Abstract
Objectives:
The aim of this systematic review is to identify published randomized controlled trials (RCTs) that evaluated the use of acetylcholinesterase inhibitors for delirium in older adults (≥60 years).
Methods:
A literature search was conducted of PubMed, MEDLINE, EMBASE, PsycINFO, and Cochrane collaboration databases for RCTs in any language that evaluated the use of acetylcholinesterase inhibitors for delirium in older adults (≥60 years). Also, bibliographic databases of the published articles were searched for additional studies.
Results:
A total of 7 RCTs that evaluated the use of acetylcholinesterase inhibitors for delirium in older adults (≥60 years) were identified. In 5 of the 7 studies, there was no benefit for the acetylcholinesterase inhibitor in either the prevention or the management of delirium. In one study, there was a trend toward benefit for the active drug group on the incidence of delirium and the length of hospital stay, but both outcomes did not attain statistical significance. One study found a longer duration of delirium and a longer length of hospital stay in the active drug group when compared to the placebo group. The acetylcholinesterase inhibitors were well tolerated in 4 of the 7 studies. In 1 study, the mortality rate was found to be almost 3 times higher in the group receiving haloperidol and rivastigmine when compared to the group receiving haloperidol and placebo.
Conclusion:
Current evidence does not suggest efficacy of acetylcholinesterase inhibitors for the prevention or management of delirium in older adults.
Keywords: acetylcholinesterase inhibitors, delirium, older adults, prevention, management
Introduction
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) identifies delirium as a condition associated with a disturbance in attention, awareness, and cognition. 1 Although the rates of delirium are low in older individuals living in the community at 1% to 2%, their incidence increases significantly in hospital settings. 2,3 During admission to hospitals, the incidence rate of delirium is approximately 11% to 42%. The incidence rate of delirium during a hospital stay is over 50%. 3,4 In intensive care units (ICUs), the incidence of delirium in the elderly population is greater than 70%. 2,3 Postoperative delirium is very common in older adults with an incidence rate that varies between 15% and 60%. 2,3 It is currently estimated that in the United States, between one-tenth and one-half of all individuals ≥65 years in age who require hospitalization are affected by delirium. 5
The presence of delirium is associated with poorer patient outcomes including a worsening of their nutritional status, cognition, and medical comorbidities. 3 In addition, delirium results in a greater burden of care on the nursing staff, longer lengths of hospital stay, and greater cost of hospitalization. 6,7 Furthermore, delirium increases the risk of dementias, hospitalization, institutionalization, and death. 3,8,9 The average medical care cost for individuals with delirium is more than 2½ times the cost for individuals without delirium. 10
Acetylcholine is noted to be an important substrate for the modulation of cognition in humans. 11,12 Current evidence indicates that there is a disruption of the normal activity of the cholinergic system in the brain of individuals with delirium. 13,14 Additionally, it has been noted that drugs with anticholinergic properties can precipitate delirium, especially in the older individuals. 15,16
Available data from several studies indicate that acetylcholinesterase inhibitors that have shown efficacy in individuals with Alzheimer’s disease may also benefit individuals with delirium. 17 –21 However, the only meta-analysis that evaluated the efficacy of acetylcholinesterase inhibitors for delirium included only 1 randomized controlled trial (RCT) that compared donepezil to placebo. 22 The investigators found no significant difference between the treatment and the placebo groups for the duration of delirium from this 1 study. The mean duration of postoperative delirium for the donepezil group was 1.0 day (standard error, 0.0), whereas for the placebo group it was 1.3 days (standard error, 0.19). There were no other outcomes measured for the patients who developed delirium. The investigators concluded that there is no evidence from controlled trials that donepezil is effective in the treatment of delirium. In a recent systematic review that was not specific to studies conducted in older adults, the investigators did not find any evidence for the efficacy of acetylcholinesterase inhibitors in either the prevention or the management of delirium. 23
Given this conflicting information available in the literature, we wanted to systematically evaluate the evidence for the use of acetylcholinesterase inhibitors in the prevention and management of symptoms of delirium in older adults (≥60 years) from RCTs. If there is good evidence for the efficacy of acetylcholinesterase inhibitors in the prevention or management of symptoms of delirium in older adults, then these drugs could be used in lieu of or in addition to the antipsychotics. 24 Antipsychotics appear to have some efficacy in the prevention and management of symptoms of delirium, but their use is associated with serious adverse effects including cerebrovascular adverse effects and death, especially in individuals with cognitive impairment. 25
Search Strategy
This systematic review was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 26 The purpose of this review is to evaluate the data on the efficacy and tolerability of acetylcholinesterase inhibitors for delirium in the older adults (≥60 years) from RCTs. We performed a literature search of PubMed, MEDLINE, EMBASE, PsycINFO, and Cochrane collaboration databases through June 30, 2015, using the following key words: acetylcholinesterase inhibitors, donepezil, rivastigmine, galantamine, delirium, and RCT. The search was not restricted by the age of the participants or the language of publication of the study. However, in the final analysis, we only included studies involving humans that were published in English-language journals or had official English translations. In addition, we reviewed the bibliographic databases of the published articles for additional studies.
