | 27 | 10 | 40.0 | 0 | Poor | 1 | 2 |
| 14 | 25 | 3 | 13.3 | 0 | Mild | 1 | 2 |
| 15 | 22 | 2 | 13.3 | 0 | Mild | 1 | 2 |
| 16c | 21 | 1 | 6.7 | 10 | Normal | 2 | 1 |
| 17 | 26 | 5 | 5.0 | 0 | Poor | 2 | 3 |
| 18 | 16 | 0 | 33.3 | 0 | Poor | 2 | 1 |
| 19 | 14 | 7 | 26.7 | 80 | Mild | 3 | 1 |
| 20 | 25 | 7 | 66.7 | 50 | Poor | 0 | 1 |
| 21 | 20 | 2 | 5.0 | 0 | Poor | 3 | 2 |
| 22 | 20 | 0 | 25.0 | 4.2 | Poor | 0 | 1 |
| 23 | 24 | 5 | 75.0 | 0 | Poor | 2 | 3 |
| 24 | 25 | 11 | 25.0 | 36 | Poor | 1 | 2 |
| 25c | 24 | 2 | 20.0 | 0 | Normal | 2 | 3 |
Abbreviations: MMSE, Mini-Mental State Examination; WPM, words per minute; mBNT, min-Boston Naming Test; LAT, left anterior temporal; RAT, right anterior temporal; NA, not available.
a %Correct of either the 15-item mBNT or the 20-item extended mBNT.
b %Correct of either the 20-item or the 24-item famous faces test.
c Patients with suicidal behavior: case reports 1 = #25; 2 = #16; 3 = #2; 4 = #5; and 5 = #6.
In order to compare the visual images across studies and institutions, we applied a neuroimaging visual rating scale for involvement in the right and left anterior temporal lobes in comparison to the rest of the brain. 20 To assess temporal atrophy, we used a 4-point rating scale (grade 0 = no atrophy; grade 1 = mild widening of the sulci without evident volume loss of the gyri; grade 2 = substantial widening of the sulci and volume loss of the gyri; and grade 3 = severe end-stage atrophy). In the presence of multiple scans per patient, the most abnormal scan scores were chosen for analysis.
Statistical Analysis
Data analysis used SPSS version 20 (IBM, Inc [Armonk, New York]) to calculate simple parametric (t test) and nonparametric (chi-square [χ 2]) differences between the SD and the AD groups and between the patients with SD with and without suicidal behavior.
Results
Patients
There were no significant differences between the SD and the AD groups on major variables such as age, gender, age of onset and duration, mean years of education, and MMSE on presentation (see Table 2). Although the mean duration was longer and the MMSE scores were higher in the patients with SD compared to those with AD, these differences did not reach statistical significance.
Table 2.
Comparison of Semantic Dementia and AD Groups.
| SD, n = 25 | AD, n = 111 | Significance | |
|---|---|---|---|
| Age | 62.4 ± 6.7 | 61.3 ± 8.3 | ns |
| Men/women | 11/14 | 52/59 | ns |
| Education, years | 14.8 ± 2.2 | 15.1 ± 3.7 | ns |
| Age of onset | 58.7 ± 6.6 | 55.5 ± 8.3 | ns |
| Duration, years | 3.7 ± 2.6 | 3.2 ± 2.4 | ns |
| Mini-Mental State Examination | 24.9 ± 4.3 | 23.4 ± 3.4 | ns |
| During dementia | |||
| Suicidal behavior | 5 (20.0%) | 1 (0.9%) | χ 2 = 13.41; P < .001 |
| Depression | 12 (48.0%) | 27 (24.3%) | t = 4.49; P < .05 |
| Other psychiatric/ behaviorala | 10 (40.0%) | 26 (23.4%) | ns |
| Medical history | |||
| Suicidal behavior | 3 (12.0%) | 0 (0%) | χ 2 = 8.63; P < .01 |
| Depression | 9 (36.0%) | 21 (18.9%) | ns |
| Other psychiatric/ behaviorala | 2 (8.0%) | 10 (9.0%) | ns |
| Family history | |||
| Suicidal behavior | 1 (4.0%)b | 2 (1.8%) | ns |
| Depression | 2 (8.0%) | 3 (2.7%) | ns |
| Other psychiatric/ behaviorala | 1 (4.0%) | 4 (3.6%) | ns |
Abbreviations: AD, Alzheimer’s disease; ns not significant; SD, semantic dementia; χ 2, chi-square.
