Abstract
Background:
Dementia is an age-related disorder associated with elderly population, resulting from interaction of lifestyle risk factors with genetic, vascular, and other risk factors to affect risk of disease. Alzheimer’s disease (AD) is the most common form of dementia, estimated to be affecting 4.4% of the population older than 65 years of age. Apolipoprotein E (ApoE) ∊4 allele is a known genetic risk factor for AD, which not only predisposes and influences the severity of pathological changes in the brain, thereby modifying the age at onset, but also promotes cognitive decline early in nondemented older people.
Objectives:
To review the published evidence on ApoE polymorphism with the susceptibility to AD and frequency of ApoE ∊4 genotype (∊4/-) and homozygotes (∊4/4) among patients diagnosed with AD as compared to controls in Indian Population.
Materials and Methods:
In the present study, MEDLINE was reviewed for articles published till June 2013 supplemented by citation analysis from retrieved articles to select case–control studies. A meta-analysis was performed to demonstrate the association of ApoE gene with vascular dementia by random effects to demonstrate models. The association was assessed by odds ratio (OR) with 95% confidence intervals (CIs). Study Selection: Case–control studies, using clinical criteria for AD with ApoE polymorphism determined for allele and genotype in both cases and controls. Statistical Analysis: A meta-analysis was performed to demonstrate the association of ApoE gene with AD by random effects to demonstrate models. The association was assessed by OR with 95% CIs. We also looked for publication bias and performed sensitivity analysis to investigate the influence of each individual study.
Results:
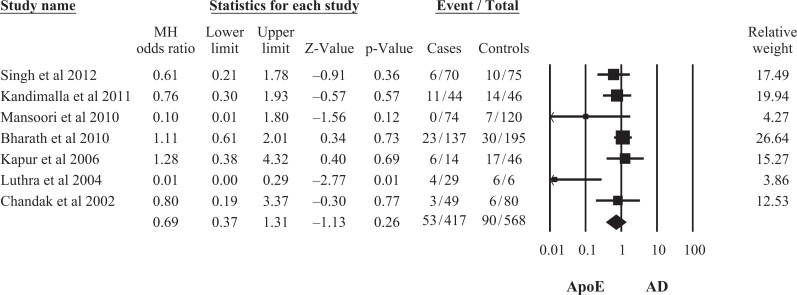
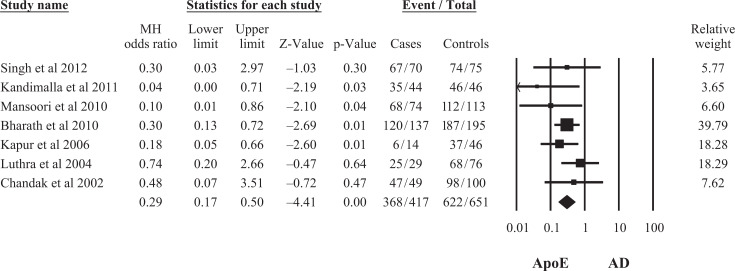
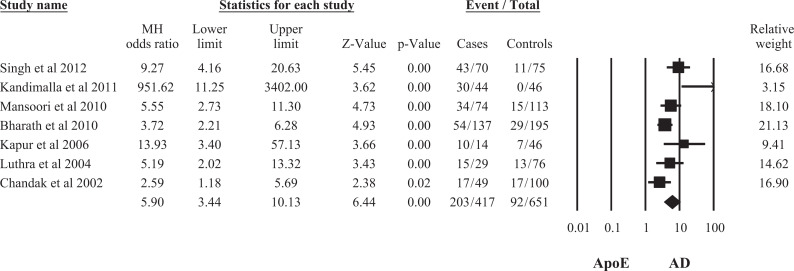
A total of 7 studies representing data from 417 patients with AD and 651controls in the Indian population were eligible. The ApoE ∊2/4, ∊3/4, and ∊4/4 genotypes (OR = 3.93, 95% CI: 1.60-9.68; OR = 4.18, 95% CI: 2.54-6.87; OR = 4.81, 95% CI: 1.95-11.86, respectively) as well as ApoE ∊4 allele (OR = 5.90, 95% CI: 3.44-10.13) were associated with an increased risk of AD, whereas ApoE ∊2/3, ∊3/3 genotypes (OR = 0.52, 95% CI: 0.32-0.83; OR = 0.28, 95% CI: 0.19-0.42), and ApoE ∊3 allele (OR = 0.29, 95% CI: 0.17-0.50) were found to be marginally significant protective factors for AD. There was no significant difference in ApoE ∊2/2 genotype and ApoE ∊2 allele frequency (OR = 0.42; 95% CI: 0.11-1.68; OR = 0.69, 95% CI: 0.37-1.31, respectively) in patients with AD and controls.
Conclusions:
These results indicate that all genotypes of ApoE ∊4 allele, that is, ∊2/4, ∊3/4, and ∊4/4, are associated with an increased risk of AD, whereas ApoE ∊2/2, ∊2/3, and ∊3/3 are protective for AD.
