Abstract
Background:
We hypothesized that neuropsychological tests could help in identifying preclinical stages of vascular cognitive impairment, when aspirin use might be associated with lower dementia incidence.
Methods:
We used data of Canadian Study of Health and Aging (CSHA) which was a longitudinal study of Canadians older than 65 years and was done in 3 waves, 1991 to 1992 (CSHA-1), 1996 to 1997 (CSHA-2), and 2001 to 2002.
Results:
CSHA-1 participants with vascular dementia performed worse in copying pentagons and writing subtests of modified Mini-Mental State Examination test than participants with probable Alzheimer’s disease. Salicylates use was associated with lower incident dementia among normal cognition CSHA-1 participants who had low scores in copying pentagons and writing subtests after controlling for age, sex, education, and vascular risk factors (odds ratio = 0.25, 95% confidence interval: 0.073-0.86, P = .028).
Conclusions:
Two simple neuropsychological tests might help in identifying preclinical stages of vascular cognitive impairment, and salicylates use was associated with lower dementia incidence.
Keywords: dementia, aspirin, prevention, vascular cognitive impairment, vascular dementia, cerebrovascular disorders
Introduction
Longitudinal studies have given conflicting results about the association between aspirin use and dementia incidence. In some studies, aspirin acted as a protective factor, 1 in some as a risk factor, 2 and in some, there was no association. 3,4
Our hypothesis was that aspirin might be associated with lower dementia incidence when overt or hidden vascular pathologies (such as infarcts, 5 microinfarcts, 6 and atherosclerosis 7 ) underlie cognitive decline. To identify individuals with vascular cognitive decline, neuropsychological tests were one of the measures which could be used. 8 Neuropsychological tests at which participants with vascular dementia (VaD) performed worse than participants with Alzheimer’s disease (AD) could be assumed to help in identifying early stages of vascular cognitive impairment. If our hypothesis was true, individual with low scores in those neuropsychological tests would be at early stages of vascular cognitive impairment and would have lower dementia incidence if they were aspirin users.
We had access to Canadian Study of Health and Aging (CSHA) data set to test our hypothesis. The CSHA was a population-based longitudinal study of older Canadians who were sampled across Canada in 1991 to 1992 and were followed for 10 years.
Methods
Study Population
The CSHA was a community-based cohort study of older Canadians (≥65 years old) who were sampled from 36 urban and surrounding rural areas in the 10 Canadian provinces. The first study, CSHA-1, started in 1991 and sampled about 9000 participants from community-living Canadians and 1300 Canadians (≥65 years) from institutional settings. Community samples were screened for cognitive impairment (by the use of modified Mini-Mental State Examination [3MS]) and, if positive, were evaluated by physicians and neuropsychologists to determine their cognitive status. Institution-living participants were not screened, and all of them were evaluated by physicians and neuropsychologists because of high likelihood of cognitive impairment among them. Based upon this process, participants were classified as normal, cognitive impairment no dementia (CIND; defined by investigators for participants who had memory impairment or other cognitive or behavioral problems but did not meet dementia criteria), AD, VaD, other dementia, and not classified dementia. Diagnostic and Statistical Manual of Mental Disorders, Third Edition Revised (DSM-III-R) criteria were used for the diagnosis of dementia, 9 National Institute of Neurological and Communicative Disorders and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria were used for the diagnosis of AD, 10 and the International Classification of Diseases, Tenth Revision, was used to define subcategories of vascular and other dementias. 11
The second wave of CSHA, CSHA-2, started 5 years later in 1996 to 1997 and was done according to the same rules as CSHA-1. The only exception was complete clinical and neuropsychological examination which was done not only according to CSHA-1 rules but also for all CSHA-2 participants who were examined at CSHA-1. Also, the same diagnostic criteria were used, but in addition, participants’ cognitive status was determined once more by the use of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). 12 The CSHA had another wave in 2001 to 2002, CSHA-3, which is not used in this study. Details of the sampling methodology, the screening, and the diagnostic criteria used by the CSHA study have been published elsewhere. 13
A substudy was designed in the CSHA to investigate possible risk factors for AD and dementia. A self-administered questionnaire was completed by CSHA-1 participants who were negative in screening or who were diagnosed as cognitively normal in the clinical examination phase. The risk factor questionnaire included questions of sociodemographic, occupational, and environmental exposures; medical, drug, and family histories; smoking; alcohol, coffee, and tea consumptions; and regular physical exercise. Among drugs, a question was about regular (at least once a week for several weeks) use of analgesics, and if was answered positively, detailed questioning was done about different kinds of analgesics (such as aspirin, Anacin, Bufferin, etc). Participants were assumed to be users of salicylates if they had used aspirin or other aspirin-containing analgesics at least once a week. The same definition was used for other nonsteroidal anti-inflammatory drugs (NSAIDs).
