Abstract
Background:
Patients with Parkinson disease are at high risk of developing dementia. During the course of the disease, a substantial number of patients will experience a cognitive decline, indicating the dynamics of the underlying neuropathology. Magnetic resonance imaging (MRI) has become increasingly useful for identifying structural characteristics in radiological brain anatomy existing prior to clinical symptoms. Whether these changes reflect pathology, whether they are aging related, or both often remains unclear. We hypothesized that aging-associated brain structural changes would be more pronounced in the hippocampal region among patients with Parkinson disease having mild cognitive deficits relative to cognitively unimpaired patients.
Methods:
Using MRI, we investigated 30 cognitively healthy patients with Parkinson disease and 33 patients with nondemented Parkinson disease having mild cognitive impairment. All participants underwent structural MRI scanning and extensive clinical and neuropsychological assessments.
Results:
Irrespective of the study participants’ cognitive status, older age was associated with reduced cortical thickness in various neocortical regions. Having mild cognitive impairment was not associated with an increased rate of cortical thinning or volume loss in these regions, except in the hippocampus bilaterally.
Conclusion:
Patients with Parkinson disease having mild cognitive impairment show an accelerated age-dependent hippocampal volume loss when compared with cognitively healthy patients with Parkinson disease. This may indicate pathological processes in a key region for memory functioning in patients with Parkinson disease at risk of developing dementia. Structural MRI of the hippocampal region could potentially contribute to identifying patients who should receive early treatment aimed at delaying the clinical onset of dementia.
Keywords: Parkinson disease, mild cognitive impairment, hippocampus, aging
Introduction
Parkinson disease (PD) is one of the most common neurodegenerative disorders, often primarily presenting with motor symptoms, such as bradykinesia, rigidity, and tremor, resulting from the progressive loss of dopaminergic innervation of the striatum. 1 Nonmotor symptoms associated with PD are less well recognized and they often remain untreated, although they frequently accompany or precede motor symptoms. 2 Although a substantial number of patients with PD may even initially present with depression or sleep disturbance, cognitive dysfunction is largely perceived as a common symptom of late-stage disease. About 80% of patients with advanced PD may develop dementia. 3 Parkinson disease dementia severely interferes with a patient’s daily functioning abilities, reduces the quality of life for both the patient and caregivers, and remains a challenge with respect to treatment as well as the socioeconomic impact. In the absence of potent treatment options, an important clinical goal is to detect mild cognitive symptoms earlier in the course of the disease, as this may ideally allow clinicians to delay the onset of dementia. There is an increasing evidence that mild cognitive deficits may be present already in the initial PD stages. 4 Executive functioning impairment has been reported consistently. 5,6 Loss of cognitive flexibility, low selective attention, and reduced inhibition performance 5 may in part reflect a disruption in dopaminergic frontostriatal pathways. 7 However, the complex PD neuropathology involves changes in various neurotransmitter systems, and a cholinergic deficit is also closely associated with the cognitive impairment in PD. 8,9 Pathological studies suggest that nigrostriatal pathway dysfunction, Lewy body–type degeneration, and coincident Alzheimer-type pathology may contribute to the cognitive deterioration in patients with PD. 10 Therefore, many studies investigating patients with PD having cognitive impairment focus not only on executive functioning abilities but also on other cognitive domains, such as memory. As the cognitive deterioration progresses, severe memory dysfunction could mask the differences between the memory impairment in PD and other neurodegenerative diseases, such as Alzheimer’s disease (AD). Patients with PD may benefit more than patients with AD from semantic cueing, which would imply impairment in accessing rather than storing new information. 10,11 This could reflect a better hippocampus functioning among patients with PD relative to patients with AD. 11 Recent metabolic imaging data indicate distinct neuronal network changes underlying cognitive deterioration in PD dementia when compared to AD. 12 Patients with PD dementia show a milder medial temporal lobe glucose hypometabolism than patients with AD. 13 Utilizing volumetric assessments of structural magnetic resonance imaging (MRI) data, Burton and colleagues found greater hippocampal atrophy in AD than in patients with PD dementia. 14 However, others show that an AD-like pattern of brain atrophy (including hippocampal atrophy) predicts cognitive decline in PD. 15 Hippocampal atrophy can be detected during PD development, although the progression to PD dementia may not simply reflect further hippocampal changes. 16 From a neuropathological perspective, it seems interesting that about 30% of patients with PD dementia show hallmarks of AD—β-amyloid deposition and neurofibrillary tangles—on autopsy. 17
A recent study suggests that memory dysfunction in newly diagnosed earlier stage patients with PD is mainly explained by deficits in encoding new information, 18 and there is evidence that hippocampal atrophy correlates with impaired memory performance. 19,20 In light of the research results’ variability, the question arises when and how pathological processes would affect key brain regions of the neural networks underlying memory in nondemented patients with PD.