Keywords: ApoE, AD, meta-analysis, polymorphism
Introduction
Dementia is an age-related disorder associated with elderly population. 1 In the coming decades, increased prevalence of Alzheimer’s disease (AD) may pose burden on the society and halt care services, given the rapid increase in aging population in developed and developing countries. According to the Alzheimer’s Disease International, it is estimated that there are currently 30 million people with dementia in world which will increase to 100 million by 2050. 2 It is a life course illness resulting from the interaction of lifestyle risk factors with genetic, vascular, and other factors to affect risk of disease. 3
Apolipoprotein E (ApoE) has been recognized as an independent risk factor for developing late-onset AD4,5 as well as early familial forms. 6 The ApoE ∊4 allele is a known genetic risk factor for AD, which not only predisposes and influences the severity of pathological changes in the brain, thereby modifying the age at onset, 7 but also promotes cognitive decline early in nondemented older people. 8,9 It is estimated that the lifetime risk of developing AD increases to 29% for carriers with 1 ApoE ∊4 allele and 9% for those with no ApoE ∊4 allele. 10 Although factors contributing to the susceptibility of ApoE ∊4 to AD is not clear till now, differential binding capacity for amyloid β (Aβ) peptide and τ protein among ApoE alleles with increased binding in ApoE ∊4 explains increased deposition of Aβ peptide in brain in transgenic mouse models having ApoE ∊4 alleles. 11 Apolipoprotein E also has a prominent role in the transport and metabolism of plasma cholesterol. 12 Carriers of the ∊4 allele of the ApoE gene (ApoE ∊4) have higher total and low-density lipoprotein cholesterol levels than noncarriers. 13 Many animal models of AD show that high cholesterol levels increase Aβ protein concentration and may increase ApoE expression, explaining the mechanism of action of ApoE by which it increases the risk of AD. 14,15 Some studies have found association of ApoE ∊4 with increased peripheral lipid levels, decreased cerebral glucose metabolism, and increased Aβ deposition and neurofibrillary tangles formation in the brain, 16 along with contemporary environmental conditions such as high intake of carbohydrates and fat, low fiber, and reduced physical activity, 17 increases the susceptibility of ApoE4 carriers to developing AD.
Apolipoprotein E, a polymorphic gene on chromosome 19, has attracted immense attention lately, being a independent genetic factor for modifying the risk of late- and early-onset AD. Also, ApoE genotyping increases the specificity of the clinical diagnosis of AD. 18 There are 3 common alleles (∊2, ∊3, and ∊4) that form 6 genotypes (∊2/2, ∊2/3, ∊2/4, ∊3/3, ∊3/4, and ∊4/4). Epidemiological studies show that ApoE ∊3 allele is most frequent. 19
Studies have reported low prevalence of AD in Indian population when compared to the Western population. 20 Apolipoprotein E ∊4 has been found to be more common in North European population when compared to population residing in Mediterranean region of France and Italy and in Asia. 17 Similarly, frequency of ApoE ∊4 allele has been found vary widely across India (from 0.07 21 to 0.127 22 ). In the view of the above-mentioned facts, the purpose of conducting this meta-analysis is to summarize and reproduce the conclusive evidence based on published articles on ApoE polymorphism with the susceptibility to AD and frequency of ApoE ∊4 genotype (∊4/-) and homozygotes (∊4/4) among patients diagnosed with AD when compared to controls in Indian population.
Materials and Methods
A protocol was followed for this meta-analysis per current practices for conducting systematic reviews and meta-analysis of the literature. Studies that fulfilled all the following criteria were included in the present meta-analysis.
Criteria for Study Identification
Patients of all age groups with clinically diagnosed and confirmed AD as defined by the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association were included. 23
Case–control or cohort study in Indian population with healthy persons without dementia as controls.
Allele frequencies in all cases and the control group in Hardy-Weinberg equilibrium.
The ApoE polymorphism determined for allele and genotype in both cases and controls.
Search Strategy
A comprehensive search was conducted using both electronic and manual methods to obtain all published case–control studies; MEDLINE (via PubMed) was searched till June 2013 to identify eligible studies. Additional articles were also obtained from reference citations within the retrieved articles. The search was conducted using key words apolipoprotein, ApoE, gene polymorphism, and AD. The search was limited to English language, humans, and study designs such as longitudinal, cohort, and cross-sectional studies performed in patients with AD and controls.
The articles obtained after electronic and manual searches were screened initially for the title and abstract based on the above-mentioned inclusion criteria per the protocol developed. Full text was retrieved for the potentially eligible articles and screened by 2 independent reviewers to determine whether it fulfilled the eligibility criteria. All uncertainties were resolved by consensus.
Statistical Analysis
The presence of heterogeneity in effect size (odds ratio [OR]) between studies was examined with the help of test of significance (Q-statistics), and if heterogeneity in effect size was significantly present between the studies, then degree of heterogeneity was evaluated by I 2 test, taking the values in the range of 0% to 100%. Random effect model was applied to pool the overall OR, as heterogeneity was detected across studies. The Mantel-Haenszel (MH) method was used to pool the OR of various studies to eliminate the confounding effect.
