Abstract
The possible roles played by growth arrest and DNA damage-inducible gene 34 (GADD34) in Alzheimer’s disease (AD) are so far less understood. In this study, we found that GADD34 was increased in the brains of AD transgenic J20 mice. The deposition of β-amyloid (Aβ) peptide is the main component of neurotic plaques in AD brain. Thus, we examined the effect of Aβ in the expression of GADD34 in human SH-SY5Y cells in vitro. Amyloid β (Aβ1-42) treatment led to increased expression of GADD34. Pretreatment with 50 nmol/L of c-Jun N-terminal kinases (JNK) inhibitor SP600125 abolished the upregulation of GADD34. c-Jun silencing by transfection with c-Jun small-interfering RNA abolished the effects of Aβ1-42 on the expression of GADD34. Importantly, chromatin immunoprecipitation studies verified the ability of c-Jun to bind to the GADD34 promoter, and this ability was increased more than 3-fold by Aβ1-42. These data suggest that the induction of GADD34 by Aβ is mediated by JNK/c-Jun pathway. Finally, depletion of GADD34 significantly rescued Aβ-induced cell apoptosis as evidenced by a marked decrease in the number of terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells. Consistently, knockdown of GADD34 attenuated caspase 3 activation induced by Aβ1-42.
Keywords: Alzheimer’s disease, growth arrest and DNA damage-inducible gene 34 (gadd34), JNK, c-Jun, apoptosis
Introduction
Alzheimer’s disease (AD) is the most frequent dementing disorder in the elderly individuals and is characterized by a progressive decline in memory and other cognitive domains. 1 The hallmark neuropathological lesions in AD are the presence of neurotic plaques and neurofibrillary tangles, which are secondary to the deposition of β-amyloid peptide (Aβ) and hyperphosphorylated τ protein. 2 The pathogenic mechanisms that lead to AD are unclear. The most accepted pathogenic process in AD derives from the “amyloid cascades hypothesis.” In summary, it states that accumulation of Aβ species in the brain, either by increased production or by reduced protein clearance, leads to several secondary pathological changes in neurons and glial cells culminating in widespread neurodegeneration (dystrophic neurites and intracellular neurofibrillary tangles) and cell death. 3 One unifying hypothesis suggests that oxidative processes play contributory or acceleratory roles in many of the salient features of AD. Evidence of increased oxidative stress in AD-affected brains has come from studies showing increased lipid peroxidation, 4 increased carbonyl modifications of proteins, 5 and increased oxidation of mitochondrial and genomic DNA. 6 Generation of reactive oxygen species has been reported to induce the activation of mitogen-activated protein kinases, including c-Jun N-terminal kinases (JNK), P38, and ERK. Increased JNK phosphorylation was found to localize to amyloid deposits in AD model mice. 7 Activation of JNK and of their substrate, c-Jun, plays an essential role in Aβ-induced neuronal apoptosis. 8
The cumulative damage over time, especially to DNA, is thought to contribute to selective neuronal cell loss because unrepaired DNA can lead to apoptosis. 9 In addition, endoplasmic reticulum (ER) stress of neurons has been extensively associated with the evolution of pathological changes in AD. 10 Growth arrest and DNA damage-inducible gene 34 (GADD34) is a protein induced by cell damage, and it is a major regulator of translation during cell stress. 11 Studies of GADD34 gene-deficient mice have shown that GADD34 promotes cell survival and the recovery from protein synthesis inhibition induced by ER stress. 12 Because GADD34 was originally cloned after genotoxic stresses, GADD34 appears to possess another function that relates to genotoxic stresses. The GADD genes also have a growth inhibitory function and one or a combination of these GADD genes suppress cell growth. 13 Moreover, GADD34 works under DNA damaging stress. A recent study has demonstrated that GADD34 expression is increased in response to stress induced by ischemia. 14 GADD34 was reported to play an important role in cell protection in mutant huntingtin-expressing cells through mediating cytoprotective autophagy. 15 With regard to the regulation of GADD34 expression, little information is reported. The GADD34 promoter region contains “TGAGTCA” motifs, which is completely identical to the signal-transducing transcription factor c-Jun binding site. However, the expression patterns and possible roles of GADD34 in AD are so far less understood. In this study, we reported that GADD34 was increased in both AD transgenic J20 mice and Aβ-treated SH-SY5Y cells. Further study revealed that c-Jun was involved in the upregulation of GADD34 induced by Aβ through transactivating the promoter region of GADD34.