In this study, we first compared CSHA-1 participants having VaD with CSHA-1 participants having AD in their 3MS domain scores to identify domains more affected by VaD. In order to test the replicability of findings, we compared CSHA-2 participants having VaD with CSHA-2 AD ones in their CSHA-2 3MS domain scores. We called common VaD-affected domains as vascular domains.
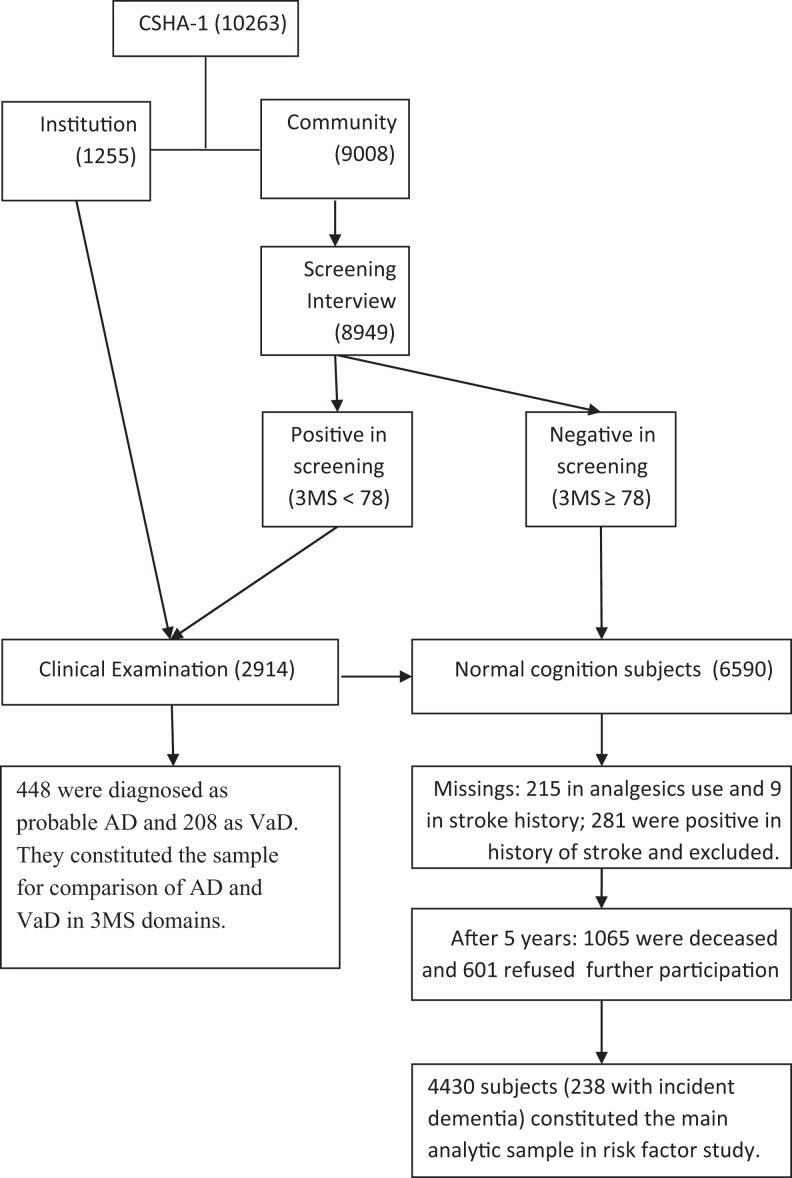
Then, among risk factor study participants, those who got low scores in the vascular domains were compared with other CSHA-1 participants in their dementia incidence. Our hypothesis was finding interaction between salicylates use and vascular domain scores in dementia incidence: there would be an association between lower dementia incidence and salicylates use when participants had low scores in vascular domains, and when participants had normal scores in vascular domains, there would be no association between salicylates use and dementia incidence. Flow chart of the study and analyses samples can be found in Figure 1. To make stroke recurrence less probable as a contributing factor in dementia incidence, participants with stroke history were excluded from our salicylate-dementia association analyses.