a See text for range of psychiatric/behavioral changes reported.
b Patient no. 4. In addition, Patient no. 2’s father may have died of suicide, but this was not known for certain. If included in the SD total family history of suicidal behavior (n = 2, 8%), the group differences would still be nonsignificant.
Neuropsychiatric Assessment
In all, 5 (20%) patients with SD had suicidal behavior during the dementia, compared to 1 (0.9%) of the patients with AD (χ 2 = 13.41; P < .001). Among the patients with SD, 3 (12%) had a prior psychiatric history (before developing dementia) of suicidal behavior, all of whom were included among the 5 with suicidal behavior after developing dementia. In all, 3 of these patients died after suicide attempt after developing SD symptoms. Only 1 patient in the AD group had active suicidal behavior consisting of a suicide plan and threats but never acted upon. Among the patients with AD, none had psychiatric history of suicidal behavior (vs the 3 in the SD group: χ 2 = 4.49; P < .05). There was no difference between both the groups for family history of suicidal attempts, depression, or other psychiatric symptoms or disorders among first-degree relatives.
Depressive symptoms beyond suicidal behavior were present in 12 (48%) of the patients with SD compared to 27 (24.3%) of the patients with AD (χ 2 = 4.49; P < .05). Among the patients with SD, 9 (36%) had psychiatric history of depression, all of whom were included among the 12 with depression after developing dementia. All the patients with SD with a history of premorbid depression appeared to have a recurrence, often after many years, of depression once they developed dementia. In comparison to the patients with SD, 21 (18.9%) of the patients with AD had psychiatric history of depression (nonsignificant difference), 20 (18.02%) of whom were included among the 27 with depression after developing dementia. In general, all 12 patients with SD and all 27 patients with AD having depression were treated with antidepressant medications (starting before any suicidal behavior), primarily selective serotonin reuptake inhibitors.
The caregivers reported a range of other psychiatric symptoms in the patients with depression with either SD or AD. Among the patients with SD, caregivers reported obsessive preoccupations (including constant rumination over their semantic deficits), compulsive-like behavior (including pathological gambling and stereotypical movements), cognitive rigidity, emotional bluntness, puerile and inappropriate behavior, and aggressive behavior. Among the patients with AD, caregivers reported apathy and social isolation, mood instability, paranoid behavior, agitation, and panic attacks.
Neurocognitive Assessment Among the Patients with SD
There were no clear differences between the patients with SD with and without suicidal behavior except for greater insight among those with suicidal behavior (t = 7.01, 23 df, P < .001; Table 1). Symptoms related to left-sided dysfunction on language tests and on right-sided dysfunction in the form of face recognition difficulty were equally distributed among those with and without suicidal behavior (categorical words per minute: 3.6 ± 3.21 vs 5.55 ± 3.85; %correct mBNT: 28.0 ± 14.44 vs 31.74 ± 18.56; %correct face recognition: 3.33 ± 5.17 vs 15.8 ± 23.12; suicide patients vs nonsuicidal patients with SD, respectively).
Neuroimaging Assessment
Using our neuroimaging visual rating scale, 14 (56%) of the patients with SD had left-sided predominance of brain atrophy, 9 (36%) had right-sided predominance, and 2 (8%) had bilaterally symmetrical temporal atrophy. Of the 5 patients with SD and suicidal behavior, 4 had left-sided predominance, and 1 had right-sided predominance. Of the 20 nonsuicidal patients with SD, 10 (50%) had left-sided predominance, 8 (40%) had right-sided predominance, and 2 (10%) were bilaterally symmetrical. Despite these differences, the visual rating scores did not reach statistical significance (left anterior temporal atrophy: 2.0 ± 0.04 vs 1.9 ± 0.97; right anterior temporal atrophy: 1.4 ± 0.89 vs 1.65 ± 0.75; suicide patients vs nonsuicidal patients with SD, respectively).