Materials and Methods
Cell Culture, Treatment, and Transfection
SH-SY5Y human neuroblastoma cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum containing glutamine (2 mmol/L) and antibiotics (penicillin/streptomycin).
Oligomeric Aβ is the most toxic form of the peptide. It was prepared following the instructions as described previously. 16 Briefly, amyloid β (Aβ1-42) peptide (American Peptide) was dissolved in hexafluoroisopropanol (Sigma) for 2 days at room temperature (RT), followed by dissolving in dimethyl sulfoxide. Cells were treated with 25 µmol/L Aβ1-42 for 48 hours. Human c-Jun and a nonspecific control small-interfering RNA (siRNA) were acquired from Santa Cruz Biotech and were transfected into SH-SY5Y cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Primary Neurons Isolated From Mice
Primary cortical neurons were obtained from C57/BL6 mice embryos on prenatal day 17 (E17), as previously described. 17 Neurons were maintained in neurobasal culture medium (Gibco, Germany) supplemented with 0.5 mmol/L glutamine, 1% antibiotic, and 3% B27 in a humidified atmosphere containing 5% CO2 at 37°C for 7 days prior to experimentation. Cell cultures were then incubated in fresh medium with Aβ1-42 (20 µmol/L). Lentiviral particles of GADD34 RNAi were purchased from Santa Cruz. It was used to infect neurons according to the protocol described previously. 18
Animals
Alzheimer’s disease transgenic J20 mice were used in this study. The mice were from the Jackson laboratory. These mice express a mutant form of the human amyloid protein precursor bearing both the Swedish (K670N/M671L) and the Indiana (V717F) mutations (APPSwInd). Mice 6 months old and of mixed gender were used. Results from all animals were compared with those from appropriate age-matched, wild-type (WT) controls. The animal protocol was approved by the Central South University Animal Care and Use Committee, in accordance with Institutional Animal Care and Use Committee standards.
Real-Time Polymerase Chain Reaction
RNA from mouse brain tissue samples or SY-SH5Y cells was isolated using TRIzol reagent (Invitrogen) following the instructions. Total RNA of 2 µg was reverse transcribed to first-strand complementary DNA using M-MuLV reverse transcriptase (New England Biolabs Inc) and mixture of random and oligo-dT primers. Real-time polymerase chain reaction (PCR) was conducted with either SYBR green or Roche universal probe reagents (Universal ProbeLibrary, Roche Applied Science) on a StepOne-Plus Real-Time PCR System (Applied Biosystems). Gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the ΔΔCt method. The following primers were used in this study: human GADD34: forward, 5′-gcttctggcagaccgaac-3′; reverse, 5′-gtagcctgatggggtgctt-3′; Human GAPDH: forward, 5′-ccacatcgctcagacaccat-3′, reverse, 5′-ccaggcgcccaatacg-3′; Mouse GADD34: forward, 5′-tctctaacgccacagttaccc-3′; reverse, 5′aaccgcctctcactgacg-3′; Mouse GAPDH: forward, 5′-tgtgtccgtcgtggatctga-3′; reverse 5′-cctgcttcaccaccttcttga-3′.