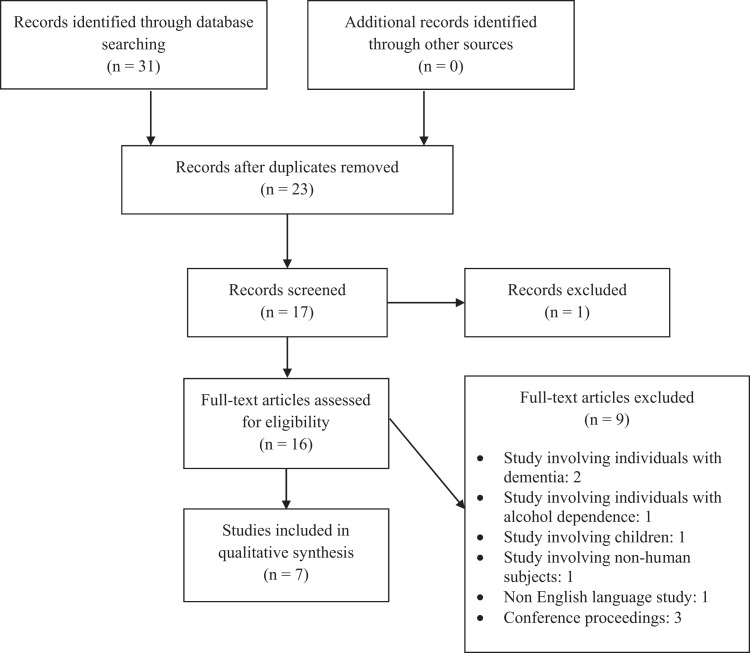
Two of the authors (RRT and DJT) reviewed all the abstracts and full-text articles from the citations obtained via the search of the databases. The decision on which studies to be included or excluded from the final analysis was done after a review of the full-text articles by all the authors. Disagreements between the authors were resolved by a consensus. The quality of the included studies was assessed using the criteria developed by the Center for Evidence-Based Medicine (Figure 1). 27
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Results
This systematic review of the literature identified a total of 7 RCTs that evaluated the efficacy of acetylcholinesterase inhibitors for the prevention and management of delirium in older adults (≥60 years). 28 –34 Six of the 7 studies were of good quality based on the criteria developed by the Center for Evidence-Based Medicine. 28 –33 Available data from 1 published study was limited (Table 1). 34
Table 1.