Case Reports
Patient No. 1 (Note 1)
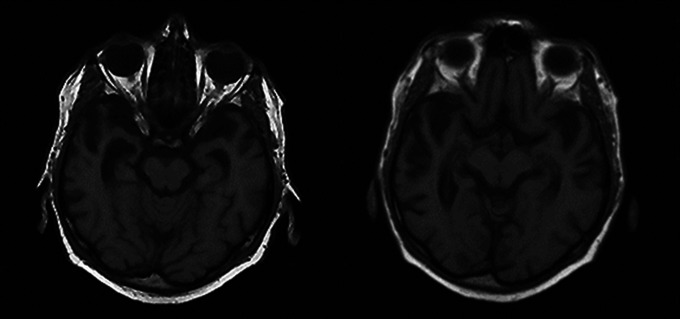
A 63-year-old, right-handed man was hospitalized after recurrent attempted suicides, this time via overdose of medications. He had also attempted suicide by hanging. The patient stated that he felt hopeless due to his “bad brain” and inability to think because of the loss of words. For many years, he had progressive difficulty naming and even knowing objects. This preoccupation consumed him, and his conversation was usually focused on his impaired ability to understand words. His history was negative for depression or suicide attempts, and his family history was negative for any similar illness or mood disorder. On examination, he was unable to name common items such as fruits and clothing items, had lost the meaning of many words, and frequently did not know what objects were. The patient was also unable to read irregular words (surface alexia) or to recognize famous faces. The rest of his examination showed adequate recognition memory and intact visuospatial skills and calculations. His neurological examination was otherwise intact. An MRI of the brain showed moderate bilateral anterior temporal atrophy, somewhat greater on the right (see Figure 1). The patient died during his hospitalization of complications from deep vein thrombosis and anticoagulation.
Figure 1.
Brain magnetic resonance imaging (MRI) scans (T1-weighted) of patient no. 1 showing significant bilateral atrophy of the anterior temporal lobes, more prominent on the right.
Patient No. 2
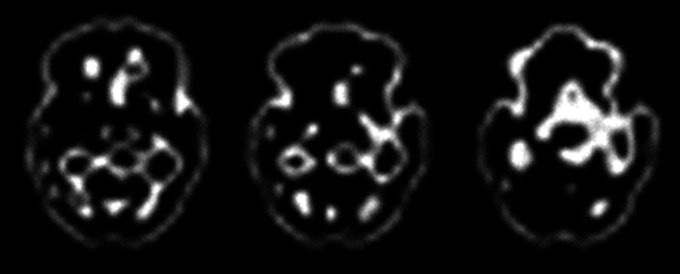
A 62-year-old, right-handed man had a 5-year history of progressive word comprehension difficulty and impaired reading and writing. He was very distressed and obsessively focused over his loss of words; this was a constant topic of his conversation. He stated that he felt handicapped by his inability to comprehend and communicate, that he had become a burden for his family, and that he wished to die. His medical history was positive for prior depression and suicidal gestures. On family history, he disclosed that his father died at age 62 years. The cause for his death was unknown, but suicide was a possibility. In addition to an anxiously depressed affect, his examination was consistent with SD. He was very impaired on confrontational naming and had prominent difficulty with single-word comprehension. For example, he could relate what a dog was, but he did not know what words such as cat, horse, or even comb meant. The patient had problems with reading irregular words and made regularization errors. He had intact recognition memory, perfect 3-dimensional constructions, and excellent calculations, but face recognition was impaired. The rest of his neurologic examination was normal. His MRI showed inferolateral anterior temporal atrophy on the left, and his PET scan confirmed left greater than right anterior temporal hypometabolism (see Figure 2). He was treated by psychiatrist for depression and suicidal ideation, but unfortunately, he committed suicide via drug overdose just before a religious day.
Figure 2.
Brain fluorodeoxy-glucose positron emission tomography (PET) scan of patient no. 3 showing hypometabolism in the left anterior temporal region (region without activity).