Figure 1.
Flow chart of CSHA participants showing this study analyses samples. AD indicates Alzheimer’s disease; CSHA, Canadian Study of Health and Aging; 3MS, modified Mini-Mental State Examination; VaD, vascular dementia.
Statistical Methods
Quantitative variables are shown as mean ± standard deviation (SD). Mann-Whitney test was used to compare AD and VaD in their 3MS domain scores. For univariate analysis, t test, χ2 test, and Mann-Whitney test (when t test assumptions were not met) were used. To compare AD and VaD in their test scores, with adjustment for confounding variables, zero-inflated Poisson regression was used. Interaction between salicylates use and vascular domain scores is shown according to the recommended guideline. 14 Delta method was used for calculation of confidence intervals (CIs) for parameters of interaction. 15 Logistic regression was used to assess the association between incident dementia and multiple variables. Age, sex, and education were entered in the model in addition to main variables of interest. Among vascular risk factors, only variables that had significant association with dependent variable (dementia incidence) were also entered. Analyses were done with SPSS version 21 and SAS version 9.2. P value less than .05 was assumed significant.
Results
Comparison of Participants Having Probable AD With VaD Ones in 3MS Domains
A total of 448 CSHA-1 participants were diagnosed as probable AD and 208 participants were diagnosed as VaD; however, not all of them were able to fill all the 3MS domains. Table 1 shows that participants with VaD performed worse than participants with AD in four 3MS domains: naming, read and obey, writing, and copying pentagons. In CSHA-2, 376 participants were diagnosed as probable AD and 153 participants were diagnosed as VaD. CSHA-2 participants with AD performed worse than participants with VaD in the 3MS domains, except mental reversal, 3-stage command, writing, and copying pentagons (data are now shown). Writing and copying pentagons were domains that in both CSHA waves, participants with VaD did worse.
Table 1.
Comparison of CSHA-1 Participants Having Probable AD With VaD Ones in Their Scores on Different Domains of 3MS.
| 3MS Domains | Mean Rank | P a | Range | Median | ||
|---|---|---|---|---|---|---|
| AD (n) | VaD (n) | AD | VaD | |||
| Date and place of birth | 248.54 (360) | 293.15 (164) | .001 | 0-5 | 4 | 4 |
| Registration | 244.16 (352) | 276.34 (155) | .013 | 0-3 | 2 | 3 |
| Mental reversal | 260.07 (361) | 269.44 (164) | .507 | 0-8 | 4 | 4 |
| First recall | 250.18 (361) | 292.64 (165) | .002 | 0-9 | 2 | 2 |
| Temporal orientation | 247.83 (359) | 293.02 (164) | .001 | 0-15 | 2 | 4 |
| Spatial orientation | 250.63 (360) | 288.55 (164) | .006 | 0-5 | 4 | 5 |
| Naming | 258.28 (348) | 251.14 (163) | .594 | 0-5 | 4 | 4 |
| Four-legged animals | 258.46 (360) | 271.36 (164) | .361 | 0-10 | 4 | 4 |
| Similarities | 254.04 (359) | 279.42 (164) | .035 | 0-6 | 0 | 0 |
| Repetition | 255.03 (359) | 274.24 (162) | .169 | 0-5 | 2 | 3 |
| Read and obey | 236.44 (322) | 231.85 (147) | .721 | 0-3 | 2 | 2 |
| Writing | 229.49 (313) | 207.61 (132) | .092 | 0-5 | 4 | 3 |
| Copy 2 pentagons | 237.56 (321) | 219.44 (142) | .175 | 0-10 | 4 | 3 |
| Three-stage command | 252.79 (356) | 271.20 (160) | .172 | 0-3 | 2 | 2 |
| Second recall | 251.70 (359) | 283.09 (163) | .024 | 0-9 | 1 | 2 |
Abbreviations: AD, Alzheimer disease; CSHA-1, first wave of Canadian Study of Health and Aging; 3MS, modified Mini-Mental State Examination; VaD, vascular dementia.
aStatistical test: Mann-Whitney test.