Quality of the Studies Reviewed.
| Name of the Study | Randomization | Similar Groups Initially? | Equal Treatments? | All Participants Accounted for? | Analyzed in Groups to Which They Were Randomized? | Objective/“Blind” Treatments? |
|---|---|---|---|---|---|---|
| Liptzin et al 28 | Yes | Yes | Yes | Yes | Yes | Yes |
| Sampson et al 29 | Yes | Yes | Yes | Yes | Yes | Yes |
| Gamberini et al 30 | Yes | Yes | Yes | Yes | Yes | Yes |
| Overshott et al 31 | Yes | Yes | Yes | Yes | Yes | Yes |
| van Eijk et al 32 | Yes | Yes | Yes | Yes | Yes | Yes |
| Marcantonio et al 33 | Yes | Yes | Yes | Yes | Yes | Yes |
| Zaslavsky et al 34 | Yes | Unclear | Unclear | Yes | Unclear | Yes |
Of the 7 studies, 5 were conducted in surgical settings. 28 –30,33,34 Three of these 5 studies included individuals who were awaiting skeletal joint repair surgeries. 28,29,33 Of the 2 other studies, one involved patients with cardiac disease 30 and one included individuals awaiting elective surgical procedures. 34 The study by Overshott et al was conducted in a medical setting. 31 The study by van Eijk et al included individuals in ICU who had medical and/or surgical problems (Table 2). 32
Table 2.
Summary of the Included Studies.
| Name of the Study, Year, Country | Total Number of Participants | Age of the Participants | Type of Setting | Comparators | Duration of Trial |
|---|---|---|---|---|---|
| Liptzin et al, 2005, United States 28 | 88 | Mean age, 67.2 years | Surgical | Donepezil (10 mg/d) versus placebo | 14 days prior to surgery and 14 days post surgery |
| Sampson et al, 2007, United Kingdom 29 | 33 | Mean age, 67.8 years | Surgical | Donepezil (5 mg/d) versus placebo | Immediately after surgery and for 3 days thereafter |
| Gamberini et al, 2009, Switzerland 30 | 120 | ≥65 years | Surgical | Rivastigmine (4.5 mg/d) versus placebo | Sixth postoperative day |
| Overshott et al, 2010, United Kingdom 31 | 15 | ≥65 years | Medical | Rivastigmine (3 mg/d) versus Placebo | 28 days |
| van Eijk et al, 2010, the Netherlands 32 | 104 | Mean age, 69 years | ICU (medical and surgical) | Haloperidol (3-7.5 mg/d) plus rivastigmine (3-12 mg/d) versus haloperidol (3-7.5 mg/d) plus placebo | Unclear |
| Marcantonio et al, 2011, USA 33 | 16 | ≥70 years | Surgical | Donepezil (5 mg/d) versus placebo | 6 weeks |
| Zaslavsky et al, 2012, USA 34 | 28 | >70 years | Surgical | Rivastigmine (9.5 mg/24 hours patch) versus placebo | 3 days |
Abbreviation, ICU, intensive care unit.
Four of the 7 studies involved rivastigmine, 30 –32,34 and 3 studies used donepezil. 28,29,33 All 3 studies involving donepezil were conducted in surgical patients. Two of the 4 rivastigmine studies were conducted in surgical patients. 30,34 Six of the 7 studies compared an acetylcholinesterase inhibitor with placebo. 28 –31,33,34 In one study, rivastigmine or placebo was added to treatment with haloperidol. 32
The number of participants in these studies varied from 15 to 120. The duration of these studies varied from 3 days to 6 weeks. Four of the 7 studies targeted the prevention and management of delirium in older adults. 28 –30,33 Two studies targeted the management of delirium, 31,32 and 1 study evaluated the prevention of delirium. 34
Three of the 4 studies that evaluated the efficacy of acetylcholinesterase inhibitors in the prevention and management of delirium did not find benefit for the active drug when compared to placebo for either the prevention or the management of delirium. 28,30,33 The study by Sampson et al showed a trend toward benefit for the donepezil group when compared to the placebo group on the incidence of delirium and the length of hospital stay, but both outcomes did not attain statistical significance. 29 One of the 2 studies that evaluated the efficacy of an acetylcholinesterase inhibitor in the management of delirium found no benefit for the active drug in reducing the duration of delirium. 30 In the second study, the investigators found that the duration of delirium and the length of hospital stay were longer in the haloperidol plus rivastigmine group when compared to the haloperidol plus placebo group. 32 The only study that exclusively evaluated the efficacy of an acetylcholinesterase inhibitor for the prevention of delirium found no benefit for the active drug when compared to placebo. 34 There was no evidence for differential efficacy of acetylcholinesterase inhibitors when comparing studies involving patients in surgical settings 28 –30,33,34 versus patients in medical settings (Table 3). 31–32
Table 3.