Patient No. 3
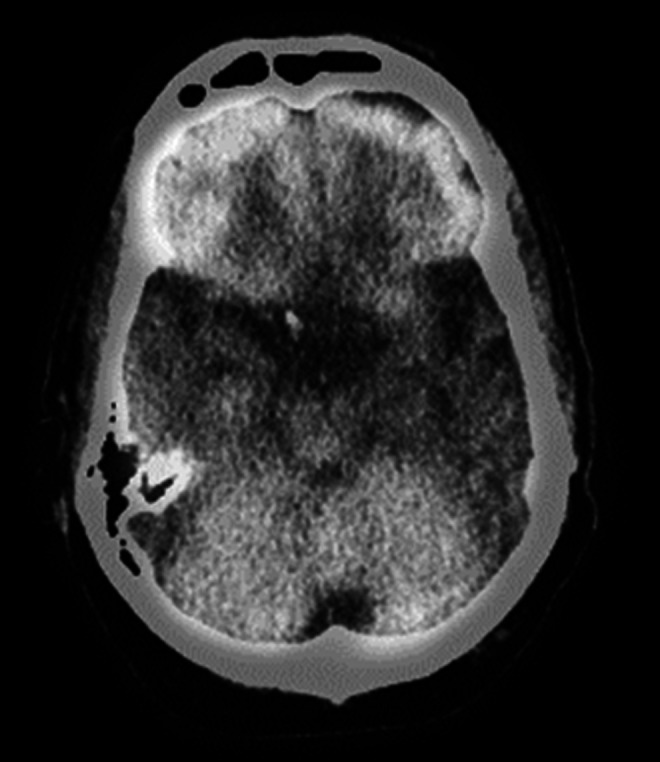
A 57-year-old right-handed woman had a 7-year history of progressive word-finding difficulty. She described her condition as “A name can be mentioned and sounds familiar but I cannot visualize a face.” She had preserved insight and was very distressed and constantly obsessed over her deficits. The patient was tearful and reported lifelong depression, which worsened with her disorder. Her medical history also included periods described as “hypomania” but no prior suicidal behavior. Her family history was negative for neurodegenerative or neuropsychiatric diseases. On examination, the patient was very anxious and displayed impaired word generation and confrontational naming. On testing of word comprehension, she did not recognize or know the meaning of specific words that she missed. Memory was normal except for a mild memory retrieval deficit, and she had good visuospatial constructions. There was some impairment in face recognition. The neurological examination was otherwise normal. Her brain MRI revealed asymmetric anterior temporal lobe atrophy, worse on the left, and her SPECT showed hypoperfusion in the left perisylvian region, particularly in the left temporal lobe (see Figure 3). The patient developed increasing anxiety and depression with suicidal ideation and subsequently committed suicide by shooting herself.
Figure 3.
Brain SPECT scan of patient no. 3 showing hypoperfusion in the left anterior temporal region (region without activity). The SPECT scans are technically fuzzy. SPECT indicates single-photon emission computed tomography.
Patient No. 4
This 60-year-old woman was referred for assessment because of depression with a plan to kill herself. She had a history of suicide attempts and was considered an active suicide risk. During the assessment, she complained of 4 to 5 years of something being “wrong in my head,” being “bad with words” and unable to understand what she reads. Her husband added that she had difficulty in knowing the meaning of words. She was continually preoccupied with her word loss and stated that, “I have the feeling I won't live long.” The patient had a history of depression for the prior 25 years with suicide attempts, including cutting her wrists and shooting herself in the stomach. The patient's mother also had a major depressive disorder and a probable suicide at age 51. On examination, the patient had prominent word-finding difficulties. For instance, she stated that a picture of a camel was a “horse”; a glove was a “hand, in case you get yucky”; a cookie was a “carrot, no, noodle”; and a pencil was a “spoon.” In addition to word comprehension difficulty, she had surface alexia on reading. Recognition memory, visuospatial constructions, and praxis were intact; however, she had face recognition difficulty. The rest of the neurological examination was normal. On MRI, there was focal parenchymal atrophy primarily in the anterior aspect of the left temporal lobe, and a SPECT showed corresponding hypoperfusion in the left temporal lobe. Subsequent to this evaluation, the patient continued under psychiatric care for her depression and suicidal ideation.