Western Blot Analysis
Cell lysates were prepared using lysis buffer (50 mmol/L Tris–HCl, pH 7.6), 0.02% sodium azide, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mg/mL aprotinin, 1 mg/mL antipain, and 1 mmol/L sodium orthovanadate). After centrifugation at 12000g for 10 minutes at 4°C, the supernatant was used for Western blot analysis. Bicinchoninic acid assay (Pierce, Rockford, Illinois) was used to determine the protein concentration. Proteins were subject to 10% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane by standard procedure as described previously. 19 After blocking by 5% nonfat milk (in Tris-buffered saline solution with Tween 20), transferred blots were sequentially probed with primary antibodies and secondary antibodies. Blots were developed by the enhanced chemiluminescence technique (Santa Cruz Biotechnology) according to the manufacturer’s instruction.
Chromatin Immunoprecipitation Studies
In brief, 2 × 107 cells were used for chromatin immunoprecipitation (CHIP) studies as described previously. Native protein–DNA complexes were cross-linked by treatment with 1% formaldehyde for 10 minutes at RT. Briefly, equal aliquots of isolated chromatin were subjected to immunoprecipitation with a mouse anti-c-Jun antibody (Cell Signaling) or mouse immunoglobulin G (IgG) control. The DNA associated with specific immunoprecipitates or with control IgG was isolated and used as a template for the PCR to amplify the GADD34 promoter sequences containing the c-Jun binding site. The primers used were: 5′-GCTCGGAAATTACGTGAGATCG-3′ and 5′-GCGCCAACATCGTCCACGCG-3′. As a specificity control, the actin promoter was amplified from the same templates using the following primers: 5′-GAGCACAGAGCCTCGCCTTT-3′; 5′-AGACAAAGACCCCGCCGGTT-3′.
Statistics
All data were presented as mean ± standard deviation. Student’s t test was used for 2-pair comparison. One-way analysis of variance was used to assess statistical significance of differences among treatment groups. Differences between means were considered significant when P < .05.
Results
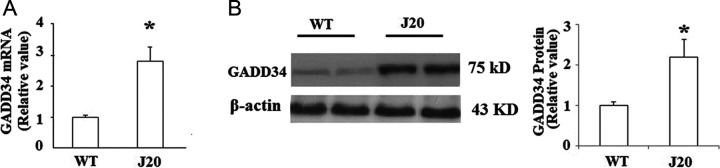
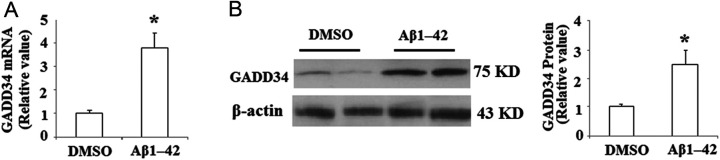
First, we examined the expression patterns of GADD34 in brains of AD transgenic J20 mice. Messenger RNA (mRNA) levels of GADD34 was measured by real-time PCR, and the result indicated that the expression level of GADD34 was significantly increased in the whole brains of J20 mice compared to WT controls (Figure 1A). Consistently, Western blot analysis of the brain tissue displayed that the expression of GADD34 was obviously increased at protein levels (Figure 1B). Alzheimer’s disease is a multiple pathological factor disease; however, Aβ has been considered to play a critical role in the process of AD. In order to examine whether the increase in GADD34 in J20 mice was induced by overburden of Aβ or other factors, the expression of GADD34 was investigated in SY-SH5Y human neuroblastoma cells after treatment with 25 µmol/L Aβ1-42 for 48 hours, and the results displayed that treatment with Aβ1-42 led to a significant induction of GADD34 at both mRNA and protein levels (Figure 2A and B).
Figure 1.
The expression level of growth arrest and DNA damage-inducible gene 34 (GADD34) was increased in J20 mice. A, Real-time polymerase chain reaction (PCR) analysis revealed that the messenger RNA (mRNA) level of GADD34 was significantly increased in J20 brains compared with controls (*P < .01 vs controls, Student’s t test, n = 5). B, Representative immunoblot and quantification analysis revealed that the protein level of GADD34 was significantly increased in J20 brains compared with age-matched controls (*P < .01 vs control, Student’s t test, n = 5); β-actin was used as an internal loading control.