The Forest plot (graphical method) was applied to present the results of meta-analysis. Publication bias was examined by funnel plot, by plotting MH log OR on x-axis and its precision on y-axis. As large studies are expected to be published regardless of their effect size, and small studies tend to be published when they show comparatively large effect size, an inverse correlation exists between effect size and study size. The rank correlation test, developed by Begg and Mazumdar, 24 explores the association between effect size and their variances (which is inversely related to study size) to examine the fact that publication bias tends to induce a correlation between these 2 factors. As this test is a nonparametric method that involves no normality assumptions and has lack of power, the possibility of publication bias cannot be ruled out completely, even when the test is nonsignificant. Hence, in the present study, emphasis has been given on both the methods, funnel plot and rank correlation test, to examine the publication bias. Sensitivity analysis was conducted to investigate the influence of each individual study. The statistical software used for analysis was Comprehensive Meta-Analysis: a computer program for meta-analysis version 2, developed by a team of experts from United States and United Kingdom.
Results
The primary search identified 18 studies on the association of ApoE polymorphism with AD in the literature, of which 11 studies were excluded. A total of 7 studies that met our selection criteria were included in the meta-analysis as 1 study only had abstract available, 5 studies were done in general population, 2 studies were done in dementia other than AD, 2 studies’ controls were not taken, and 1 study was done in American Indians. All the studies undertaken for meta-analysis were case–control studies.
Seven 18,25 –30 studies included in the final meta-analysis represented data from 417 patients with AD and 651 controls in the Indian population. The study characteristics have been summarized in Table 1. On pooling of the data by random effect model from 7 studies, the association of ApoE ∊2 allele frequency was found not to be statistically significant with AD (OR = 0.69, 95% confidence interval [CI]: 0.37-1.31), whereas association of both allele ApoE ∊3 and ApoE ∊4 frequencies were statistically significant with AD (OR = 0.29, 95% CI: 0.17-0.50; OR = 5.90, 95% CI: 3.44-10.13; Figures 1 –3). This indicates that ApoE ∊4 allele was a significant risk factor for AD due to its higher frequency, whereas ∊3 allele had a weak protective role in the development of the disease.
Table 1.
Characteristics and Results of All Case–Control Studies Included in the Meta-analysis.
| Source | No. of Patients | Age, y | Gender, M/F | ApoE Genotypes, No. of Patients | |||||
|---|---|---|---|---|---|---|---|---|---|
| ∊2/2 | ∊2/3 | ∊2/4 | ∊3/3 | ∊3/4 | ∊4/4 | ||||
| Singh et al 18 | |||||||||
| Case | 70 | 50-85 | NR | 00 | 04 | 02 | 23 | 40 | 01 |
| Control | 75 | Age matched | NR | 00 | 09 | 01 | 55 | 10 | 00 |
| Kandimalla et al 25 | |||||||||
| Case | 44 | 61.84 | 26/18 | 00 | 07 | 04 | 07 | 21 | 05 |
| Control | 46 | 60.84 | 32/14 | 00 | 14 | 00 | 32 | 00 | 00 |
| Mansoori et al 26 | |||||||||
| Case | 74 | 66.48 | 50/24 | 00 | 00 | 00 | 40 | 28 | 06 |
| Control | 113 | 64.08 | 72/41 | 00 | 06 | 01 | 92 | 14 | 00 |
| Bharath et al 27 | |||||||||
| Case | 137 | 64.08 | 72/65 | 00 | 10 | 13 | 73 | 37 | 04 |
| Control | 195 | 64.88 | 120/75 | 01 | 24 | 05 | 141 | 22 | 02 |
| Kapur et al 28 | |||||||||
| Case | 14 | NR | 7/7 | 00 | 00 | 06 | 04 | 02 | 02 |
| Control | 46 | NR | NR | 05 | 11 | 01 | 23 | 03 | 03 |
| Luthra et al 29 | |||||||||
| Case | 29 | 66.6 | NR | 01 | 02 | 01 | 11 | 12 | 02 |
| Control | 76 | 63.2 | NR | 06 | 09 | 01 | 48 | 11 | 01 |
| Chandak et al 30 | |||||||||
| Case | 49 | 71.9 | NR | 00 | 03 | 00 | 29 | 15 | 02 |
| Control | 100 | 73.3 | NR | 01 | 04 | 01 | 78 | 16 | 00 |
Abbreviations: ApoE, apolipoprotein E; NR, not reported.
Figure 1.
Meta-analysis of apolipoprotein E (ApoE) ∊2 allele and risk of Alzheimer’s Disease (AD) in the Indian Population.
Figure 2.
Meta-analysis of apolipoprotein E (ApoE) ∊3 allele and risk of Alzheimer’s Disease (AD) in the Indian Population.
Figure 3.
