Abstract
Background
The COVID-19 pandemic has had a profound global impact on health-care systems and patient outcomes. However, the specific effects of the pandemic on cancer incidence rates in the United States during its initial year remain unknown.
Methods
In this study, we analyzed data from the Surveillance, Epidemiology, and End Results–22 registries, which encompass approximately 50% of the US population. We investigated changes in monthly incidence rates stratified by various factors, including cancer type, stage, age group, sex, race and ethnicity, socioeconomic status, rural-urban status, and registry locations. We compared the incidence rates observed during the pandemic with those from the previous year.
Results
Our findings revealed a decline in incidence rates for all cancer sites combined starting in March 2020, coinciding with the implementation of stay-at-home orders. This decline reached its lowest point in April 2020 and persisted at a lower level until May 2020. Notably, compared with April 2019, the incidence rates in April 2020 dropped by 48.1% and did not consistently return to prepandemic levels. The reduction in cancer rates was more pronounced in urban and affluent counties. Across all cancer types, there was a statistically significant decrease in incidence rates during the pandemic, with the largest declines observed in thyroid (71.2%), prostate (57.9%), breast (54.9%), and colon and rectum cancers (54.1%). Furthermore, these decreases were primarily observed in early stage rather than late-stage disease.
Conclusions
The COVID-19 pandemic had a statistically significant impact on cancer outcomes. Monitoring long-term consequences of the pandemic on cancer incidence, stage at diagnosis, and mortality trends will be crucial.
The COVID-19 pandemic delayed medical care access in the United States (1). Studies using US cancer registry data have shown substantial declines in the number of new cancer diagnoses in 2020, the first year of the pandemic (2,3). These studies have indicated that declines in case counts were attributable to pandemic-related delays in cancer care, such as delays in cancer screening and diagnosis, rather than to changes in registry operations or interruptions in data collection efforts. However, these findings are not generalizable to the overall US population because they are based on hospital- or facility-based registries, electronic pathology reports, or non-nationwide data (3,4). Furthermore, none of these studies reported incidence rates that account for the population denominator using 2020 data.
The stay-at-home orders that were implemented in many countries in March 2020 in response to the COVID-19 pandemic had a clinically significant impact on medical care services. Many outpatient clinics and medical offices either closed or limited their services, resulting in delayed or reduced access to routine health care, preventive screenings, and follow-up appointments for various health conditions, including cancer (5). Herein, we examine incidence rates using 2020 data for all cancer sites combined, by cancer type, and by demographic subgroups (eg, sex, age, race and ethnicity, socioeconomic status, rural-urban status, and registry locations) to assess the pandemic’s impact on cancer incidence rates in the US population. To accomplish this, we report monthly incidence rates in 2020 (ie, pandemic) and compare them with monthly incidence rates in 2019 (ie, prepandemic). We also report annual age-adjusted incidence rates in 2020 compared with 2019 by stage at diagnosis to assess how the pandemic impacted disease severity.
Methods
Data were obtained from 22 cancer registries in the Surveillance, Epidemiology, and End Results (SEER) Program, representing about 48% of the US population (6). We calculated monthly incidence rates for 2019 and 2020 for all sites combined and by sex, age at diagnosis (0-19, 20-59, 60-69, 70-79, 80 years and older), race and ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian and Pacific Islander, non-Hispanic American Indian and Alaska Native, and Hispanic), county-level poverty (<10% poverty, 10%-19.9% poverty, 20% or higher poverty), county-level rural-urban continuum (nonmetropolitan: not adjacent to metropolitan; nonmetropolitan: adjacent to metropolitan; metropolitan < 250 000 population; metropolitan 250 000-1 million population; metropolitan > 1 million population), and registry location. County-level poverty data came from the US census and from the most recent American Community Survey 2017-2021. Rural-urban continuum codes were available from the US Census 2013 (7). Data on non-Hispanic American Indian and Alaska Native populations were restricted to purchased or referred care delivery areas to reduce racial misclassification for reporting incidence rates by non-Hispanic American Indian and Alaska Native.