Patient No. 5 (Note 1)
A 71-year-old language teacher was hospitalized for major depression with suicide plan, requiring hospitalization and electroconvulsive therapy. On evaluation, he disclosed a ≥2-year history of a slowly progressive loss of words and an inability to understand words in his different languages. The depression included awareness of the magnitude of his word loss associated with the feeling that life was no longer worth living. He constantly ruminated and expressed hopelessness about the future. The patient had had depression 25 to 30 years prior to admission with an attempted suicide with a rifle. His family history was negative for any known familial illnesses. On examination, confrontational naming was decreased, and many words, such as cuff, lapel, or eyelashes, had lost their meaning in all of his languages. His verbal memory testing was compromised by the difficulty with anomia. The patient was able to do visuospatial constructions and calculations but had difficulty with face recognition. His basic neurological examination was intact. A review of serial MRI studies showed evidence of progressive anterior temporal atrophy, left greater than right, and a PET scan showed hypometabolism of the left temporal lobe.
Discussion
We report an association of SD with suicidal behavior, including completed suicides in 2 patients. In addition, the patients with SD, compared to comparably impaired patients with AD, had more ongoing depression and more premorbid suicidal behavior. The patients with SD and suicidal behavior had insight into their deficits and tended to obsess about them. These findings indicate that among patients with dementia, those with SD pose a special risk of committing suicide, particularly in the presence of depression along with an accompanying awareness and fixation on their decreased cognitive competency. Most intriguing and unexplained is the relationship and significance of a history of suicidal behavior long preceding the development of SD. Moreover, 4 of the 5 patients with SD with suicidal behavior had a long history of depression (1 with additional hypomania) of variable duration, and 3 of them may have had depression in the period prior to the first cognitive symptoms of SD.
The role of dementia as a risk factor for suicide is controversial. The general risk of suicide in people with dementia has been low with suicide attempts occurring in less than 1% of those with dementia. 2,21,22 This overall risk of suicide in dementia appears to be comparable to that of the age-matched general population. 3,23 Two cohort studies of those over 60 years, however, report increased suicidal behavior in specific populations with dementia. One of these cohort studies found an increased risk of suicide among patients with dementia who had been admitted to a psychiatric hospital. 24 Another cohort study found an increased risk of suicide among US Veterans diagnosed with dementia. 5
Risk factors for suicide in dementia include depression and hopelessness, preserved insight with a recent diagnosis and an early-stage of the disorder, and a younger age of onset of dementia. 3–5,21,24 Depression is strongly associated with suicide in older people, 23,25,26 and depressive symptoms remain a risk factor for suicidal ideation in dementia and AD. 2,21 Some investigators speculate that there may be an inverse relationship between cognitive functioning and suicidal ideation, 27 and suicide may be more likely in mild dementia when patients are still able to execute an act of suicide. 3,28–30 Preserved insight is another risk of suicidal behavior as reflected in the increased suicide rates shortly after diagnosis when patients become distressed over the consequences of having dementia. 3,5,24 Moreover, patients with mild cognitive impairment or with mild AD with preserved insight may be at risk if they experience a sense of hopelessness for the future. 21,31,32 Finally, younger age also increases the risk of suicide. Among patients who had been diagnosed with dementia during hospital admission, the risk of suicide was greatest in younger patients (those aged 50–69 years), 24 and a 9-year British survey of those receiving mental health services also found a high-proportional risk of suicide in younger, compared to older, patients with dementia. 4
This study indicates that the type of dementia is an additional important risk factor for suicide in dementia. Suicidal ideation may be more common with non-AD dementias rather than AD. 6 Despite the fact that there is increased Alzheimer pathology in older people who committed suicide and in patients with AD with depression, 33,34 there is a relative infrequency of suicide in AD. Among patients with dementia, those with Huntington’s disease are especially prone to suicide, particularly given their increased frequency of depression, 13,35 and most recently, frontotemporal dementia, a frontotemporal lobar degeneration like SD, has been implicated in suicide. 36 Our study adds SD as a dementia syndrome with a high risk of suicidal behavior.