Figure 2.
Amyloid β (Aβ) 1-42 induced alterations of growth arrest and DNA damage-inducible gene 34 (GADD34) in SH-SY5Y cells. A, SH-SY5Y cells were treated with Aβ1-42 (25 µmol/L) for 48 hours; real-time polymerase chain reaction (PCR) analysis revealed that the messenger RNA (mRNA) level of GADD34 was significantly increased after Aβ1-42 treatment (*P < .01, Student’s t test, n = 5). B, SH-SY5Y cells were treated with Aβ1-42 (25 µmol/L) for 48 hours; representative immunoblot and quantification analysis revealed that the protein level of GADD34 was significantly increased after Aβ1-42 treatment (*P < .01, Student’s t test, n = 5).
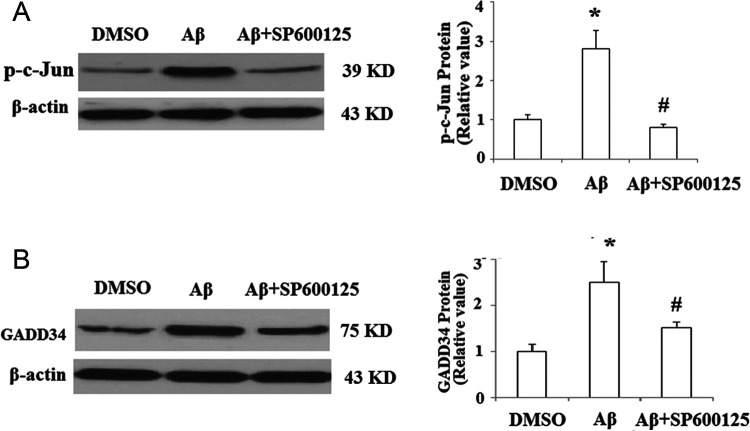
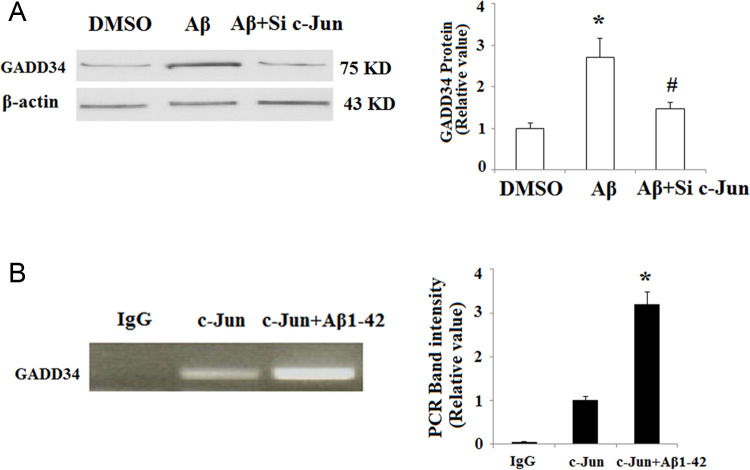
We next investigated the underlying mechanisms of the regulation of GADD34 by Aβ1-42. Promoter analysis revealed that GADD34 promoter region contained “TGAGTCA” motifs, which was completely identical to the signal-transducing transcription factor c-Jun binding site. In addition, activation of c-Jun was associated with Aβ neurotoxicity in the previous study. 20 In order to examine whether c-Jun mediated Aβ1-42-induced upregulation of GADD34, the activation pattern of c-Jun was investigated in SY-SH5Y cells after Aβ1-42 treatment. The phosphorylation of c-Jun by JNK plays a critical role in its transcriptional activity. 21 As shown in Figure 3A, exposure to Aβ1-42 for 48 hours significantly increased phosphor-c-Jun. Importantly, Western blot results revealed that pretreatment with 50 nmol/L of JNK inhibitor SP600125 abolished the upregulation of GADD34 induced by Aβ1-42 (Figure 3B). Furthermore, c-Jun silencing by transfection with c-Jun siRNA also abolished the effects of Aβ1-42 on the expression of GADD34 (Figure 4A). To confirm whether c-Jun binds to GADD34 promoter region at site, we performed a ChIP assay using an anti-c-Jun antibody. The ChIP studies verified the ability of c-Jun to bind to the GADD34 promoter. In addition, we found that binding of c-Jun to GADD34 promoter was increased more than 3-fold after treatment with Aβ1-42 compared with sham control (Figure 4B).