We used the Vintage 2020 population estimates projected from the 2010 census as the denominator for calculating incidence rates (8). To calculate rates for a given month, we calculated population at risk in a given month based on the annual (ie, mid-year July 1) county population estimates. Specifically, we use July 1 population as an estimate of person-years at risk because we cannot calculate person-years at risk directly. We first obtained annual county population estimates by age, sex, race, county, calendar year, and Hispanic origin. To estimate the rates, we first divided the annual population estimates by 365 days for a given stratum by any combination of the factors above and then converted population size from day to month to obtain population at risk in each month. The monthly incidence rates were age standardized to the 2000 US population in 5-year age groups. We selected cancers with screening recommendations (female breast, prostate, colorectal), cancer types detected mainly by symptoms (lung and bronchus [although lung cancer has screening guidelines, most lung cancers are still symptom detected], esophageal, pancreas), cancers with frequent incidental detection (bladder, thyroid), and a hematologic cancer (non-Hodgkin lymphoma). Stage distribution using combined Summary Stage (https://seer.cancer.gov/tools/ssm/) was reported by cancer type. Cancer stage was categorized as in situ, localized, regional, and distant.
The analyses were conducted using SEER*Stat software version 8.4.1 (9). Authors NH and DM analyzed the data. The decrease (or percent change) in the April 2020 rate compared with the April 2019 rate was calculated using SEER*Stat. Specifically, we used SEER*Stat estimates of ratio (rate [2020 April]/rate [2019 April]) and the respective confidence interval (95% lower confidence interval, 95% upper confidence interval). Percent change was calculated as (ratio-1) x 100. The lower and upper limits of the confidence interval for percent change were calculated as (95% upper confidence interval-1) x 100 and (95% lower confidence interval-1) x 100, respectively (10). We used the same formula to calculate decreases in annual incidence rate by stage and cancer site.
Data and code used in this project cannot be directly shared by authors. The data and statistical software used are publicly available through the National Cancer Institute’s SEER Program (6,9).
Results
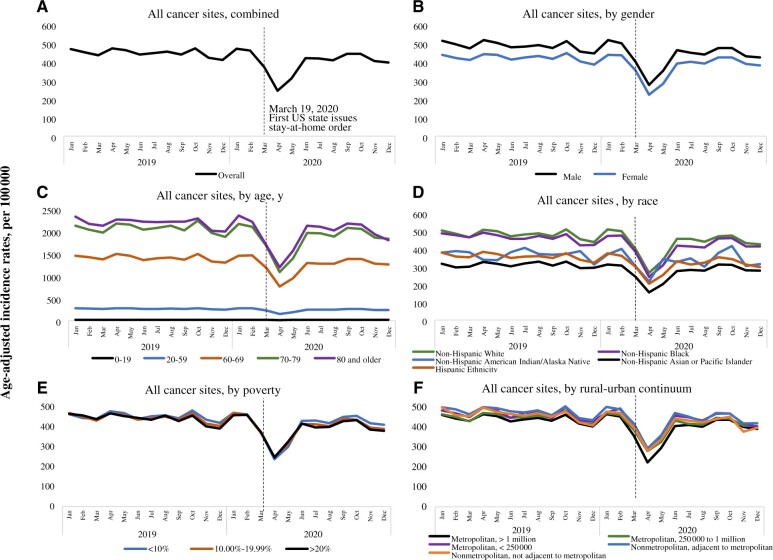
Figure 1, A, shows monthly incidence rates for all cancers combined from 2019 and 2020. Incidence rates for all cancer sites combined started declining soon after the March 2020 stay-at-home order was declared, reaching a nadir in April 2020, and they continued to be low up until May 2020. Compared with April 2019, incidence rates dropped by 48% (95% confidence interval [CI] = 47% to 49%) in April 2020: from 472.3 (April 2019) to 245.2 (April 2020). Incidence rates came back up in June 2020. After the first decline in March 2020, however, incidence rates never returned to prepandemic levels.
Figure 1.
Monthly incidence rates for all cancer sites combined and by gender, age, race, poverty, and rural-urban continuum. 2019-2020. Surveillance, Epidemiology, and End Results–22.