CSHA-1 participants with AD were significantly older (84.46 ± 6.8 vs 80.92 ± 7.23, P < .001) and more were female (78.1% vs 55.8%, P < .001) than participants with VaD, but there was no significant difference in education. To see whether the difference between the 2 groups was due to sex and age confounding effects, a multivariate zero-inflated Poisson regression was run. A dependent variable was made by the addition of copying pentagons and writing scores (copying pentagon_writing variable with 0-15 range), and independent variables were sex, age, and clinical diagnosis. The model-derived mean of participants with AD was 4.07, which was significantly more than the mean of participants with VaD (1.21; P = .002). In a validation analysis, CSHA-2 participants with probable AD had higher copying pentagons–writing scores than participants with VaD (P = .016), after controlling for sex and age.
Salicylates Association With Dementia Incidence
Basic characteristics of the participants can be found in Table 2. One hundred three participants were missing in copying pentagons–writing scores and were significantly older (75.39 ± 7.24 vs 73.53 ± 6.15; P = .01) and less educated (67.0% vs 34.8% had less than 10 years education; P < .001).
Table 2.
Basic Characteristics of Risk Factor Study Sample.
| Age (mean ± SD) | 73.57 ± 6.18 |
|---|---|
| Female sex (%, n/N) | 60.2, 2669/4430 |
| ≥10 years education (%, n/N) | 64.5, 2846/4415 |
| ≥1 E4 allele (%, n/N) | 23.1, 279/1209 |
| Copying pentagon–writing score (mean; SD; median) | 14.14; 1.48; 15a |
| NSAIDs | |
| Regular salicylates (%, n/N) | 16.1, 713/4430 |
| Regular nonsalicylates (%, n/N) | 3.5, 155/4430 |
| CSHA-1 3MS score (mean ± SD) | 90.52 ± 6.05 |
| CSHA-2 3MS score (mean ± SD) | 87.28 ± 10.96a |
| Outcome | |
| Demented (%, n/N) | 5.4, 238/4430 |
Abbreviations: CSHA-1, first wave of Canadian Study of Health and Aging; 3MS, modified Mini-Mental State Examination; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation.
aCopying pentagon–writing score was missing in 103 participants, and CSHA-2 3MS score was missing in 36 participants.
Participants who developed dementia at CSHA-2 had low copying pentagons–writing scores compared with participants who did not (13.46 ± 1.98 vs 14.18 ± 1.44, P < .001), even when analysis was restricted to participants with normal 3MS scores at CSHA-1 (13.66 ± 1.69 vs 14.23 ± 1.34, P < .001). Salicylates use did not have any effect on dementia prevention: 6.5% of users versus 5.2% of nonusers developed dementia within 5 years of follow-up (P = .17). In a logistic regression when both salicylates use and copying pentagons–writing scores were entered as predictors, salicylates use did not predict dementia incidence (P = .38). When their interaction was added, both salicylates use (P = .02) and their interaction (P = .02) changed to significant predictors of dementia incidence.
We analyzed dementia incidence among salicylates users versus nonusers categorized by their copying pentagons–writing scores. Until score of 12, dementia incidence was lower among salicylates users (4.7% vs 12.7%), and from 13 to 15, it was higher (6.3% vs 4.3%), which is equivalent to a significant interaction term seen in the mentioned logistic regression.
Table 3 shows logistic regression of dementia prediction with different predictors when copying pentagons–writing scores were treated as a dummy variable (scores less than 13 rescored to 1 and scores 13 or greater rescored to zero). When only salicylates use and copying pentagon–writing score were entered as independent variables, salicylates use was not a significant predictor. When their interaction term was added, both the interaction term and salicylates use became significant. Adjustment by age, sex, education, physical exercise, and smoking (diabetes mellitus and hypertension were not added to the model because of no significant association with dementia incidence in bivariate analyses) did not alter the significance of the interaction term. Other NSAIDs were not a significant predictor of dementia incidence alone or in interaction with copying pentagons–writing scores (Table 3). Table 4 shows the effect modification of copying pentagons–writing scores in salicylates-dementia association.
Table 3.