Meta-analysis of apolipoprotein E (ApoE) ∊4 allele and risk of Alzheimer’s Disease (AD) in the Indian Population.
On genotypic analysis, it was found that ApoE ∊2/3, ∊3/3, ∊2/4, ∊3/4, and ∊4/4 had a strong association with AD (OR = 0.52, 95% CI: 0.32-0.83; OR = 0.28, 95% CI: 0.19-0.42; OR = 3.93, 95% CI: 1.60-9.68; OR = 4.18, 95% CI: 2.54-6.87; OR = 4.81, 95% CI: 1.95-11.86), while ApoE ∊2/2 was not significantly associated with AD (OR = 0.42; 95% CI: 0.11-1.68). The ApoE ∊2/3 and ∊3/3 genotypes were found to be marginally significant protective factors for AD, whereas ApoE ∊2/4, ∊3/4, and ∊4/4 genotypes had strong association with increased risk of AD (Table 2). Also, ApoE ∊2/3 and ∊3/3 genotypes were found to be significantly higher in the controls, whereas ApoE ∊2/4, ∊3/4, and ∊4/4 genotypes were higher in AD. These results suggest that all genotypes of ApoE ∊4 allele, that is, ∊2/4, ∊3/4, and ∊4/4, were significantly associated with an increased risk of AD, whereas ApoE ∊2/3 and ∊3/3 were protective against AD.
Table 2.
Meta-analysis of ApoE Genotypes and Risk of AD in the Indian Population.
| Genotype | Random Effects Model, OR (95% CI) | Fixed Effects Model, OR (95% CI) | Heterogeneity Test, P Value | Degree of Heterogeneity, I 2 in % | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| ApoE ∊2/2 | 0.42 (0.11-1.68) | 0.42 (0.11-1.68) | 0.42 (0.11-1.68) | 0.41 (0.10-1.60) | .98 | .98 | 0.00 | 0.00 |
| ApoE ∊2/3 | 0.52 (0.32-0.83) | 0.52 (0.32-0.83) | 0.52 (0.32-0.83) | 0.48 (0.30-0.76) | .62 | .60 | 0.00 | 0.00 |
| ApoE ∊3/3 | 0.28 (0.19-0.42) | 0.28 (0.19-0.42) | 0.31 (0.23-0.40) | 0.30 (0.23-0.40) | .07 | .07 | 49.19 | 49.22 |
| ApoE ∊2/4 | 3.93 (1.60-9.67) | 3.93 (1.60-9.67) | 4.00 (1.86-8.61) | 4.10 (2.04-8.25) | .32 | .32 | 13.81 | 13.86 |
| ApoE ∊3/4 | 4.16 (2.56-6.77) | 4.18 (2.54-6.87) | 3.92 (2.82-5.44) | 4.38 (3.20-6.01) | .09 | .07 | 45.85 | 48.00 |
| ApoE ∊4/4 | 4.81 (1.95-11.86) | 4.81 (1.95-11.86) | 4.81 (1.95-11.86) | 5.84 (2.48-13.78) | .85 | .83 | 0.00 | 0.00 |
Abbreviations: AD, Alzheimer’s disease; ApoE, apolipoprotein E; OR, odds ratio; CI, confidence interval.
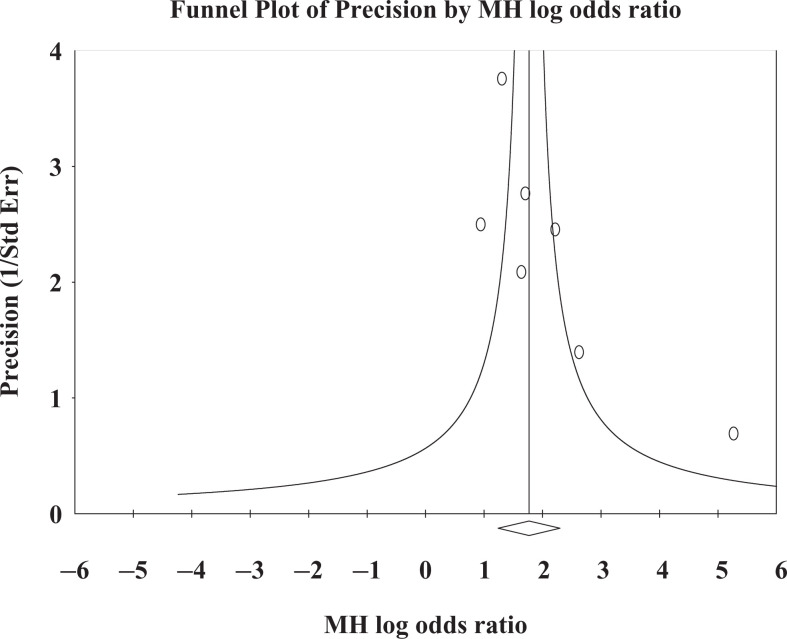
In this study, both random effect and fixed effect models were applied to pool the ORs. On comparison of both models, almost similar results were observed. Publication bias was examined by funnel plot, by plotting study size (usually standard error or precision) on the vertical axis and effect size on the horizontal axis. Funnel plot showed considerably low publication bias, plotted for pooling of effect size (OR) of ApoE ∊4 allele (Figure 4). Begg and Mazumdar’s correlation test also showed that nonsignificant publication bias (P = .13) exists in the present study. It may be due to low statistical power of the included studies.
