Abstract
Introduction:
Mutations in the gene for presenilin 1 (PSEN-1) cause familial, early-onset Alzheimer’s disease (EOAD). Diagnosis of EOAD is often a challenge because of the high frequency of atypical presentations. Clinical manifestation of EOAD may vary depending on underlying mutation; specific genetic mutations influence development of specific clinical phenotypes; however, intrafamilial phenotypic heterogeneity has also been noted in some pedigrees.
Case presentation:
We report a case of a 36-year-old woman presenting with progressive behavioral disturbances, dementia, involuntary movements, pyramidal signs, epilepsy, and a family history of early-onset dementia accompanied by involuntary movements. On genetic testing, the mutation at codon 424 (Leu→Arg) in PSEN-1 gene was identified.
Conclusion:
Our case describes a new phenotype of a known mutation of PSEN-1 at codon 424.
Keywords: Alzheimer’s disease, PS1, mutation, phenotype, phenotype of codon 424 (Leu→Arg), PSEN-1 gene mutation
Case Presentation
A 36-year-female who is right handed, caucasian, nonsmoking, married, and a grocery seller first presented to the Outpatient Memory Clinic in the Department of Neurology at the University Hospital in Krakow in March 2011. The first symptoms started 2 years prior to presentation and included the following: cognitive decline and gait problems. The family observed changes in her behavior, such as intermittent explosiveness, apathy, and aggression. She had to give up her job 6 months prior to presentation because of increasing intellectual and physical disability. Her medical history was insignificant. Her family history was remarkable for a number of early deaths. Her father died at the age of 37 years in an institution. Little was known about the course of his disease. According to his wife, his first symptoms started about 3 to 4 years before he died. She described personality changes, memory problems, and motor disturbances. His sister had similar symptoms and died at the age of 40. Their father also died in his 40s, who was diagnosed with a “psychiatric disease” at the time of his death.
Neurological examination of the presented patient revealed discreet grimacing, smacking, and puckering of the lips, more pronounced involuntary and choreic-like movements in the upper and lower extremities, and dystonia in the right upper extremity. Voluntary movements were slow and affected. Power was generally full; however, she had difficulty in maintaining muscular constriction in her upper extremities. The gait was irregular and unsteady with sporadic lurching to one side. She had global cognitive decline with word-finding difficulty and visuospatial deficit. The patient’s Mini-Mental State Examination 1 was 18 of 30 and the Clock Test was 1 of 10 points. 2 A lack of awareness of her disabilities was observed. She was admitted to the hospital with suspicion of Huntington’s disease (HD).
No abnormalities were detected upon extensive workup including cell blood count, chemistry panel, thyroid hormones, vitamin B12, copper metabolism, antinuclear, and antiperoxidase antibodies, with the exception of a high cholesterol level (6,17 mmol/L). Cerebrospinal fluid examination was normal (1 cell/mm3; cell type: lymphocytes, protein level 14 g/L), including a workup for Lyme disease and viral infection. Electroencephalogram showed a generalized diffuse slowing and a decrease in reactivity of basic rhythm. Nerve conduction studies and electromyography revealed no abnormalities. Ophthalmic examination was normal.
Computed tomography showed moderate general atrophy with enlargement of ventricles and subarachnoid space. Probable HD was diagnosed. The blood was sent for genetic testing and simvastatin was introduced to treat the hypercholesterolemia. She was discharged from the hospital.
After discharge, she was followed at the Neurology Outpatients Clinic in June 2011. One month before the follow-up visit, she had 3 epileptic seizures, and Carbamazepine 300 mg twice daily was introduced. The genetic testing requested for HD was negative.
On neurological examination at that time, she had pronounced myoclonic jerks on her face and extremities, often smacking and puckering of the lips, mild weakness in the left upper extremity, and voluntary movements were jerky. Dysdiadochokinesis was present, Romberg’s sign was negative, the gait was wide based with occasional staggering, and tandem gait was impaired. During the examination, she was agitated and aggressive. She could hardly communicate due to aphasia.
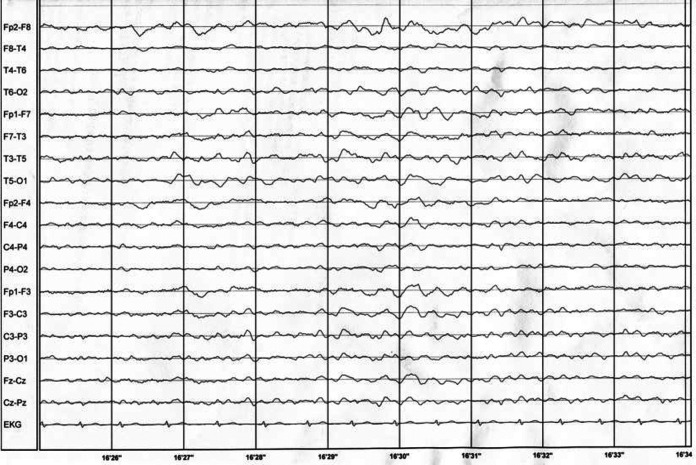
She was again admitted to the hospital with suspicion of familial prion disease. Blood laboratory tests were within normal ranges. Apolipoprotein E (ApoE) polymorphism was checked; she carried ApoE3/ApoeE3 isoforms. Electroencephalogram showed generalized diffuse slowing and decrease in reactivity of basic rhythm (Figure 1). Magnetic resonance imaging showed cortical and subcortical atrophy with thin corpus callosum.
Figure 1.
Electroencephalogram performed during second hospital stay.