The reasons for increased suicidal behavior in SD are unclear, but this study and the literature suggest possible mechanisms. Our patients clearly have awareness and insight for their semantic deficits and are obsessionally preoccupied with them. They feel a sense of decreased mental competence with hopelessness and difficulty projecting themselves into the future. In addition, about 28% of patients with SD experience depression, especially those with greater left than right temporal lobe atrophy. 35 Patients with SD who are prone to depression may be predisposed to a nihilistic interpretation that they, in effect, are losing their mind or their self. 37 Even more speculative is the potential relationship to pathology, such as greater left than right anterior temporal involvement and possible relative sparing of the Von Economo neurons that are involved in other frontotemporal lobar degenerations. 38 Finally, our patients also have a significant history of premorbid suicidal behavior often with depression. This raises the possibility that depression could be a prodrome or early manifestation of SD, as in HD, or, less likely, that depression is a risk factor for SD.
There are a number of potential problems with this study. First, it is retrospective review of a clinical population. As such, the neuropsychiatric assessments were not specifically designed to evaluate the risk factors for suicidal behavior, and the neurocognitive testing was not uniform. These patients, however, all underwent evaluation in a university clinic that screened for suicidal behavior and depression. Second, there can be inconsistency in the definitions of suicidal behavior and depression. Consequently, we used definitions, such as active suicidal behavior rather than “suicidal ideation,” which we could operationalize as outlined in the methods of this article. Third, the neuroimaging was heterogenous and from many centers and scanners. We endeavored to solve this problem by applying a visual rating scale across the clinical images. Finally, the relationship of premorbid suicidal behavior to SD remains unexplained. This is a major unexpected finding of this study and needs to be a focus of future research.
In conclusion, patients with SD may be at risk of suicide, particularly in the presence of depression, retained insight, and obsessional rumination over their loss of semantic competence. This information can lead to the early management of risk factors such as depression and prompt intervention to prevent suicides in these patients. The intriguing relationships been depression, suicidal behavior, and SD need further clarification and investigation, including these patients’ distressing sense of mental incompetence as well the significance of early life suicidal behavior and depression as a marker for the development of SD.
Note
The study is included, in part, in other publications.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute on Aging R01 AG034499 (MFM).
References
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider B, Maurer K, Frolich L. Dementia and suicide. Fortschr Neurol Psychiatr. 2001;69(4):164–169. [DOI] [PubMed] [Google Scholar]
- 3. Haw C, Harwood D, Hawton K. Dementia and suicidal behavior: a review of the literature. Int Psychogeriatr. 2009;21(3):440–453. [DOI] [PubMed] [Google Scholar]
- 4. Purandare N, Voshaar RC, Rodway C, Bickley H, Burns A, Kapur N. Suicide in dementia: 9-year national clinical survey in England and Wales. Br J Psychiatry. 2009;194(2):175–180. [DOI] [PubMed] [Google Scholar]
- 5. Seyfried LS, Kales HC, Ignacio RV, Conwell Y, Valenstein M. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seyfried LS, Kales HC, Ignacio RV, Conwell Y, Valenstein M. Investigation of Alzheimer's disease-related pathology in community dwelling older subjects who committed suicide. J Affect Disord. 2007;99(1-3):127–132. [DOI] [PubMed] [Google Scholar]
- 7. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 9. Kertesz A, Jesso S, Harciarek M, Blair M, McMonagle P. What is semantic dementia? A cohort study of diagnostic features and clinical boundaries. Arch Neurol. 2010;67(4):483–489. [DOI] [PubMed] [Google Scholar]
- 10. Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(Pt 1):300–306. [DOI] [PubMed] [Google Scholar]