Summary of Results From the Included Studies.
| Name of the Study | Delirium Rating Scales | Outcomes | Tolerability |
|---|---|---|---|
| Liptzin et al 28 | CAM; DSI | There was no difference in the incidence (P = .69), duration of delirium (P = .12), and the length of hospital stay (P = .09) between the 2 groups | Donepezil was as well tolerated as placebo, although one-fourth of the study participants discontinued the medications in both groups after randomization |
| Sampson et al 29 | DSI | The incidence of delirium (P = .08) and the length of hospital stay (P = .09) showed a trend toward benefit for the donepezil group when compared to the placebo group, although both outcomes did not attain statistical significance | Donepezil was as well tolerated as placebo with no serious adverse events reported |
| Gamberini et al 30 | CAM | There was no difference between the 2 groups on the incidence (P = .8), duration of delirium (P = .3), or the length of hospital stay (P = .3) | Rivastigmine was as well tolerated as placebo |
| Overshott et al 31 | CAM | More individuals in the rivastigmine group responded to treatment (P = .03) when compared to the placebo group, but the duration of delirium was no different (P = .5) between the 2 groups | Rivastigmine was as well tolerated as placebo |
| van Eijk et al 32 | CAM-ICU; DSI | The duration of delirium (P = .06) and the length of hospital stay (P = .0.06) were longer in the haloperidol plus rivastigmine group compared to the haloperidol and placebo group | Three times more individuals died in the rivastigmine group when compared to the placebo group (P = .07) |
| Marcantonio et al 33 | CAM; DSI; MDAS | The incidence (P = .9) and duration of delirium (P = 1.0) were no different between the 2 groups | The total number of side effects, insomnia, and diarrhea were greater in the donepezil group when compared to the placebo group (P = .04) |
| Zaslavsky et al 34 | CAM | There was no difference in the incidence of delirium (P = 0.8) between the 2 groups | The study was stopped prematurely as there were concerns about mortality with rivastigmine from a previous study |
Abbreviations: CAM, Confusion Assessment Method; CAM-ICU, Confusion Assessment Method for the intensive care unit; DSI, Delirium Symptom Interview; MDAS, Memorial Delirium Assessment Scale.
The acetylcholinesterase inhibitors were well tolerated in 4 of the 7 studies, with the side-effect profile of the drug being similar to that of placebo. 28 –31 In the study by van Eijk et al, rivastigmine or placebo was added to treatment regimen with haloperidol in critically ill patients in ICUs. 32 In this study, the mortality rate was found to be almost 3 times higher in the group receiving haloperidol and rivastigmine when compared to the group receiving haloperidol and placebo (22% vs 8%, P = .07). The reason for the higher mortality rate in the rivastigmine group is unclear, and investigators opined that it could be possibly due to chance. In the study by Marcantonio et al, the side effects were greater in the donepezil group when compared to the placebo group. 33 The study by Zaslavsky et al 34 was stopped prematurely due to the increased risk of death noted with rivastigmine in older adults with delirium that was identified in the study by van Eijk et al. 32
Discussion
The data available from this systematic review indicate that acetylcholinesterase inhibitors cannot be recommended for the prevention or management of delirium in older adults. Although 6 of the 7 RCTs evaluated in this review were of good quality, these studies were underpowered to detect a difference between the active drug and placebo. Additionally, there was significant heterogeneity among the various studies. These studies were conducted in 4 different countries and used different validated methods to identify and manage delirium. These studies also used different dose equivalents of drugs, dosing strategies, and duration for the prescription of the drugs. Also, the disease burden among the participants in these studies was varied. Furthermore, one of the 2 larger studies included in this review had to be terminated prematurely due to the increased risk of death noted in the haloperidol and rivastigmine group when compared to the haloperidol and placebo group. 32