Cerebrospinal fluid examination was normal including 14-3-3 protein. Pathogenic mutations in presenilin (PSEN) 2 (PSEN-2), APP, and PRP genes were excluded by sequencing. The Sanger sequencing of the whole coding sequence of the PSEN-1 gene led to the identification of a heterozygous Leu→Arg substitution at codon 424. Donepezil was introduced 10 mg per day, and she was discharged from the hospital.
Discussion
Alzheimer’s disease (AD) is a progressive neurodegenerative disease manifested by memory loss, behavioral, and mood disturbances. About 5% of all cases with AD are characterized by early-onset Alzheimer's disease (EOAD). The age range for the onset of disease is wide (24-60 years), and cognitive decline may be accompanied by behavioral disturbances, movement disorders, pyramidal signs, or seizures. The most common mutations associated with EOAD are located on the 3 genes: APP, PSEN-1, or PSEN-2. Diagnosis of EOAD is often a challenge because of the high frequency of atypical presentations. 3 Known diversity of pathogenic PSEN-1 mutations, 185 to these data, 4 may in part be explained by heterogeneity of clinical phenotypes.
We report the clinical presentation of EOAD associated with a known mutation at codon 424 (Leu→Arg) in PSEN-1 gene. What is interesting about this case is that, despite all the neurological signs described in other patients with various PSEN-1 mutations, this case presents with a new phenotype in the proband carrying the mutations at codon 424 (Leu Arg) in the PSEN-1 gene that was not previously described.
This mutation was first reported in a family originating from Poznan, Poland. 5 The proband had progressive memory and language impairment, which started at the age of 30. A year later he developed sporadic myoclonic jerks and mild left-side hemiparesis. Five other members of his family had progressive dementia with myoclonic jerks in their 30s. They all died 4 to 8 years after the onset of the disease.
Our patient presents with similar symptoms; behavioral disturbances, progressive cognitive impairment; and involuntary movements that appeared at approximately the same time, which were then followed by weakness in the upper extremity. Additionally, she quickly developed dystonia, ataxic gait, and epilepsy.
It was suggested that the clinical manifestation of EOAD may vary depending on the underlying mutation 6 ; specific genetic mutations usually influence development of specific clinical phenotypes. However, interfamilial phenotypic heterogeneity has been noted in some pedigrees. 3
There are other mutations affecting 424 codon; Leu→His 7 and Leu→Val 8 were reported. The clinical presentation for patients with those mutations was similar. The patients presented with frontotemporal dementia with Parkinsonism.
Apolipoprotein E genotype was also suggested to influence the onset and progression of AD; however, the data are controversial. 9 -11 Our patient carried the E3/E3 genotype. In previously described patients, the genotypes were not provided, therefore we cannot speculate whether ApoE may modify the phenotype in affected patients.
The family history and clinical presentation of our patient strongly suggested HD when she was first seen at the Memory Clinic. After a few months, the clinical symptoms suggested familial prion disease. Finally, based on genetic tests EOAD was diagnosed.
The EOAD is rare and mutations in PSEN-1 are responsible only for 30% to 78% of all cases. 12 One should always include familial EOAD in the differential diagnosis of quickly progressing dementia with an onset before 40 years of age, even if the patient is presenting with signs and symptoms suggestive of HD or familial prion disease.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Folstein MF, Folstein SE, McHugh PR. “Mini Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12 (3):189–198. [DOI] [PubMed] [Google Scholar]
- 2. Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37 (8):725–729. [DOI] [PubMed] [Google Scholar]
- 3. Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer's disease associated with mutations of the presenilin-1gene. J Neurol. 2006;253 (2):139–158. [DOI] [PubMed] [Google Scholar]
- 4. Cruts M, Theuns J, Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Human Mutation. 2012;33 (9):1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kowalska A, Forsell C, Florczak J, et al. A Polish pedigree with Alzheimer's disease determined by a novel mutation in exon 12 of the presenilin 1 gene: clinical and molecular characterization. Folia Neuropathol. 1999;37 (1):57–61. [PubMed] [Google Scholar]
- 6. Gómez-Tortosa E, Barquero S, Barón M, et al. Clinical-genetic correlations in familial Alzheimer's disease caused by presenilin 1 mutations. J Alzheimers Dis. 2010;19 (3):873–884. [DOI] [PubMed] [Google Scholar]
- 7. Zekanowski C, Golan MP, Krzyśko KA, et al. Two novel presenilin 1 gene mutations connected with frontotemporal dementia-like clinical phenotype: genetic and bioinformatic assessment. Exp Neurol. 2006;200 (1):82–88. [DOI] [PubMed] [Google Scholar]
- 8. Robles A, Sobrido MJ, García-Murias M, et al. Clinical picture of a patient with a novel PSEN1 mutation (L424V). Am J Alzheimers Dis Other Demen. 2009;24 (1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM. Most rapid cognitive decline in APOE epsilon4 negative Alzheimer's disease with early onset. Psychol Med. 2009;39 (11):1907–1911. [DOI] [PubMed] [Google Scholar]
- 10. Vermeiren AP, Bosma H, Visser PJ, et al. The association between APOE ∊4 and Alzheimer-type dementia among memory clinic patients is confined to those with a higher education. The DESCRIPA Study. J Alzheimers Dis. 2013;35 (2):241–246. [DOI] [PubMed] [Google Scholar]
- 11. Sando SB, Melquist S, Cannon A, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurol. 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen JC, Beck JA, Campbell TA, et al. Early onset familial Alzheimer's disease: Mutation frequency in 31 families. Neurology. 2003;60 (2):235–239. [DOI] [PubMed] [Google Scholar]