Structural MRI may become an increasingly important tool in PD diagnostic procedures. An example is visualizing the loss of dopaminergic neurons in the substantia nigra pars compacta using ultra-high-field MRI, 21 but researchers will also be able to more precisely investigate other brain regions that are involved in the various motor and nonmotor symptoms of PD. With respect to memory functioning, the medial temporal lobe and specifically the hippocampus are of particular scientific interest. Compared with the vast amount of structural MRI data from patients with AD or its preclinical stages, the medial temporal lobe region has been investigated less frequently among patients with PD. Cognitively healthy patients with PD and demented PD show hippocampal atrophy when compared with healthy people. 22 –24 These authors and others did not find a clear volumetric difference in the hippocampus between patients with PD with or without dementia. 14,24,25 Dalaker and colleagues 26 revealed larger left inferior lateral ventricle and third ventricle volumes in nondemented patients with mild cognitive impairment relative to cognitively unimpaired patients with PD. Overall, there is an increasing evidence for changes in brain structure associated with specific neuropsychological deficits in PD. 27 The relationship of hippocampal volume loss and verbal memory deficits remains a consistent finding. 27 However, the majority of studies did not investigate patients with PDlongitudinally, and the main focus was often to reveal differences in radiological anatomy between a patient group (cognitively impaired or nonimpaired patients with PD) and healthy elderly people. Bouchard and colleagues 28 showed that the hippocampal volumes of older but not younger patients with nondemented PD differed from the hippocampus volumes of healthy control participants. This highlights the possible presence of additional aging-related hippocampal atrophy processes. 29,30
In this study, we investigated middle-aged and elderly cognitively unimpaired patients with PD (“PD” group) and patients with nondemented PD having mild cognitive impairment (“PD-MCI” group) using structural MRI. We hypothesized that age and cognitive impairment would be associated with reduced volume in brain regions most susceptible to the neuropathological changes underlying mild cognitive impairment, specifically the hippocampus.
Participants and Methods
Participants
All study participants were consecutively recruited through the Movement Disorder outpatient center at the University hospital’s Department of Neurology. The University’s ethics committee approved the study, and written informed consent was obtained.
Participants across all ages and disease severities meeting the criteria for “idiopathic PD,” according to the UK PD Brain Bank criteria, 31 or the diagnosis “idiopathic PD with MCI” (PD-MCI), according to the consensus guidelines developed by Litvan and colleagues, 32 were included in this study. The sample was part of a larger ongoing study of natural progression of cognitive impairment in PD (DEMPARK/LANDSCAPE [Parkinson’s Disease and Dementia: A Longitudinal Study] study). 33 Patients were excluded if they had an identifiable cause of parkinsonism or signs for atypical parkinsonian disorders, psychosis, met the criteria for “idiopathic PD with dementia” according to Emre and colleagues, 34 conditions that prevented an MRI scan, or other relevant conditions interfering with the study protocol. All patients were assessed during best medical on state.
In both groups, only patients without significant signs of vascular brain disease were considered. The included patients did not show white matter lesions in their structural brain MRI scans, or they had focal white matter lesions only (Scale for age-related white matter changes [ARWMC-scale] scale, 35 score <2 points), and were free of focal lesions in gray matter.