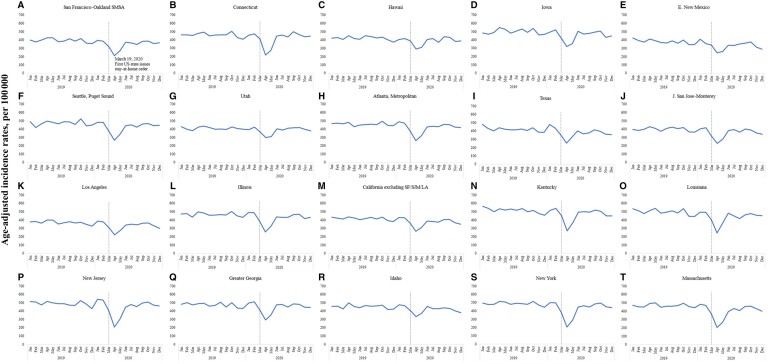
Stay-at-home orders were implemented around March across most states in the United States. To assess their impact on cancer incidence rates, we analyzed the reduction in incidence rates by comparing April 2020 with April 2019, rather than calculating the rate reduction for each month throughout the entire calendar year. We observed consistent patterns in the decline of cancer incidence rates in 2020 across sex, race and ethnicity, and age at diagnosis (Figure 1, B-F, and Table 1). Our analysis revealed that the incidence rate decreased statistically significantly more in urban counties, with a decrease of 52% (95% CI = 51% to 53%) compared with a relatively smaller decrease of 43% (95% CI = 40% to 47%) in rural counties. Also, the incidence rate decreased statistically significantly more in more affluent counties, with a drop of 51% (95% CI = 50% to 52%) compared with a relatively smaller decrease of 44% (95% CI = 41% to 46%) in less affluent counties. Furthermore, there was substantial variability in incidence rates by registry location (Figure 2 and Table 1). Registries located in the Northeast region of the country had the largest decrease in cancer rates. For example, the largest decrease was observed in New York (60%, 95% CI = 59% to 62%), and the smallest decrease was observed in Utah (30%, 95% CI = 22% to 36%).
Table 1.
Monthly rates in April 2019 (prepandemic) as compared with April 2020 (pandemic) for all cancers combined overall, by demographic subgroups, and by cancer type
| 2019 April | 2020 April | Percent changea (95% CIb) | |
|---|---|---|---|
| All cancers combined | 472.2 | 245.2 | −48.1% (−47.4% to −48.7%) |
| Sex | |||
| Male | 516.6 | 275.5 | −46.7% (−45.7% to −47.6%) |
| Female | 442.1 | 223.3 | −49.5% (−48.5% to −50.4%) |
| Race and ethnicity | |||
| Hispanic | 381.8 | 204.1 | −46.5% (−44.6% to −48.4%) |
| Non-Hispanic Asian and Pacific Islander | 338.3 | 216.7 | −35.9% (−22.1% to −47.5%) |
| Non-Hispanic American Indian and Alaska Native | 324.4 | 154.2 | −52.5% (−49.8% to −54.9%) |
| Non-Hispanic Black | 490.4 | 242.3 | −50.6% (−48.6% to −52.5%) |
| Non-Hispanic White | 505.0 | 263.9 | −47.7% (−46.9% to −48.6%) |
| Age, y | |||
| 0-19 | 21.2 | 14.0 | −34.1% (−25.8% to −41.6%) |
| 20-59 | 281.1 | 148.8 | −47.1% (−45.8% to −48.3%) |
| 60-69 | 1488.5 | 764.8 | −48.6% (−47.4% to −49.8%) |
| 70-79 | 2175.9 | 1089.5 | −49.9% (−48.7% to −51.2%) |
| 80 and older | 2264.0 | 1192.6 | −47.3% (−45.6% to −49.0%) |
| County-level poverty | |||
| Poverty <10% | 478.9 | 237.7 | −51.1% (−49.9% to −52.3%) |
| Poverty 10%-19.99% | 468.9 | 249.3 | −47.3% (−46.4% to −48.1%) |
| Poverty >20% | 468.3 | 247.0 | −43.5% (−41.0% to −46.0%) |
| Urban-rural continuum | |||
| Nonmetropolitan, not adjacent to metropolitan | 497.9 | 282.1 | −43.3% (−39.7% to −46.8%) |