Logistic Regressions of Dementia Incidence With Different Sets of Predictors.
| Predictors | P | OR | 95% CI for OR | |
|---|---|---|---|---|
| Step 1 | Salicylate | .400 | 1.16 | 0.82-1.64 |
| Copy–write | .000 | 2.65 | 1.93-3.65 | |
| Step 2 | Salicylate | .032 | 1.50 | 0.001-0.62 |
| Copy–write | .000 | 3.27 | 0.71-0.82 | |
| Salicylate* copy–write | .009 | |||
| Step 3a | Salicylate | .047 | 1.50 | 1.01-2.24 |
| Copy–write | .000 | 2.38 | 1.61-3.52 | |
| Salicylate* copy–write | .007 | |||
| Step 4b | Salicylate | .069 | 1.68 | 0.96-2.95 |
| Copy–write | .002 | 2.24 | 1.34-3.73 | |
| Salicylate* copy–write | .040 | |||
| Step 5 | Other NSAIDs | .92 | 0.96 | 0.42-2.21 |
| Copy–write | .000 | 2.68 | 1.94-3.70 | |
| Other NSAIDs* copy–write | .72 |
Abbreviations: CI, confidence interval; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio; Copy-write, Copying pentagons-writing scores of modified mini-mental state examination test.
aAdjusted by age, sex, education, physical exercise, and smoking; 209 cases were missing compared with steps 1 and 2.
bAdjusted by age, sex, education, physical exercise, smoking, and ApoE4 status. This analysis was done with 1099 cases because of additional missing data due to ApoE4 variable. *means interaction in this table.
Table 4.
Modification of Salicylates Effect on Dementia by Copying Pentagons–Writing Scores.
| Nonsalicylates Users | Salicylates Users | ORs for Salicylates Use Within Copy–Writing Strata | |||
|---|---|---|---|---|---|
| N With/Without Dementia | OR (95% CI) | N With/Without Dementia | OR (95% CI) | ||
| Copy–writing score ≥13 | 138/3096 | 1 | 38/569 | 1.500 (1.005-2.238); P = .047 | 1.500 (1.005-2.238); P = .047 |
| Copy–writing score <13 | 51/350 | 2.382 (1.614-3.516); P < .001 | 4/81 | 0.603 (0.182-1.997); P = .408 | 0.250 (0.073-0.860); P = .028 |
Abbreviations: CI, confidence interval; OR, odds ratio; RERI, relative excess risk due to interaction.
Measure of effect modification on additive scale: RERI: −2.279 (−3.624 to −0.934); P = .001. Measure of effect modification on multiplicative scale: −3.779 (−9.638 to 2.079); P = .206. ORs are adjusted by age, sex, education, smoking, and physical exercise.
Among participants who had copying pentagons–writing scores less than 13, the incidence of dementia was 4.7% among salicylates users and 12.7% among nonusers (Figure 2). In a logistic regression, this association was shown in an odds ratio of 0.34 (95% CI: 0.12-0.97; P = .04). Adjustment by age, sex, education, smoking, and physical exercise resulted in an odds ratio of 0.25 (95% CI: 0.073-0.86 P = .028).
Figure 2.
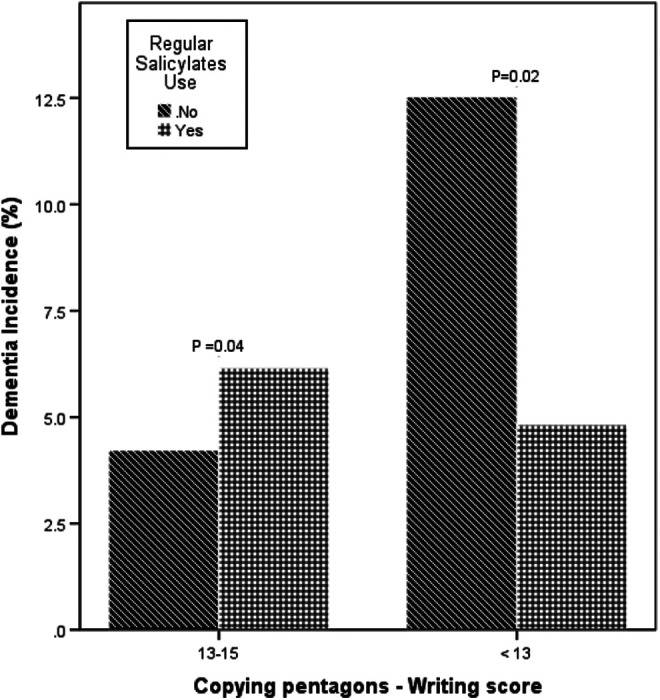
Salicylates use is associated with lower dementia incidence among participants with low copying pentagon–writing score.