Clinical Assessments and MRI Scanning
We assessed demographic and clinical data including disease duration, modified Hoehn-Yahr score, 36 and Unified PD Rating Scale. 37 In addition, all participants completed the extensive neuropsychological test battery of the DEMPARK/LANDSCAPE study protocol. 33
We obtained T1-weighted brain images using a 1.5-T Siemens Magnetom Sonata MRI (Siemens Medical Solutions, Erlangen, Germany) with an 8-array head coil (MPRAGE, sagittal, time to echo (TE): 3.9 millisecond, repetition time: 2180 millisecond, field of view: 280 × 256 mm, phase-FOV: 91.6%, resulting in 0.7 × 0.7 mm in-plane resolution and 1-mm slice thickness). The MRI data were analyzed for group differences in cortical thickness and volumes of subcortical structures using the FreeSurfer software package 38 (http://surfer.nmr.mgh.harvard.edu). We utilized the recon-all program for skull stripping, Talairach transformation, segmentation of subcortical white matter and deep gray matter volumetric structures, 39 and delineation of white and gray matter surfaces. Automatic cortical parcellation is based on the Destrieux anatomical atlas. 40 Subcortical and deep gray matter volumetric segmentation is also an automated procedure, which assigns a neuroanatomical label to each voxel in an MRI volume based on a probabilistic atlas of the brain. With respect to accuracy, the technique is comparable to manual labeling approaches. 41 It is also robust to anatomical variability, including ventricular enlargement typically associated with neurological diseases and aging. 41
Cortical thickness was measured as the distance between gray and white matter boundaries at each point (vertex) across the cortex. Using continuity and intensity information from the volumetric MRI data for segmentation and deformation procedures, cortical thickness maps are generated based on spatial intensity gradients. In order to align cortical folding patterns of multiple patients for group analyses, the recon-all stream includes a nonrigid spherical averaging method. The cortical thickness maps were smoothed with a Gaussian kernel of 10 mm full width at half-maximum. The procedures have been detailed elsewhere, 41 –43 and the accuracy of the technique has been confirmed using histological measures and manual approaches. 44,45
Statistical Analyses
Demographic and neuropsychological data were analyzed using independent samples t tests for continuous variables and χ2 tests for categorical measures. In the FreeSurfer analyses, we used a generalized linear model (GLM) for the assessment of aging effects on cortical thickness, applying a false discovery rate correction (0.05) for multiple comparison correction. For the calculation and visualization of group statistics on brain morphometry data, we utilized the “Query, Design, Execute, Contrast” program within the FreeSurfer tool kit. Hippocampal volume differences were analyzed using SPSS statistical software (version 18.0; SPSS Inc, Chicago, Illinois) and a GLM, with hippocampal volume (left + right) as the dependent variable and group (PD-MCI, PD) as a fixed factor. We additionally investigated the effects of age on hippocampal volume by using age as a covariate and by determining Pearson correlation coefficients between hippocampus volume and age.
Results
Study Cohort
A total of 31 cognitively healthy patients with PD (6 women, 25 men, mean [SD] age 65.7 [7.5] years) and 32 patients with PD-MCI (8 women, 24 men, mean age 68.3 [6.8] years) were enrolled. A total of 15 patients were screened and rejected, because they did not meet inclusion criteria. Mini-Mental State Examination and Parkinson Neuropsychometric Dementia Assessment results were significantly worse for PD-MCI than for patients with PD (P < .001). The PD-MCI also performed significantly worse in all other neuropsychological tests except for the Boston Naming Test and the constructional praxis subtest of the Consortium to Establish a Registry for Alzheimer’s Disease test battery. There were no significant differences between groups concerning age, sex, disease duration, and educational level. All patients were right-handed. For extended clinical and neuropsychological data, see Tables 1 and 2.
Table 1.
Baseline Demographic and Clinical Data of Patients With PD and PD-MCI.