| Nonmetropolitan, adjacent to metropolitan | 500.7 | 292.6 | −41.6% (−38.8% to −44.2%) |
| Metropolitan, <250 000 population | 497.7 | 290.0 | −41.7% (−39.0% to −44.4%) |
| Metropolitan, 250 000 to 1 million population | 471.2 | 280.0 | −40.6% (−38.8% to −42.3%) |
| Metropolitan, >1 million population | 465.1 | 223.1 | −52.0% (−51.2% to −52.8%) |
| Registry | |||
| Atlanta, metropolitan | 483.2 | 260.8 | −46.0% (−41.1% to −50.5%) |
| California, excluding San Francisco, San Jose Monterey, Los Angeles | 438.7 | 264.9 | −39.6% (−37.5% to −41.7%) |
| Connecticut | 478.4 | 218.2 | −54.4% (−50.4% to −58.1%) |
| Greater Georgia | 489.8 | 294.1 | −40.0% (−36.4% to −43.3%) |
| Hawaii | 453.1 | 292.0 | −35.6% (−26.9% to −43.2%) |
| Idaho | 499.4 | 333.8 | −33.2% (−25.5% to −40.1%) |
| Illinois | 497.6 | 257.3 | −48.3% (−46.0% to −50.5%) |
| Iowa | 549.9 | 321.3 | −41.6% (−36.7% to −46.1%) |
| Kentucky | 535.4 | 270.1 | −49.5% (−45.9% to −53.0%) |
| Los Angeles | 400.2 | 221.5 | −44.7% (−41.5% to −47.6%) |
| Louisiana | 512.4 | 245.1 | −52.2% (−48.5% to −55.6%) |
| Massachusetts | 491.0 | 205.5 | −58.1% (−55.4% to −60.7%) |
| New Jersey | 516.3 | 208.2 | −59.7% (−57.4% to −61.8%) |
| New Mexico | 411.3 | 246.7 | −40.0% (−32.9% to −46.4%) |
| New York | 515.4 | 205.0 | −60.2% (−58.7% to −61.7%) |
| San Francisco-Oakland SMSA | 426.6 | 211.9 | −50.3% (−46.3% to −54.1%) |
| San Jose-Monterey | 429.3 | 228.7 | −46.7% (−40.9% to −52.1%) |
| Seattle, Puget Sound | 497.5 | 265.3 | −46.7% (−42.9% to −50.2%) |
| Texas | 439.3 | 252.8 | −42.5% (−40.6% to −44.2%) |
| Utah | 423.5 | 298.6 | −29.5% (−22.3% to −36.1%) |
| Cancer types | |||
| Breast, female | 137.3 | 61.9 | −54.9% (−53.2% to −56.5%) |
| Prostate | 128.9 | 54.3 | −57.9% (−56.3% to −59.4%) |
| Lung and bronchus | 52.6 | 29.9 | −43.1% (−41.0% to −45.1%) |
| Colon and rectum | 38.6 | 17.7 | −54.1% (−51.9% to −56.2%) |
| Non-Hodgkin lymphoma | 20.3 | 11.6 | −42.9% (−39.3% to −46.3%) |
| Urinary bladder | 19.8 | 11.4 | −42.4% (−38.9% to −45.8%) |
| Thyroid | 15.0 | 4.3 | −71.2% (−68.3% to −73.9%) |
| Pancreas | 14.2 | 10.8 | −24.0% (−18.9% to −28.8%) |
| Esophagus | 4.2 | 2.6 | −36.5% (−27.9% to −44.1%) |
April 2020 compared with April 2019. CI = confidence interval.
Rates are per 100 000 population. We used Surveillance, Epidemiology, and End Results Program SEER*Stat estimates of ratio = [rate (2020)/rate (2019)] and respective confidence interval (lower confidence interval, upper confidence interval). Percent change was calculated as (ratio-1) x 100. The lower and upper limits of the confidence interval for percent change were calculated as (upper confidence interval-1) x 100 and (lower confidence interval-1) x 100, respectively.
Figure 2.
Monthly incidence rates by Surveillance, Epidemiology, and End Results (SEER) registry site. 2019-2020. SEER-22. LA = Los Angeles; SF = San Francisco; SJM = San Jose Monterey.