- 11. Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67(10):1752–1756. [DOI] [PubMed] [Google Scholar]
- 12. Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci. 2010;293(1-2):35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 16. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. [DOI] [PubMed] [Google Scholar]
- 17. Mendez MF, Kremen SA, Tsai PH, Shapira JS. Interhemispheric differences in knowledge of animals among patients with semantic dementia. Cogn Behav Neurol. 2010;23(4):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendez MF, Ghajarnia M. Agnosia for familiar faces and odors in a patient with right temporal lobe dysfunction. Neurology. 2001;57(3):519–521. [DOI] [PubMed] [Google Scholar]
- 19. Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria". Conscious Cogn. 2011;20(4):1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koedam EL, Lehmann M, van der Flier WM, et al. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21(12):2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim WS, Rubin EH, Coats M, Morris JC. Early-stage Alzheimer disease represents increased suicidal risk in relation to later stages. Alzheimer Dis Assoc Disord. 2005;19(4):214–219. [DOI] [PubMed] [Google Scholar]
- 22. Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry. 2002;52(3):193–204. [DOI] [PubMed] [Google Scholar]
- 23. Harwood D, Hawton K, Hope T, Jacoby R. Psychiatric disorder and personality factors associated with suicide in older people: a descriptive and case–control study. Int J Geriatr Psychiatry. 2001;16(2):155–165. [DOI] [PubMed] [Google Scholar]
- 24. Erlangsen A, Zarit SH, Conwell Y. Hospital-diagnosed dementia and suicide: a longitudinal study using prospective, nationwide register data. Am J Geriatr Psychiatry. 2008;16(3):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conwell Y, Lyness JM, Duberstein P, et al. Completed suicide among older patients in primary care practices: a controlled study. J Am Geriatr Soc. 2000;48(1):23–29. [DOI] [PubMed] [Google Scholar]
- 26. Waern M, Rubenowitz E, Runeson B, Skoog I, Wilhelmson K, Allebeck P. Burden of illness and suicide in elderly people: case–control study. BMJ. 2002;324(7350):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heisel MJ, Flett GL, Besser A. Cognitive functioning and geriatric suicide ideation: testing a mediational model. Am J Geriatr Psychiatry. 2002;10(4):428–436. [PubMed] [Google Scholar]
- 28. Cohen D, Llorente M, Eisdorfer C. Homicide–suicide in older persons. Am J Psychiatry. 1998;155(3):390–396. [DOI] [PubMed] [Google Scholar]
- 29. Rubin EH, Veiel LL, Kinscherf DA, Morris JC, Storandt M. Clinically significant depressive symptoms and very mild to mild dementia of the Alzheimer type. Int J Geriatr Psychiatry. 2001;16(7):694–701. [DOI] [PubMed] [Google Scholar]
- 30. Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):222–228. [PubMed] [Google Scholar]
- 31. Harwood DG, Sultzer DL. “Life is not worth living": hopelessness in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2002;15(1):38–43. [DOI] [PubMed] [Google Scholar]
- 32. Ayalon L, Mackin S, Arean PA, Chen H, McDonel Herr EC. The role of cognitive functioning and distress in suicidal ideation in older adults. J Am Geriatr Soc. 2007;55(7):1090–1094. [DOI] [PubMed] [Google Scholar]
- 33. Rubio A, Vestner AL, Stewart JM, Forbes NT, Conwell Y, Cox C. Suicide and Alzheimer's pathology in the elderly: a case–control study. Biol Psychiatry. 2001;49(2):137–145. [DOI] [PubMed] [Google Scholar]
- 34. Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry. 2008;16(2):168–174. [DOI] [PubMed] [Google Scholar]
- 35. Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. [DOI] [PubMed] [Google Scholar]
- 36. Alberici A, Cottini E, Cosseddu M, Borroni B, Padovani A. Suicide risk in frontotemporal lobe degeneration: to be considered, to be prevented. Alzheimer Dis Assoc Disord. 2012;26(2):194–196. [DOI] [PubMed] [Google Scholar]
- 37. Mendez MF, Ramirez-Bermudez J. Cotard syndrome in semantic dementia. Psychosomatics. 2011;52(6):571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brune M, Schobel A, Karau R, et al. Neuroanatomical correlates of suicide in psychosis: the possible role of von Economo neurons. PLoS One. 2011;6(6):e20936. [DOI] [PMC free article] [PubMed] [Google Scholar]