Figure 3.
JNK/c-Jun inhibitor SP600125 attenuates amyloid β (Aβ) 1-42-induced upregulation of growth arrest and DNA damage-inducible gene 34 (GADD34). A, SH-SY5Y cells were treated with Aβ1-42 in the presence or absence of 50 nmol/L of specific c-Jun N-terminal kinases (JNK) inhibitor SP600125; the level of phosphor-c-Jun was measured by Western blot analysis (*P < .01, compared to nontreatment; #P < .01, compared to Aβ1-42 treatment, n = 4). B, SH-SY5Y cells were treated with Aβ1-42 in the presence or absence of 50 nmol/L of specific JNK inhibitor SP600125, the amount of GADD34 protein was measured by Western blot analysis (*P < .01, compared to nontreatment; #P < .01, compared to Aβ1-42 treatment, n = 4).
Figure 4.
c-Jun N-terminal kinases (JNK)/c-Jun mediates amyloid β (Aβ) 1-42-induced induction of growth arrest and DNA damage-inducible gene 34 (GADD34). A, SH-SY5Y cells were transfected with c-Jun small-interfering RNA (siRNA) and incubated with Aβ1-42; the amount of GADD34 protein was measured by Western blot analysis (*P < .01, compared to nontreatment; # P < .01, compared to Aβ1-42 treatment, n = 4). B, C-Jun binding to GADD34 promoter was determined by chromatin immunoprecipitation analysis (*P < .05 vs c-Jun group, n = 4).
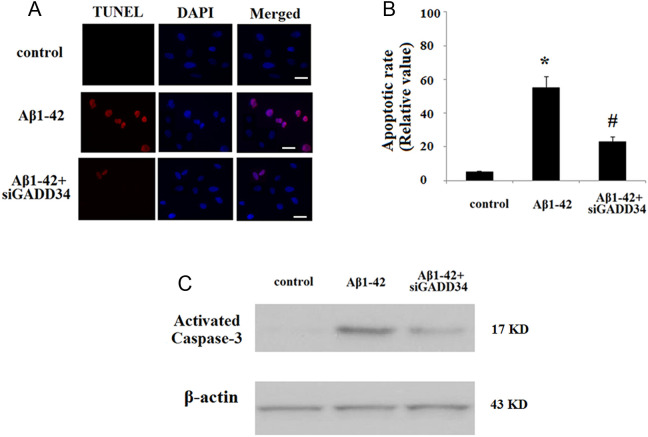
As we have shown that Aβ upregulates GADD34 in cultured human neuroblastoma cells, we were interested to determine whether endogenous depletion of GADD34 would modulate Aβ1-42-induced cell death in mice. To deplete GADD34 levels in primary neurons, we utilized a viral vector system that delivers RNAi of GADD34 into the cells. Of significant interest, depletion of endogenous GADD34 in primary neurons significantly rescued Aβ-induced cell death as evidenced by a marked decrease in the number of terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells (Figure 5A and B). Caspase 3 is a critical executioner of apoptosis. Our result indicated that Aβ1-42 led to the activation of caspase 3; however, knockdown of GADD34 attenuated caspase 3 activation (Figure 5C).
Figure 5.
Growth arrest and DNA damage-inducible gene 34 (GADD34) silencing prevents amyloid β (Aβ)-induced apoptosis in mouse primary neurons. Scale bar, 50 µmol/L. A, Cells were stained using a terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) assay kit. Nuclear DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). B, Quantitative analysis of the apoptotic rate (*P < .01, compared to nontreatment; # P < .01, compared to Aβ1-42 treatment, n = 5). C, Western blot analysis revealed that GADD34 silencing attenuated Aβ-induced activation of caspase 3. Representative bands from 3 independent experiments.