Discussion
Our study shows that salicylates use may be associated with lower dementia incidence among older participants who have low scores in a simple neuropsychological test and are 2 to 3 times more prone to develop dementia compared with normal cognition participants.
Among the published longitudinal studies, there is controversy about association of aspirin use with dementia incidence. Three published articles have investigated the role of aspirin in the prevention of dementia, AD, and VaD among participants who were participating in the CSHA. Hebert et al reported a higher incidence of VaD among participants who had used aspirin. 16 They followed participants from CSHA-1 to CSHA-2 and excluded CSHA-2–mixed participants with AD + VaD dementia from their analysis. They did not do any analysis about incident all-cause dementia. Lindsay et al showed that no association existed between CSHA-1 salicylates use and CSHA-2 AD incidence. 17 Like the other article, they did not do any analysis about all-cause dementia incidence. Cote et al reported inconsistent association between salicylates use and all-cause dementia incidence. 18 Salicylates were associated with lower incident dementia when participants with CIND were included in addition to normal cognition participants. Similarly, we did not find any association between salicylates use and incident dementia among normal cognition participants, but we found salicylates use to be associated with lower dementia incidence among a subgroup of normal cognition participants who had low scores in 2 neuropsychological tests. These tests, which were affected specifically among participants with VaD rather than AD ones, may be reflective of vascular brain pathologies.
This inconsistency is also seen in results of other cohorts. In a cohort of Swedish octogenarian twins, aspirin was associated with lower dementia incidence. 1 In another cohort of Swedish participants, aspirin was associated with an increased risk of all-cause dementia. 19 And, still some cohorts showed no association between aspirin use and dementia incidence. 3
Nowadays, there is recommendation to shift researches and actions against dementia from the late stage (overt dementia) to earlier stages, 20 even preclinical one, and relevant guidelines have been published. 21 Neuropsychological tests are widely available and nonexpensive diagnostic modalities which can help identify dementia-prone participants in preclinical stages. They have been shown to perform as accurate as magnetic resonance imaging findings in the prediction of dementia conversion among participants with mild cognitive impairment. 22
Community clinical–pathological studies have shown vascular pathologies to have nearly the same prevalence as AD pathologies, 23 and many participants who were clinically diagnosed as probable AD had mixed AD and vascular pathologies. 24 Contrary to AD pathologies, there are preventive modalities for vascular pathologies such as antithrombotics, antihypertensive drugs, and statins. Identification of vascular cognitive impairment in the preclinical or mild cognitive impairment stages can help prevent incident dementia and clinical AD.
We did not have imaging or pathology data for our sample, but we showed that in these 2 tests, the scores of participants with VaD were lower than scores of patients with probable AD. In other words, these 2 simple neuropsychological tests might be impaired in preclinical stages of vascular cognitive impairment. Visuoexecutive scores in Montreal Cognitive Assessment test, which included copying cubes, were worse among participants with stroke and transient ischemic attack compared with participants complaining of memory problems. 25 Copying pentagons involves at least 2 cognitive domains: visuospatial and executive functions. Posterior parietal cortices are involved in visuospatial processing, 26 whereas frontal–subcortical circuits are involved in executive functions. 27 Although hypometabolism of posterior parietal cortices has been shown in both AD and VaD, brain areas such as frontal pole and basal ganglia were hypometabolic only in VaD, not in AD. 28 Therefore, it is anticipated that copying pentagons are more severely impaired in VaD compared with AD because of decreased functional activity in both systems rather than one.
Salicylates increased dementia incidence among normal cognition participants with normal copying pentagons–writing scores. It is possible that at least a subset of these participants had preclinical AD pathologies, such as amyloid angiopathy, and salicylates have been shown to be associated with cerebral microbleeds, 29 which in turn is associated with the development of dementia. 30
Strengths of this study were community sampling, longitudinal design, and good follow-up. Limitations were those of a post hoc analysis, lack of imaging data, and drug usage information which was gathered through questions asked from participants or their relatives, not through pharmacy records.