How do these data compare with the data on antipsychotic medications for the prevention of or in the management of delirium in older adults? In a meta-analysis by Lonergan et al that included data from 3 studies, the investigators found that the reduction in delirium scores was similar between the group of individuals who were treated with low-dose haloperidol (<3.0 mg/d) and groups treated with olanzapine or risperidone (odds ratio: 0.63, 95% confidence interval [CI]: 1.029-1.38, P = .25). 35 Low-dose haloperidol did not have a higher incidence of adverse effects when compared to the atypical antipsychotics, but high-dose haloperidol (>4.5 mg/d) was associated with a greater incidence of extrapyramidal adverse effects when compared with olanzapine. A meta-analysis that included data from 5 trials found that prophylaxis with antipsychotics reduces the incidence of delirium in older individuals receiving surgical procedures by about 50% when compared to placebo (relative risk: 0.51, 95% CI: 0.33-0.79, P < .01). 36 Data from 4 of the 5 studies indicated that prophylaxis with antipsychotic medications resulted in a reduction in the incidence of delirium (number needed to treat, 4.00-12.6). When compared to the data on antipsychotic medications, the data for using acetylcholinesterase inhibitors for the prevention or management of delirium in older adults is significantly weaker, with 5 of the 7 studies showing no benefit for the drugs and 1 study showing a worse outcome.
Despite a search strategy that was optimized toward identifying studies using acetylcholinesterase inhibitors for the prevention or management of delirium in older adults, the data we obtained were identical to the data obtained by Friedman and colleagues in their systematic review. 23 Our conclusions are also consistent with those of Friedman and colleagues.
Given the lack of evidence for the efficacy of acetylcholinesterase inhibitors for the prevention or management of delirium in older adults from 2 systematic reviews, is there a place for using these drugs for delirium in older adults? Unless data from larger well-conducted RCTs that are powered to detect a clear difference between acetylcholinesterase inhibitors and other active drugs or placebo are demonstrated, these drugs cannot be recommended for routine use in either the prevention or the management of delirium in older adults.
The strengths of this study include the systematic nature of the collection of data using different search terms from 5 large databases. Also, there were no time or language restrictions placed with the initial study search. Only 1 study was excluded from the final review due to a language restriction as it was in Russian and no official English translation was available.
The limitations of this review include the use of data exclusively from published RCTs. We only found 7 published RCTs on the use of acetylcholinesterase inhibitors in older adults with delirium. Majority of these studies were not adequately powered to detect a meaningful difference in outcomes between the active drugs and placebo. In addition, there was significant heterogeneity between the study populations, that is, surgical versus medical versus ICU patients. Five of the 7 studies involved surgical patients, whereas 1 study involved medical patients and 1 study involved patients in ICU. Also, did the adverse outcomes noted in the study by van Eijk et al occur as their study population in the ICU had greater burden of illness than individuals in the other studies? Furthermore, we did not utilize statistical methods to evaluate the heterogeneity between the included studies or assess the efficacy or tolerability of the active drugs.