| PD | PD-MCI | P Valuea | |
|---|---|---|---|
| Number of patients, n | 31 | 32 | |
| PD-MCI subtype classification, n (%) | |||
| Amnestic single domain | 3 (9%) | ||
| Amnestic multiple domain | 21 (66%) | ||
| Nonamnestic single domain | 6 (19%) | ||
| Nonamnestic multiple domain | 2 (6%) | ||
| Female/male, n (%) | 6 (19%)/25 (81%) | 8 (25%)/24 (75%) | .597 |
| Age, mean (SD), range, years | 65.7 (7.5), 52-79 | 68.3 (6.8), 54-80 | .371 |
| Duration of PD (years), mean (SD; range) | 7.3 (3.4), 2-14 | 8.8 (5.3), 2-24 | .172 |
| Laterality at disease onset (right/left), n (%) | 17 (55%)/14 (45%) | 21 (66%)/11 (34%) | .390 |
| Hoehn and Yahr stage, mean (SD) | 2.2 (0.6) | 2.5 (0.7) | .075 |
| I-I.5, n (%) | 3 (10%) | 2 (6%) | |
| II-II.5, n (%) | 18 (58%) | 13 (41%) | |
| III, n (%) | 10 (32%) | 15 (47%) | |
| IV, n (%) | 0 (0%) | 2 (6%) | |
| Treatment, n (%) | |||
| Levodopa | 21 (68%) | 25 (78%) | .124 |
| Dopamine agonists | 26 (84%) | 29 (91%) | .293 |
| Amantadine | 13 (42%) | 12 (36%) | .299 |
| Antidepressants | 5 (16%) | 9 (28%) | .440 |
| Neuroleptics | 2 (6%) | 1 (3%) | .181 |
| UPDRS III, mean (SD; range) | 20 (7), 9-37 | 22 (8), 7-39 | .338 |
| MMSE, mean (SD; range) | 29 (1), 27-30 | 27 (2), 22-30 | <.001 |
| PANDA, mean (SD; range) | 26 (4), 17-30 | 21 (4), 10-28 | <.001 |
| GDS, mean (SD; range) | 3 (3), 0-13 | 3 (3), 0-11 | 1.000 |
| AES, mean (SD; range) | 12 (6), 0-24 | 14 (8), 1-30 | .302 |
Abbrevaitions: AES, Apathy Evaluation Scale; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; PANDA, Parkinson Neuropsychometric Dementia Assessment; PD, Parkinson disease; PD-MCI, Parkinson disease with mild cognitive impairment, PD-MCI subtype classification according to the consensus guidelines by Litvan and coworkers32; UPDRS, Unified Parkinson Disease Rating Scale; SD, standard deviation.
aUnpaired 2-sided t test.
Table 2.
Neuropsychological Data of Patients With PD and PD-MCI at Baseline.
| Cognitive Functions | Tests Applied | PD | PD-MCI | P Valuea |
|---|---|---|---|---|
| Memory | CERAD (z scores) | |||
| Word list learning, mean (SD) | 0.27 (0.91) | −0.54 (1.17) | .003 | |
| Word list recall, mean (SD) | 0.16 (0.91) | −0.50 (1.12) | .015 | |
| Word list recognition, mean (SD) | 0.25 (0.73) | −0.49 (1.08) | .002 | |
| Executive functions | Modified card sorting test (t scores) | |||
| Categories, mean (SD) | 46.06 (8.22) | 32.68 (9.39) | <.001 | |
| Nonperseverative errors, mean (SD) | 45.03 (6.73) | 36.58 (7.28) | <.001 | |
| Perseverative errors, mean (SD) | 47.71 (5.29) | 41.52 (8.31) | .001 | |
| Verbal fluency tests (z scores) | ||||
| Animal, mean (SD) | 0.23 (0.87) | −0.37 (0.89) | .009 | |
| Words with S, mean (SD) | 0.79 (0.96) | 0.47 (1.08) | 2.25 | |
| Attention | Trail Making Test (z scores), mean (SD) | 0.19 (1.04) | −0.37 (1.15) | .059 |
| Stroop test (t scores) | ||||
| Words, mean (SD) | 55.31 (6.22) | 54.03 (7.35) | .468 | |
| Colors, mean (SD) | 55.55 (8.70) | 52.85 (8.48) | .223 | |
| Interference, mean (SD) | 58.93 (6.31) | 55.84 (7.56) | .090 | |
| Visuospatial function | LPS subtest 7 (t scores), mean (SD) | 58.77 (9.64) | 48.16 (11.81) | <.001 |
| LPS subtest 9 (t scores), mean (SD) | 60.20 (5.68) | 50.65 (8.57) | <.001 | |
| CERAD constructional praxis (z scores), mean (SD) | 0.05 (1.04) | 0.15 (1.00) | .207 | |
| Naming | Boston Naming Test (z scores), mean (SD) | 0.24 (0.78) | −0.06 (0.90) | .177 |
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease test battery; LPS, Leistungsprüfsystem, an intelligence test frequently used in German-speaking countries; PD, Parkinson disease; PD-MCI, Parkinson disease with mild cognitive impairment; SD, standard deviation.
aUnpaired 2-sided t test.