Figure 4.
Funnel plot of MH odds ratio for apolipoprotein E (ApoE) ∊4 in Alzheimer’s Disease (AD) in Indian population.
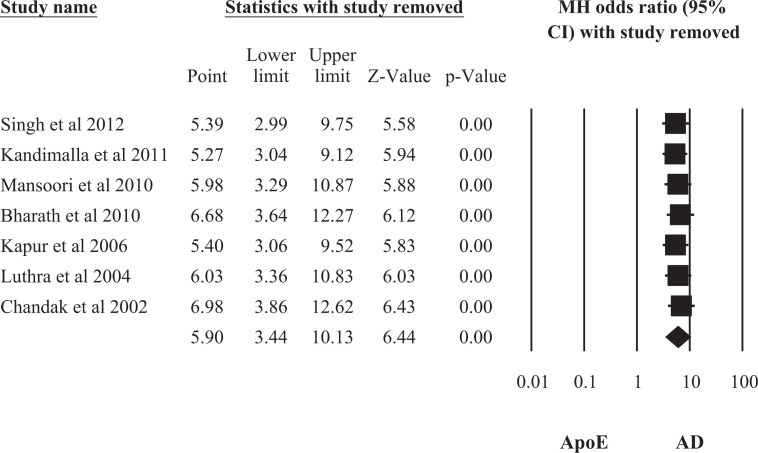
A sensitivity assessment was performed by repeating the meta-analysis systematically by excluding each individual study in turn. This assessment indicates which studies are most influential. Here, sensitivity analysis was conducted for ApoE ∊4 allele only, because it was found to be the most significant risk factor for AD. Studies were pooled using random effect model. Figure 5 shows that the OR and CI did not change materially with exclusion of any individual study. However, it does suggest that of the 7 studies, study by Chandak et al 30 was most influential.
Figure 5.
Investigating the impact of each individual study on overall pooled odds ratio (OR) of apolipoprotein E (ApoE) ∊4 allele.
Discussion
In Indian population, the prevalence of AD and other dementias has been found to be lower than the Western population. 21,31,32 Luthra et al 29 have reported ApoE ∊4 allele frequency (29.3%) among AD in Indian population almost similar to African American 6 (32.2%) and Japanese 6 (27.8%) population but lower than that of the caucasian population 6 (36.7%). Another study by Ganguli et al 21 reported 16.07% frequency of ApoE ∊4 among patients with AD in Indian population. Ward et al 17 in their meta-analysis showed prevalence of the ApoE ∊4 genotype (∊4 =/-) and homozygotes (∊4/4) among patients from Asia, Europe, North America, and South America, diagnosed with AD. They reported lowest regional estimates for ∊4 carrier status in Asia (41.9%) and South Europe (40.5%), where majority of patients with AD were not ApoE ∊4 carriers. Similar patterns were observed for ApoE ∊4/4 estimates. However, in their meta-analysis, only 1 study 30 undertaken on Indian population was included in the Asian studies. Hence, the present meta-analysis was performed in Indian population.
In the present analysis, ApoE ∊2/2 genotype and ∊2 allele frequency were not significantly associated with AD. However, ApoE ∊2/4, 3/4, and 4/4 and ApoE ∊4 allele were associated with increased risk of AD. There is suggestion of an inverse association between ApoE ∊2/3 and ∊3/3 and AD. Raygani et al 33 and Kim et al 34 also showed higher frequency of ApoE ∊4 allele in both patients with early- and late-onset AD than in controls. Similar findings have been reported by Tang et al 35 in caucasian and Hispanic population and by de Andrade et al 36 in Brazilian population. Tang et al 35 showed that relative risk of AD associated with ApoE ∊4 homozygosity was increased in African American, Caucasians, and Hispanic population, but with ApoE ∊4 heterozygotes, risk of AD increased only in caucasians and Hispanic population. Such variability is attributed to existence of possible gene–environment interaction in AD, including smoking, 37 –39 serum cholesterol level, 7 blood glucose level, and head trauma, 40 well known to modify ApoE-related risk as well as gene–gene interaction. However, any interaction between ApoE and the putative susceptibility genes remains controversial. 41 Studies show no consensus on ApoE ∊2 frequency in AD. Raygani et al 33 observed low ApoE∊2 frequency in patients with AD in Iranian population, but Kim et al 34 found no difference in ApoE ∊2 frequency between AD and control in Korean population. Also, the patients carrying ApoE ∊2 alleles showed delayed age at onset for AD. Similarly, Tang et al 35 observed disease risk associated with ApoE ∊2 among caucasians but not in Africans and Hispanics. Meta-analysis performed by Bang et al 42 showed low prevalence of ApoE ∊2 allele in AD group and significantly reduced ORs in all subtypes of AD.