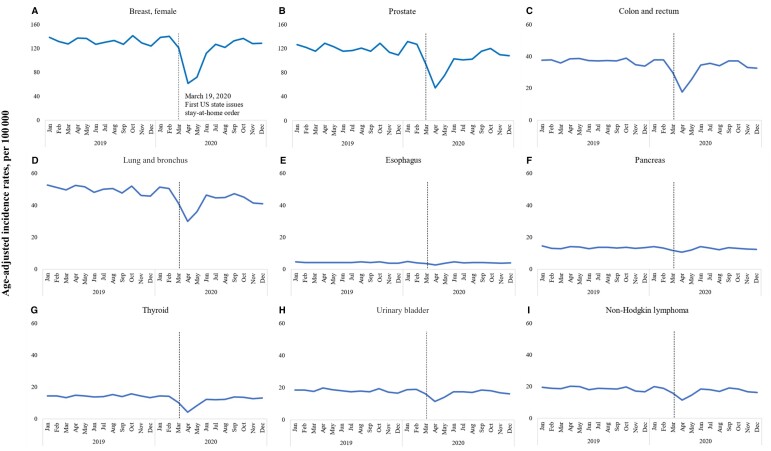
By cancer type (Figure 3 and Table 1), the data show statistically significant decreases in cancer incidence rates across all types during the pandemic, with the largest decreases observed in thyroid (71.2%, 95% CI = 68.3% to 73.9%), prostate (57.9%, 95% CI = 56.3% to 59.4%), breast (54.9%, 95% CI = 53.2% to 56.5%), and colon and rectum (54.1%, 95% CI = 51.9% to 56.2%) cancers. Lung and bronchus, non-Hodgkin lymphoma, and urinary bladder cancers decreased by approximately 40%, while the smallest decrease was seen in pancreatic cancer (24%, 95% CI = 18.9% to 28.8%).
Figure 3.
Monthly incidence rates by cancer site. 2019-2020. Surveillance, Epidemiology, and End Results–22.
Table 2 presents the percent change in age-adjusted rates of cancer for different types and stages between 2019 and 2020. Age-adjusted rates decreased consistently across all stages at diagnosis, with the largest decline seen for in situ cases, followed by cases diagnosed at localized and regional stages. This trend was most notable for cancer types with early detection programs, for example colorectal cancer, where the greatest decrease was seen for the localized stage (17.8%, 95% CI = 16.2% to 19.4%), followed by regional (8.4%, 95% CI = 6.7% to 10.1%) and distant (6.2%, 95% CI = 3.9% to 8.4%) stages. Similarly, large decreases were seen for early stage prostate (16.2%, 95% CI = 15.3% to 17.1%) and female breast (13.9%, 95% CI = 12.4% to 15.4%) cancers. For cancers diagnosed at a later stage, such as lung cancer, the drop in age-adjusted rates was relatively small.
Table 2.
Age-adjusted rates by stage at diagnosis and cancer type, Surveillance, Epidemiology, and End Results–22, 2019-2020
| Cancer type | Stage at diagnosis | 2019 age-adjusted rate | 2020 age-adjusted rate | Percent changea (95% CI) |
|---|---|---|---|---|
| Female breast | In situ | 31.6 | 27.2 | −13.9% (−12.4% to −15.4%) |
| Localized | 86.2 | 76.1 | −11.7% (−10.8% to −12.6%) | |
| Regional | 35.0 | 32.0 | −8.6% (−7.1% to −10.1%) | |
| Distant | 7.7 | 7.3 | −4.2% (−0.9% to −7.4%) | |
| Prostate | Localized | 81.8 | 68.5 | −16.2% (−15.3% to −17.1%) |
| Regional | 15.8 | 13.5 | −14.4% (−12.3% to −16.4%) | |
| Distant | 10.5 | 10.1 | −4.2% (−1.2% to −7.1%) | |
| Colon and rectum | Localized | 12.9 | 10.6 | −17.8% (−16.2% to −19.4%) |
| Regional | 13.3 | 12.2 | −8.4% (−6.7% to −10.1%) | |
| Distant | 8.2 | 7.7 | −6.2% (−3.9% to −8.4%) | |
| Lung and bronchus | Localized | 13.9 | 11.8 | −15.0% (−13.4% to −16.5%) |
| Regional | 10.1 | 8.4 | −16.9% (−15.1% to −18.6%) | |
| Distant | 22.6 | 20.4 | −9.9% (−8.6% to −11.2%) | |
| Esophagus | Localized | 0.9 | 0.8 | −15.3% (−9.1% to −21.0%) |
| Regional | 1.3 | 1.2 | −5.9% (−0.3% to −11.2%) | |
| Distant | 1.5 | 1.5 | −2.5% (2.8% to −7.5%) | |
| Pancreas | Localized | 2.3 | 2.1 | −6.5% (−2.2% to −10.6%) |
| Regional | 3.6 | 3.4 | −6.4% (−3.1% to −9.6%) | |
| Distant | 6.4 | 6.2 | −2.7% (−0.1% to −5.2%) | |
| Thyroid | Localized | 9.2 | 7.6 | −17.2% (−15.1% to −19.1%) |
| Regional | 4.4 | 3.5 | −19.3% (−16.3% to −22.1%) | |
| Distant | 0.4 | 0.3 | −12.8% (−2.3% to −22.1%) | |
| Urinary bladder | In situ | 8.7 | 7.8 | −9.9% (−7.8% to −11.9%) |
| Localized | 6.5 | 6.1 | −5.7% (−3.2% to −8.1%) | |
| Regional | 1.2 | 1.2 | −3.3% (2.8% to −9.0%) | |
| Distant | 1.0 | 1.0 | −2.7% (3.9% to −9.0%) | |
| Non-Hodgkin lymphoma | Localized | 4.8 | 4.4 | −10.2% (−7.4% to −13.0%) |
| Regional | 2.5 | 2.3 | −7.1% (−3.0% to −11.0%) | |
| Distant | 9.6 | 8.9 | −7.3% (−5.3% to −9.3%) |
2020 compared with 2019. CI = confidence interval.