Bold values indicate significant differences of group.
Magnetic Resonance Imaging Results
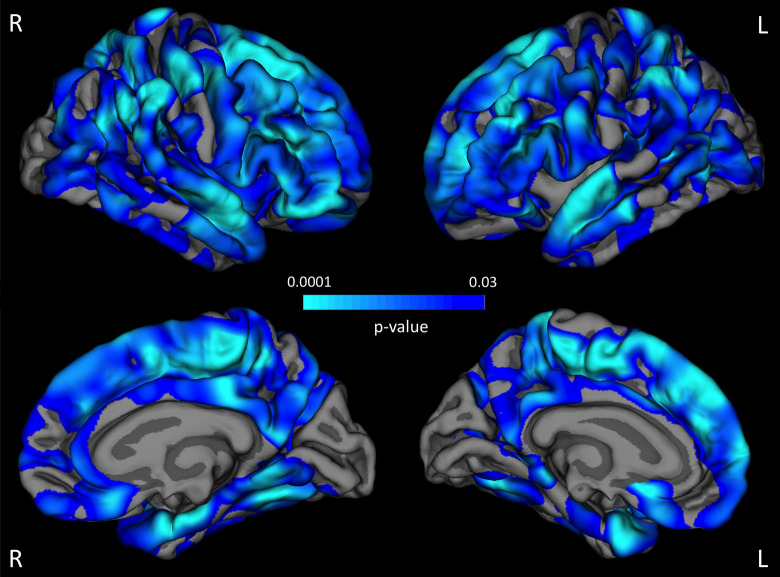
We showed that older age was associated with a thinner cortex in various brain regions. The highest correlations between cortical thickness and age were found in the frontal and temporal cortex bilaterally (Figure 1). The factor “group” (PD, PD-MCI) did not influence cortical thickness or the association between cortical thickness and age at the predefined statistical threshold in the whole-brain analysis.
Figure 1.
Aging-associated cortical thinning. Across all study participants, there are various brain regions (highlighted in blue) that showed a thinner cortex with advanced age at the predefined statistical threshold (false discovery rate [FDR] 0.05 for multiple comparison correction). The strongest associations can be detected in the frontal and temporal cortices (light blue) (see color version of this figure online).
The analysis of hippocampal volume (left + right) revealed a smaller hippocampal volume among patients with PD-MCI versus PD (F(1,61) = 4.656, P = .035). Investigating the left and right hippocampus separately showed that the result was not driven by 1 hemisphere (Table 3). Although our groups did not differ in mean age, Pearson correlation analyses showed lower hippocampal volumes with a more advanced age among patients with PD-MCI (r = −.459, P = .007), whereas patients with PD only showed a trend for this association (r = −.306, P = .1; Figure 2). This effect was again not driven by 1 hemisphere.
Table 3.
Hippocampal Volumes of the Cohort.a
| PD | PD-MCI | P Value | |
|---|---|---|---|
| Hippocampus, L | 4153 (463) | 3886 (528) | .04 |
| Hippocampus, R | 4196 (492) | 3915 (555) | .04 |
| Hippocampus, L + R | 8348 (926) | 7802 (1069) | .03 |
Abbreviations: L, left; R, right; PD, Parkinson disease; PD-MCI, Parkinson disease with mild cognitive impairment.
aVolumes are expressed as mean (SD), mm3.
Figure 2.
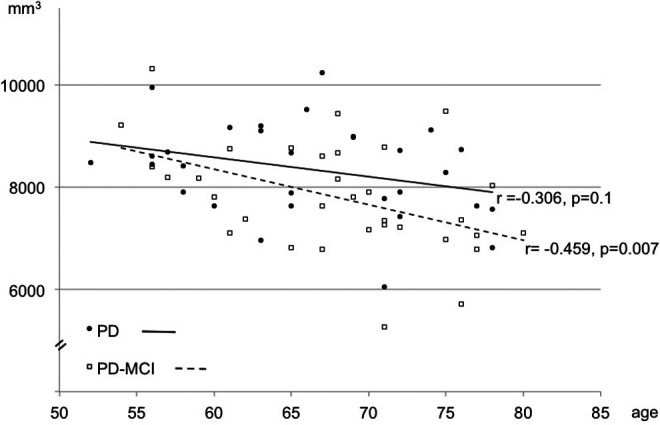
Hippocampal volume and age. In contrast to patients with Parkinson disease (PD), patients with Parkinson disease having mild cognitive impairment (PD-MCI) showed an accelerated volume loss with an increasing age (Pearson correlation analysis).