Conclusion
The data from this systematic review does indicate the efficacy of acetylcholinesterase inhibitors for the prevention or management of delirium in older adults. Although these drugs were well tolerated in 4 of the 7 studies included in this review, there was concern from one study for greater mortality with rivastigmine. Strongly positive data from larger well-conducted RCTs will be needed before these drugs can be recommended for routine use in either the prevention or the management of delirium in older adults.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Psychiatric Association. Desk Reference to the Diagnostic Criteria from DSM-5. Washington, DC: American Psychiatric Association; 2013:292–298. [Google Scholar]
- 2. Saxena S, Lawley D. Delirium in the elderly: a clinical review. Postgrad Med J. 2009;85(1006):405–413. [DOI] [PubMed] [Google Scholar]
- 3. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller MO. Evaluation and management of delirium in hospitalized older patients. Am Fam Physician. 2008;78(11):1265–1270. [PubMed] [Google Scholar]
- 5. Khan BA, Zawahiri M, Campbell NL, et al. Delirium in hospitalized patients: implications of current evidence on clinical practice and future avenues for research—a systematic evidence review. J Hosp Med. 2012;7(7):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 7. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. [DOI] [PubMed] [Google Scholar]
- 8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalisation, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing. 1999;28(6):551–556. [DOI] [PubMed] [Google Scholar]
- 10. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94(4):360–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. Gerontol A Biol Sci Med Sci. 2008;63(7):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. [DOI] [PubMed] [Google Scholar]
- 15. Lauretani F, Ceda GP, Maggio M, Nardelli A, Saccavini M, Ferrucci L. Capturing side-effect of medication to identify persons at risk of delirium. Aging Clin Exp Res. 2010;22(5-6):456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751–765. [DOI] [PubMed] [Google Scholar]
- 17. Wengel SP, Roccaforte WH, Burke WJ. Donepezil improves symptoms of delirium in dementia: implications for future research. J Geriatr Psychiatry Neurol. 1998;11(3):159–161. [DOI] [PubMed] [Google Scholar]
- 18. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437–438. [DOI] [PubMed] [Google Scholar]
- 19. Dautzenberg PL, Mulder LJ, Olde Rikkert MG, Wouters CJ, Loonen AJ. Adding rivastigmine to antipsychotics in the treatment of a chronic delirium. Age Ageing. 2004;33(5):516–517. [DOI] [PubMed] [Google Scholar]
- 20. Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G. Cholinesterase inhibition as a possible therapy for delirium in vascular dementia: a controlled, open 24-month study of 246 patients. Am J Alzheimers Dis Other Demen. 2004;19(6):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oldenbeuving AW, de Kort PL, Jansen BP, Kappelle LJ, Roks G. A pilot study of rivastigmine in the treatment of delirium after stroke: a safe alternative. BMC Neurol. 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman JI, Soleimani L, McGonigle DP, Egol C, Silverstein JH. Pharmacological treatments of non-substance-withdrawal delirium: a systematic review of prospective trials. Am J Psychiatry. 2014;171(2):151–159. [DOI] [PubMed] [Google Scholar]
- 24. Mittal V, Muralee S, Williamson D, et al. Review: delirium in the elderly: a comprehensive review. Am J Alzheimers Dis Other Demen. 2011;26(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mittal V, Kurup L, Williamson D, Muralee S, Tampi RR. Risk of cerebrovascular adverse events and death in elderly demented patients when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26(1):10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 27. Critical Appraisal Tools. Web site. http://www.cebm.net/critical-appraisal/. Accessed November 16, 2015.
- 28. Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. 2005;13(12):1100–1106. [DOI] [PubMed] [Google Scholar]
- 29. Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22(4):343–349. [DOI] [PubMed] [Google Scholar]
- 30. Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—a randomized controlled trial. Crit Care Med. 2009;37(5):1762–1768. [DOI] [PubMed] [Google Scholar]
- 31. Overshott R, Vernon M, Morris J, Burns A. Rivastigmine in the treatment of delirium in older people: a pilot study. Int Psychogeriatr. 2010;22(5):812–818. [DOI] [PubMed] [Google Scholar]
- 32. van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376(9755):1829–1837. [DOI] [PubMed] [Google Scholar]
- 33. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59(suppl 2):S282–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaslavsky A, Haile M, Kline R, Iospa A, Frempong-Boadu A, Bekker A. Rivastigmine in the treatment of postoperative delirium: a pilot clinical trial. Int J Geriatr Psychiatry. 2012;27(9):986–988. [DOI] [PubMed] [Google Scholar]
- 35. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teslyar P, Stock VM, Wilk CM, Camsari U, Ehrenreich MJ, Himelhoch S. Prophylaxis with antipsychotic medication reduces the risk of post-operative delirium in elderly patients: a meta-analysis. Psychosomatics. 2013;54(2):124–131. [DOI] [PubMed] [Google Scholar]