As seen in this meta-analysis, ApoE ∊4 is associated with an increased risk of AD. But, many people with ApoE ∊4 allele did not develop AD and AD also developed in its absence. Hence, neither ApoE genotyping is recommended for routine clinical diagnosis nor can it be used for predictive testing. 43 However, ApoE genotyping may be applied to differentiate AD and non-AD dementia. It can also play a vital role in subgrouping of both AD and non-AD dementia. 42 Two meta-analysis have been conducted 42,44 to evaluate the differential diagnostic role of ApoE genotype in dementia. In meta-analysis of 42 case–control series performed by Rubinsztein et al 44 to quantify the effects of ApoE on early- and late-onset, sporadic, and familial AD, the authors have reported association of ApoE ∊4 allele with significantly higher relative risk in early-onset AD (age < 65 years), whereas ApoE ∊2 was found to be associated with a significantly reduced risk of AD above the age of 65 years. They concluded that 60% of patients with AD older than 65 years of age and 92% of patients younger than 65 years of age would be attributable to ApoE. Another meta-analysis undertaken by Bang et al 42 including 78 case–control studies having a total 10 654 elderly controls, 7812 patients with AD, and 1272 patients with non-AD dementia, was reviewed to evaluate the role of ApoE genotyping in differential diagnosis of dementia. In their meta-analysis, the authors found low frequency of ApoE ∊4 allele in vascular dementia (VD) compared to the AD group for both caucasian and East Asian population. Also, patients with dementia carrying ApoE ∊4 allele had a 3-fold greater probability of acquiring AD than VD. Their results also showed high ApoE ∊4 allele frequency and ApoE ∊4-associated OR in familial and late-onset AD.
The current literature indicates that ApoE ∊4 exerts broad, but specific, adverse small effects on a range of neurocognitive functions in cognitively healthy adults. 45,46 Their studies show that carriers of ApoE ∊4 allele perform significantly worse on measures of episodic memory, executive functioning, and overall global cognitive ability and increase in age results in significantly larger differences between ApoE ∊4 carriers and ApoE non-∊4 carriers on measures of episodic memory and global cognitive ability in these patients.
In the present meta-analysis, heterogeneity among studies regarding the strength of risk of AD for carriers of ApoE ∊4 allele has been observed which may be due to small sample size in certain studies, 27,28 genotyping methods used, and regional variations (Table 3). Also, other than selection bias, factors such as age, sex, smoking, hypercholesterolemia, and hyperglycemia are known to modify ApoE-related risk in AD.
Table 3.
Meta-Analysis of ApoE Alleles and Risk of AD in the Indian Population.
| Alleles | Random Effects Model, OR (95% CI) | Fixed Effects Model, OR (95% CI) | Heterogeneity Test, P Value | Degree of Heterogeneity, I 2 in % | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| ApoE ∊2 | 0.70 (0.38-1.30) | 0.69 (0.37-1.31) | 0.84 (0.56-1.24) | 0.73 (0.50-1.07) | .09 | .08 | 44.51 | 46.72 |
| ApoE ∊3 | 0.29 (0.17-0.50) | 0.29 (0.17-0.50) | 0.29 (0.17-0.50) | 0.26 (0.15-0.44) | .48 | .46 | 0.00 | 0.00 |
| ApoE ∊4 | 5.87 (3.46-9.96) | 5.90 (3.44-10.13) | 5.02 (3.69-6.82) | 5.65 (4.21-7.59) | .02 | .02 | 58.98 | 60.53 |
Abbreviations: AD, Alzheimer’s disease; ApoE, apolipoprotein E; OR, odds ratio; CI, confidence interval.
Conclusion
Most studies confirm that ApoE ∊4 allele is a risk factor for AD, and when used with conventional clinical criteria, the presence and absence of ApoE ∊4 allele significantly increased diagnostic confidence by 10% to 30% in all clinical criteria with varying accuracy of AD and non-AD dementia, respectively. Our study also shows that ApoE ∊4/4 homozygotes and ApoE ∊4/-heterozygote genotype had strong association with increased risk of AD. However, one cannot conclude that the presence of ApoE ∊4 allele will lead to development of AD even if there is familial predisposition. As, there is no consensus on it, it is not clear whether ApoE polymorphism should be performed or not for every person who is at risk of AD. However, it can be proposed that ApoE genotyping may be performed in all individuals at risk of coronary arterial disease or not responding to drugs.