Discussion
When considering all study participants irrespective of cognitive status, a more advanced age was associated with decreased cortical thickness, showing strongest associations in frontal and temporal brain regions. This is in line with other studies investigating aging effects on cortical thinning patterns in healthy people 46,47 and cognitively unimpaired patients with PD. 48 The existing imaging data on cortical atrophy in early PD remain variable, 14,23,49 and our study was not aimed at detecting such possible changes associated with PD, since we did not investigate a healthy control group.
We did not detect a group difference in cortical thickness between our patients with PD and PD-MCI in a whole-brain analysis. We also did not expect global thickness changes. Beyer and colleagues 50 showed reduced cortical gray matter density in the left middle frontal gyrus, precentral gyrus, left superior temporal lobe, and right inferior temporal lobe among patients with PD-MCI when compared to patients with cognitively unimpaired PD. Although differences in morphometric brain analysis techniques could have contributed to the contrasting finding, the authors note that their results did not survive multiple comparison correction. Since we did not have a hypothesis regarding brain structure changes in specific neocortical regions other than the hippocampus, we did not consider possible group differences in cortical thickness using uncorrected data.
According to the nature of MCI as defined by current criteria, 51 memory impairment is the prominent neuropsychological feature. Patients with PD with MCI are not demented, but they are at high risk of developing dementia. 52 There is a substantial amount of neuroimaging data 53 –55 on hippocampal structure changes in patients with MCI without a movement disorder who are at high risk of developing AD. 56 We therefore hypothesized that early structural brain changes associated with memory impairment would be detectable specifically in the hippocampal region among patients with PD-MCI. Furthermore, we demonstrated a remarkable effect of aging on brain structure, suggesting accelerated aging-related decline in hippocampal volume for patients with PD-MCI. This points to the possible neuropathological changes in this region preceding PD dementia. Whether these changes are similar to neuropathological features seen in AD cannot be determined using structural MRI. Recent positron emission tomography (PET) studies in patients with PD suggest that amyloid pathology is associated with cognitive decline. However, β-amyloid plaques as well as Lewy-body pathology are not unique for a specific neurodegenerative disease. 57,58 It also remains unclear how and under which circumstances these diseases’ underlying cellular changes may overlap or coexist.
Footnotes
Authors’ Note: Christine B. Schneider and Markus Donix has authors contributed equally to the study.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rodriguez-Oroz MC, Jahanshahi M, Krack P, et al. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–1139. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. [DOI] [PubMed] [Google Scholar]
- 4. Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(pt 7):1787–1798. [DOI] [PubMed] [Google Scholar]
- 5. Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson’s disease: systematic review and meta-analysis. Mov Disord. 2011;26(13):2305–2315. [DOI] [PubMed] [Google Scholar]
- 6. Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson’s disease: a population-based study. Eur J Neurol. 2009;16(12):1278–1284. [DOI] [PubMed] [Google Scholar]
- 7. Ravizza SM, Goudreau J, Delgado MR, Ruiz S. Executive function in Parkinson’s disease: contributions of the dorsal frontostriatal pathways to action and motivation. Cogn Affect Behav Neurosci. 2012;12(1):193–206. [DOI] [PubMed] [Google Scholar]
- 8. Perry EK, Curtis M, Dick DJ, et al. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1985;48(5):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emre M. What causes mental dysfunction in Parkinson’s disease? Mov Disord. 2003;18(suppl 6):S63–S71. [DOI] [PubMed] [Google Scholar]
- 10. Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2(4):229–237. [DOI] [PubMed] [Google Scholar]
- 11. Helkala EL, Laulumaa V, Soininen H, Riekkinen PJ. Recall and recognition memory in patients with Alzheimer’s and Parkinson’s diseases. Ann Neurol. 1988;24(2):214–217. [DOI] [PubMed] [Google Scholar]
- 12. Mattis PJ, Niethammer M, Sako W, et al. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology. 2016;87(18):1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nobili F, Morbelli S, Arnaldi D, et al. Radionuclide brain imaging correlates of cognitive impairment in Parkinson’s disease (PD). J Neurol Sci. 2011;310(1-2):31–35. [DOI] [PubMed] [Google Scholar]