Discussion
The pathophysiological changes accompanying AD are complex and include alterations in gene transcription, cell signaling, energy metabolism, oxidative stress, and apoptosis. The precise signals regulating these events are not fully understood. But ER stress 22 and DNA damage 23 may contribute to cell death observed in AD. Previous studies have reported that the GADD34 is a growth arrest and DNA damage inducible gene upregulated in response to DNA damage, cell cycle arrest, and apoptosis. 24,25 Recent findings show that GADD34 works under both ER stress. 26 In addition, in vivo depletion of GADD34 using a lentiviral knockdown approach leads to a rescue of Akt activation and markedly attenuates traumatic brain injury-induced cell death. 27 These studies implied that GADD34 might have a potential role in the pathogenesis of AD. In this study, we found that GADD34 was upregulated in both AD transgenic mice and Aβ-treated neuronal cells. Further study revealed that activation of c-Jun participated in regulating the expression of GADD34. Importantly, GADD34 silencing using siRNA method attenuated the neurotoxicity of Aβ by inhibiting apoptosis and caspase 3 activation.
GADD34 is also thought to play a crucial role in the modulation of protein synthesis. As a component of the protein phosphatase 1 complex, GADD34 dephosphorylates eIF-2α. Phosphorylation of the translation initiation factor eIF-2α was reported to increase β-secretase 1 and elevate Aβ production in primary neurons and AD transgenic Tg2576 mice. 28 These data suggest a potential role of GADD34 in reducing the production of Aβ. Previously, it was reported that GADD34 was one of the proteins expressed in response to the preconditioning ischemic stimulus that protects the brain from a subsequent lethal ischemic challenge as well as being strongly translated during the reperfusion phase following the lethal ischemia. 29 These studies showed that the effect of GADD34 in brain tissues is still controversial. Notably, all the in vitro findings in this study are based on the human neuroblastoma SH-SY5Y cell line. There is no direct evidence on the neuroprotective effect of GADD34 against Aβ toxicity in primary neurons. However, treatment with Aβ1-42 has been reported to cause primary neuronal death. 30 By combining these findings together, it is suggested that GADD34 should be able to protect primary neurons from the insult of Aβ1-42.
The transcription factor c-Jun is one component of activator protein 1 (AP-1) that binds and activates transcription at AP-1 sites. Phosphorylation of c-Jun occurs in response to different kinds of stress, that is, ultraviolet irradiation and growth factors stimulation. Importantly, activation of transcriptional factor c-Jun and its stress kinase JNK has been extensively associated with AD. 31 Recently, JNK signaling is required for the formation of amyloid plaques in vivo, and inhibition of increased JNK activity associated with aging or with a pathological condition was considered to constitute a potential strategy for the treatment of AD. 32 Consistent with our finding in the current study, increased transcriptional activity of c-Jun has been found in Aβ-treated human brain endothelial cells to be responsible for Aβ-induced neuroinflammation. The JNLK/c-Jun may serve as a therapeutic target for relieving Aβ-induced inflammation. 33 In this study, we reported that activation of c-Jun could bind to the promoter region of GADD34 and participated in upregulation of GADD34. Our finding suggests a new function of JNK/c-Jun pathway in the neuropathogenesis of AD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368 (9533):387–403. [DOI] [PubMed] [Google Scholar]
- 2. Hardy J, Selkoe D. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297 (5580):353–356. [DOI] [PubMed] [Google Scholar]
- 3. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12 (10):383–388. [DOI] [PubMed] [Google Scholar]
- 4. Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55 (1):342–345. [DOI] [PubMed] [Google Scholar]
- 5. Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer's disease. Free Radic Biol Med. 2009;46 (10):1362–1375. [DOI] [PubMed] [Google Scholar]