Acknowledgment
We thank Mr Puneet Talwar, MTech, for acquisition of data and Mr Dharmender Kumar Dubey, MSc, for reviewing the studies.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Romas SN, Tang MX, Berglund L, Mayeux R. ApoE genotype, plasma lipids, lipoproteins and AD in community elderly. Neurology. 1999;53(3):517–521. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal R, Chhillar N, Mishra VN, Tripathi CB. CSF tau and amyloid β42 levels in Alzheimer’s disease—a meta analysis. Adv Alzheimers Dis. 2012;1(3):30–44. [Google Scholar]
- 3. Zekry D, Duyckaerts C, Belmin J, Geoffre C, Moulias R, Hauw JJ. Alzheimer's disease and brain infarcts in the elderly. Agreement with neuropathology. J Neurol. 2002;249(11):1529–1534. [DOI] [PubMed] [Google Scholar]
- 4. Ahn JS, Ahn K, Kim JH, et al. ApoE-epsilon4 dependent association of the choline acetyltransferase gene polymorphisms (2,384 G > A & 1,882 G > A) with Alzheimer’s disease. Clin Chem Acta. 2006;368(1-2):179–182. [DOI] [PubMed] [Google Scholar]
- 5. Altamura C, Squitti R, Pasqualetti P, et al. What is the relationship among atherosclerosis markers, apolipoprotein E polymorphism and dementia? Eur J Neurol. 2007;14(6):679–682. [DOI] [PubMed] [Google Scholar]
- 6. Farrer LA, Cupples LA, Haines JL, et al. For the APOE and Alzheimer’s disease meta analysis consortium: effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer’s disease: a meta analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 7. Evans RM, Hui S, Perkins A, lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer’s disease progression. Neurology. 2004;62(10):1869–1871. [DOI] [PubMed] [Google Scholar]
- 8. Caselli RJ, Graff-Radford NR, Reima EM, et al. Preclinical memory decline in cognitively normal apolipoprotein E-L4 homozygotes. Neurology. 1999;53(1):201–207. [DOI] [PubMed] [Google Scholar]
- 9. Craft S, Teri L, Edland SD, Kukull WA, et al. Accelerated decline in apolipoprotein E-∊4 homozygotes with Alzheimer’s disease. Neurology. 1998;51(1):149–153. [DOI] [PubMed] [Google Scholar]
- 10. Seshadri S, Drachman DA, Lippa CF. Apolipoprotein E∊4 allele and the lifetime risk of Alzheimer’s disease. What physicians know, and what they should know. Arch Neurol. 1995;52(11):1074–1079. [DOI] [PubMed] [Google Scholar]
- 11. Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96(26):15233–15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. [DOI] [PubMed] [Google Scholar]
- 13. Reily SL, Ferrell RE, Kottke BA, Kamboh MI, Sing CF. The gender specific apolipoprotein E genotype influence on the distribution of lipids and apolipoproteins in the population of Rochester, MN. I Pleiotropic effects on means and variances. Am J Hum Genet. 1991;49(6):1155–1166. [PMC free article] [PubMed] [Google Scholar]
- 14. Puglielli L, Tanzi RE, Koovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6(4):345–351. [DOI] [PubMed] [Google Scholar]
- 15. Wu CW, Liao PC, Lin C, et al. Brain region-dependent increases in β amyloid and apolipoprotein E levels in hypercholesterolemic rabbits. J Neural Transm. 2003;110(6):641–649. [DOI] [PubMed] [Google Scholar]
- 16. Lane RM, Farlow MR. lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer’s disease. J lipid Res. 2005;46(5):949–968. [DOI] [PubMed] [Google Scholar]
- 17. Ward A, Crean S, Catherine JM, et al. Prevalence of apolipoprotein E4 genotype and homozygotes, (APO E e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta analysis. Neuroepidemiology. 2012;38(1):1–17. [DOI] [PubMed] [Google Scholar]
- 18. Singh NK, Chhillar N, Banerjee BD, et al. Gene–environment interaction in Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2012;27(7):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asada T, Motonaga T, Kinoshita T. Predictors of severity of behavioral disturbance among Community-dwelling elderly individuals with Alzheimer’s disease: a 6-year follow up study. Psychiatry Clin Neurosci. 2000;54(6):673–677. [DOI] [PubMed] [Google Scholar]
- 20. Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer’s disease and other dementias in rural India. Neurology. 1998;51(4):1000–1008. [DOI] [PubMed] [Google Scholar]
- 21. Ganguli M, Chandra V, Kamboh MI, et al. Apolipoprotein E polymorphism and Alzheimer’s disease: the Indo-US cross-national dementia study. Arch Neurol. 2000;57(6):824–830. [DOI] [PubMed] [Google Scholar]
- 22. Hallman DM, Boerwinkle E, Saha N, et al. The apolipoprotein E polymorphism: a comparison of allele frequencies and effects in nine population. Am J Hum Genet. 1991;49(2):338–349. [PMC free article] [PubMed] [Google Scholar]