- 14. Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(pt 4):791–800. [DOI] [PubMed] [Google Scholar]
- 15. Weintraub D, Dietz N, Duda JE, et al. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain. 2012;135(pt 1):170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson’s disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. [DOI] [PubMed] [Google Scholar]
- 17. Sabbagh MN, Adler CH, Lahti TJ, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23(3):295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bronnick K, Alves G, Aarsland D, Tysnes OB, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25(1):114–124. [DOI] [PubMed] [Google Scholar]
- 19. Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jokinen P, Bruck A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15(2):88–93. [DOI] [PubMed] [Google Scholar]
- 21. Cho ZH, Oh SH, Kim JM, et al. Direct visualization of Parkinson’s disease by in vivo human brain imaging using 7.0 T magnetic resonance imaging. Mov Disord. 2011;26(4):713–718. [DOI] [PubMed] [Google Scholar]
- 22. Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46(3):678–681. [DOI] [PubMed] [Google Scholar]
- 23. Summerfield C, Junqué C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62(2):281–285. [DOI] [PubMed] [Google Scholar]
- 24. Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003;18(7):784–790. [DOI] [PubMed] [Google Scholar]
- 25. Apostolova LG, Beyer M, Green AE, et al. Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord. 2010;25(6):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalaker TO, Zivadinov R, Ramasamy DP, et al. Ventricular enlargement and mild cognitive impairment in early Parkinson’s disease. Mov Disord. 2011;26(2):297–301. [DOI] [PubMed] [Google Scholar]
- 27. Ibarretxe-Bilbao N, Junque C, Marti MJ, Tolosa E. Brain structural MRI correlates of cognitive dysfunctions in Parkinson’s disease. J Neurol Sci. 2011;310(1-2):70–74. [DOI] [PubMed] [Google Scholar]
- 28. Bouchard TP, Malykhin N, Martin WR, et al. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol Aging. 2008;29(7):1027–1039. [DOI] [PubMed] [Google Scholar]
- 29. Driscoll I, Hamilton DA, Petropoulos H, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13(12):1344–1351. [DOI] [PubMed] [Google Scholar]
- 30. Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–438. [DOI] [PubMed] [Google Scholar]
- 31. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balzer-Geldsetzer M, Costa AS, Kronenburger M, et al. Parkinson’s disease and dementia: a longitudinal study (DEMPARK). Neuroepidemiology. 2011;37(3-4):168–176. [DOI] [PubMed] [Google Scholar]
- 34. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707; quiz 837. [DOI] [PubMed] [Google Scholar]
- 35. Wahlund LO, Barkhof F, Fazekas F, et al. ; European Task Force on age-related white matter changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322.+ [DOI] [PubMed] [Google Scholar]
- 36. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 37. Fahn S, Elton RL, UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, eds. Recent Developments in Parkinson’s Disease. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163, 293-304. [Google Scholar]
- 38. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. [DOI] [PubMed] [Google Scholar]
- 40. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 42. Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- 43. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 44. Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. [DOI] [PubMed] [Google Scholar]
- 45. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. [DOI] [PubMed] [Google Scholar]
- 46. Fjell AM, Westlye LT, Amlien I, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19(9):2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. [DOI] [PubMed] [Google Scholar]
- 48. Jubault T, Gagnon JF, Karama S, et al. Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage. 2011;55(2):462–467. [DOI] [PubMed] [Google Scholar]
- 49. Dalaker TO, Larsen JP, Bergsland N, et al. Brain atrophy and white matter hyperintensities in early Parkinson’s disease(a). Mov Disord. 2009;24(15):2233–2241. [DOI] [PubMed] [Google Scholar]
- 50. Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petersen R. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 52. Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. [DOI] [PubMed] [Google Scholar]
- 53. Chételat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–946. [DOI] [PubMed] [Google Scholar]
- 54. Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. [DOI] [PubMed] [Google Scholar]
- 55. Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6(4):347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 57. Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25(15):2516–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]