If our study findings can be replicated in other longitudinal studies, this may provide bases for a clinical trial of using aspirin in the prevention of dementia among a subgroup of normal population who are at early stages of vascular cognitive impairment. One small clinical trial has showed efficacy of aspirin in cognitive performance improvement among participants with multi-infarct dementia. 31
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grant R-09-102 FSO 91647 from the Canadian Institutes of Health Research (CIHR).
References
- 1. Nilsson SE, Johansson B, Takkinen S, et al. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged > or =80 years. Eur J Clin Pharmacol. 2003;59(4):313–319. [DOI] [PubMed] [Google Scholar]
- 2. in t’ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–1521. [DOI] [PubMed] [Google Scholar]
- 3. Kern S, Skoog I, Ostling S, Kern J, Borjesson-Hanson A. Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open. 2012;2(5). pii: e001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang JH, Cook N, Manson J, Buring JE, Grodstein F. Low dose aspirin and cognitive function in the women’s health study cognitive cohort. BMJ. 2007;334(7601):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082–1088. [DOI] [PubMed] [Google Scholar]
- 6. Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70(5):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Arlington, VA: American Psychiatric Association; 1987. [Google Scholar]
- 10. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. Tenth Revision of the International Classification of Diseases. 10th ed. Geneva, Switzerland: World Health Organization; 1987. [Google Scholar]
- 12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 13. Canadian Study of Health and Aging: study methods and prevalence of dementia. CMAJ. 1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- 14. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. [DOI] [PubMed] [Google Scholar]
- 16. Hebert R, Lindsay J, Verreault R, Rockwood K, Hill G, Dubois MF. Vascular dementia: incidence and risk factors in the Canadian Study of Health and Aging. Stroke. 2000;31(7):1487–1493. [DOI] [PubMed] [Google Scholar]
- 17. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. [DOI] [PubMed] [Google Scholar]
- 18. Cote S, Carmichael PH, Verreault R, Lindsay J, Lefebvre J, Laurin D. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2012;8(3):219–226. [DOI] [PubMed] [Google Scholar]
- 19. Cornelius C, Fastbom J, Winblad B, Viitanen M. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology. 2004;23(3):135–143. [DOI] [PubMed] [Google Scholar]
- 20. Hachinski V. Shifts in thinking about dementia. JAMA. 2008;300(18):2172–2173. [DOI] [PubMed] [Google Scholar]
- 21. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ewers M, Walsh C, Trojanowski JQ, et al. ; North American Alzheimer’s Disease Neuroimaging Initiative (ADNI). Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33(7):1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neuropathology Group; Medical Research Council Cognitive Function and Ageing Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet. 2001;357(9251):169–175. [DOI] [PubMed] [Google Scholar]
- 24. Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke. 2010;41(10 suppl):s144–s146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pendlebury ST, Markwick A, de Jager CA, Zamboni G, Wilcock GK, Rothwell PM. Differences in cognitive profile between TIA, stroke and elderly memory research subjects: a comparison of the MMSE and MoCA. Cerebrovasc Dis. 2012;34(1):48–54. [DOI] [PubMed] [Google Scholar]
- 26. Vannini P, Almkvist O, Franck A, et al. Task demand modulations of visuospatial processing measured with functional magnetic resonance imaging. NeuroImage. 2004;21(1):58–68. [DOI] [PubMed] [Google Scholar]
- 27. Kokubo K, Suzuki K, Hattori N, Miyai I, Mori E. Executive dysfunction in patients with putaminal hemorrhage. J Stroke Cerebrovasc Dis. 2015;24(9):1978–1985. [DOI] [PubMed] [Google Scholar]
- 28. Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC. 18FDG PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. 2006;26(9):1213–1221. [DOI] [PubMed] [Google Scholar]
- 29. Vernooij MW, Haag MD, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66(6):714–720. [DOI] [PubMed] [Google Scholar]
- 30. Miwa K, Tanaka M, Okazaki S, et al. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83(7):646–653. [DOI] [PubMed] [Google Scholar]
- 31. Meyer JS, Rogers RL, McClintic K, Mortel KF, Lotfi J. Randomized clinical trial of daily aspirin therapy in multi-infarct dementia. A pilot study. J Am Geriatr Soc. 1989;37(6):549–555. [DOI] [PubMed] [Google Scholar]