- 6. Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012;120 (3):419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savage MJ, Lin YG, Ciallella JR, Flood DG, Scott RW. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition. J Neurosci. 2002;22 (9):3376–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Troy CM, Rabacchi SA, Xu Z, et al. Beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77 (1):157–164. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93 (4):953–962. [DOI] [PubMed] [Google Scholar]
- 10. Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J Neuroinflammation. 2009;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22 (5):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kojima E, Takeuchi A, Haneda M, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17 (11):1573–1575. [DOI] [PubMed] [Google Scholar]
- 13. Zhan Q, Lord KA, Alamo I, Jr, et al. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14 (4):2361–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White F, McCaig D, Brown SM, Graham DI, Harland J, Macrae IM. Up-regulation of a growth arrest and DNA damage protein (GADD34) in the ischaemic human brain: implications for protein synthesis regulation and DNA repair. Neuropathol Appl Neurobiol. 2004;30 (6):683–691. [DOI] [PubMed] [Google Scholar]
- 15. Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318 (1):33–42. [DOI] [PubMed] [Google Scholar]
- 16. Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277 (35):32046–32053. [DOI] [PubMed] [Google Scholar]
- 17. Alvira-Botero X, Pérez-Gonzalez R, Spuch C, et al. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol Cell Neurosci. 2010;45 (3):306–315. [DOI] [PubMed] [Google Scholar]
- 18. Farook JM, Shields J, Tawfik A, et al. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013;4:e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hale AT, Tian H, Anih E, et al. Endothelial kruppel-like factor 4 regulates angiogenesis and the notch signaling pathway. J Biol Chem. 2014;289 (17):12016–12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braithwaite SP, Schmid RS, He DN, et al. Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer's disease. Neurobiol Dis. 2010;39 (3):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353 (6345):670–674. [DOI] [PubMed] [Google Scholar]
- 22. Viana RJ, Nunes AF, Rodrigues CM. Endoplasmic reticulum enrollment in Alzheimer's disease. Mol Neurobiol. 2012;46 (2):522–534. [DOI] [PubMed] [Google Scholar]
- 23. Kanungo J. DNA-dependent protein kinase and DNA repair: relevance to Alzheimer's disease. Alzheimers Res Ther. 2013;5 (2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fornace AJ, Jr, Nebert DW, Hollander MC, et al. Mammalian genes co-ordinately regulated by growth arrest signals and DNA damaging agents. Mol Cell Biol. 1989;9 (10):4196–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollander C, Zhan Q, Bae I, Fornace A. Mammalian GADD34, an apoptosis and DNA damage-inducible gene. J Biol Chem. 1997;272(21):13731–13737. [DOI] [PubMed] [Google Scholar]
- 26. Kojima E, Takeuchi A, Haneda M, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17 (11):1573–1575. [DOI] [PubMed] [Google Scholar]
- 27. Farook JM, Shields J, Tawfik A, et al. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013;4:e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Connor T, Sadleir KR, Maus E, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60 (6):988–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. García L, Burda J, Hrehorovská M, Burda R, Martín ME, Salinas M. Ischaemic preconditioning in the rat brain: effect on the activity of several initiation factors, Akt and extracellular signal regulated protein kinase phosphorylation, and GRP78 and GADD34 expression. J Neurochem. 2004;88 (1):136–147. [DOI] [PubMed] [Google Scholar]
- 30. Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A. 1993;90 (17):7951–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson AJ, Cummings BJ, Cotman CW. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer's disease: association with pathology. Exp Neurol. 1994;125 (2):286–295. [DOI] [PubMed] [Google Scholar]
- 32. Mazzitelli S, Xu P, Ferrer I, Davis RJ, Tournier C. The loss of c-Jun N-terminal protein kinase activity prevents the amyloidogenic cleavage of amyloid precursor protein and the formation of amyloid plaques in vivo. J Neurosci. 2011;31 (47):16969–16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vukic V, Callaghan D, Walker D, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34 (1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]