- 23. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging. Alzheimer’s association work groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 25. Kandimalla RJL, Prabhakar S, Binukumar BK, et al. Apo-E ∊4 allele in conjunction with Aβ42 and Tau in CSF: biomarkers for Alzheimer’s disease. Curr Alzheimer Res. 2011;8(2):187–196. [DOI] [PubMed] [Google Scholar]
- 26. Mansoori N, Tripathi M, Alam R, et al. IL-6-174 G/C and ApoE gene polymorphisms in Alzheimer’s and vascular dementia patients attending the cognitive disorder clinic of the all India institute of medical sciences, New Delhi. Demet Geriatr Cogn Disord. 2010;30(6):461–468. [DOI] [PubMed] [Google Scholar]
- 27. Bharath S, Purushottam M, Mukherjee O, et al. Apolipoprotein e polymorphism and Dementia: a hospital-based study from Southern India. Demet Geriatr Cogn Disord. 2010;30(6):455–460. [DOI] [PubMed] [Google Scholar]
- 28. Kapur S, Sharad S, Kapoor M, Bala K. Apo E genotypes: risk factor for Alzheimer’s disease. J Indian Acad Clin Med. 2006;7(2):118–112. [Google Scholar]
- 29. Luthra K, Tripathi M, Grover R, Dwivedi M, Kumar A, Dey AB. Apolipoprotein E gene polymorphism in Indian patients with Alzheimer’s disease and Indian patients with Alzheimer’s disease and vascular Dementia. Dement Geriatr Cogn Disord. 2004;17(3):132–135. [DOI] [PubMed] [Google Scholar]
- 30. Chandak GR, Uma Dridevi M, vas CJ, Pannikker DM, Singh L. Apolipoprotein E and presenilin-1 allelic variation and Alzheimer’s disease in India. Hum Biol. 2002;74(5):683–693. [DOI] [PubMed] [Google Scholar]
- 31. Shaji S, Promodu K, Abraham T, Roy KJ, Verghase A. An epidemiological study of dementia in a rural community in Kerala, India. Br J Psychiatry. 1996;168(6):745–749. [DOI] [PubMed] [Google Scholar]
- 32. Van CJ, Pinto C, Panikker D, et al. Prevalence of dementia in an urban India population. Int Psychogeriatr. 2001;13(4):439–450. [DOI] [PubMed] [Google Scholar]
- 33. Raygani AV, Zahrai M, Raygani AV, et al. Association between apolipoprotein E polymorphism and Alzheimer disease in Tehran, Iran. Neurosci Lett. 2005;375(1):1–6. [DOI] [PubMed] [Google Scholar]
- 34. Kim KW, Jhoo JH, Lee KU, et al. Association between apolipoprotein E polymorphism and Alzheimer’s disease in Koreans. Neurosci Lett. 1999;277(3):145–148. [DOI] [PubMed] [Google Scholar]
- 35. Tang M-X, Maestre G, Tsai WT, et al. Relative risk of Alzheimer’s disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Human Genet. 1996;58(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 36. De-andrade FM, Larrandaburu M, Callegari-Jacques SM, Gastaldo G, Hutz MH. Association of apolipoprotein E polymorphism with plasma lipids and Alzheimer’s disease in a Southern Brazilian population. Braz J Med Biol Res. 2000;33(5):529–537. [DOI] [PubMed] [Google Scholar]
- 37. Bickeböller H, Campion D, Brice A, et al. Apolipoprotein E and Alzheimer disease: genotype-specific risks by age and sex. Am J Hum Genet. 1997;60(2):439–446. [PMC free article] [PubMed] [Google Scholar]
- 38. Farrer LA, Cupples LA, Haines JL, et al. The effect of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer’s disease. A meta analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 39. Frisoni GB, Manfredi M, Geroldi C, et al. The prevalence of apo E-epsilon 4 in Alzheimer’s disease is age dependent. J Neurol Neurosurg Psychiatry. 1998;65(1):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans RM, Emsley CL, Gao S, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54(1):240–242. [DOI] [PubMed] [Google Scholar]
- 41. Pandey P, Pradhan S, Mittal B. Presenilin gene predisposes to late-onset degenerative but not vascular dementia: a comparative study of PS1 and ApoE genes in a North Indian cohort. Dement Geriatr Cogn Disord. 2007;24(3):151–161. [DOI] [PubMed] [Google Scholar]
- 42. Bang OY, Kwak YT, Joo IS, Huh K. Important link between dementia subtype and apolipoprotein E: a Meta-analysis. Yonsei Med J. 2003;44(3):401–413. [DOI] [PubMed] [Google Scholar]
- 43. National Institute on Aging/Alzheimer’s association Working Group. Apolipoprotein E genotype in Alzheimer’s disease. Lancet. 1996;347(9008):1091–1095. [PubMed] [Google Scholar]
- 44. Rubinsztein DC, Eastern DF. Apolipoprotein E genetic variation and Alzheimer’s disease. Dement Geriatr Cogn Disord. 1999;10(3):199–209. [DOI] [PubMed] [Google Scholar]
- 45. Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein e and cognitive performance: a meta analysis. Psychol Aging. 2004;19(4):592–600. [DOI] [PubMed] [Google Scholar]
- 46. Wisdom NM, Callaham JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011;32(1):63–74. [DOI] [PubMed] [Google Scholar]