Discussion
The present study investigated the impact of the COVID-19 pandemic on cancer incidence rates in the United States. The results showed that the incidence rates for all cancer sites combined started declining in March 2020, coinciding with the implementation of various stay-at-home orders and guidance provided by states and municipalities. These measures led to notable differences in observed rates in the New York and Utah regions. The observed incidence rates reached a nadir in April 2020 and continued to be low until May 2020. Compared with April 2019, the incidence rates declined by 48.1% in April 2020 and did not return to prepandemic levels. The decline in cancer incidence rates was consistent across sex, race and ethnicity, and age at diagnosis.
The study also found that the cancer incidence rate decreased statistically significantly more in urban and more affluent counties compared with rural and less affluent counties. Urban and more affluent counties generally have better access to health-care facilities and services, including cancer screening and treatment, than rural and less affluent counties (11). However, these areas were heavily affected by the pandemic, leading to restricted health-care access. Furthermore, a considerable number of individuals chose to defer or avoid nonurgent medical appointments and procedures thereby contributing to the decline in cancer incidence rates (12).
There was also substantial variability in incidence rates by registry location, with registries located in the Northeast region of the United States having the largest decreases in cancer rates. New York State was one of the hardest-hit regions in the early stages of the COVID-19 pandemic (13,14). The state implemented strict stay-at-home orders and social distancing measures, which led to a statistically significant disruption of the health-care system (15). Hospitals were overwhelmed, and many non–COVID-19–related services were halted to free up resources for COVID-19 patients. This disruption of the health-care system likely led to a decrease in cancer screenings and diagnoses. Many people may have been hesitant to seek health-care services because of fear of contracting COVID-19 or because health-care providers were unable to provide routine care. Additionally, many health-care facilities were forced to reduce their capacity to comply with social distancing measures, which could have led to longer wait times or appointment delays, further discouraging patients from seeking cancer care. These factors may have contributed to the larger decrease in cancer incidence rates observed in New York and the registries located in the Northeast region of the country (eg, Massachusetts and New Jersey) compared with other states, during the pandemic’s early stages, whereas Utah had some of the lowest changes in rates, possibly explained by Utah’s stay-at-home orders extending to only parts of the state (15). Population density could also have played a role, by putting more strain on hospitals and non–COVID-19 medical services that were statistically significantly disrupted in a state like New York. Utah, with a lower population density, could have experienced less strain on its health-care system, resulting in a comparatively smaller disruption of cancer screening services and less impact on cancer rates.
We also demonstrated that rates decreased dramatically for screen-detected cancers between March and May 2020. This is not surprising given that cancer screening rates decreased during the pandemic (16,17). Although cancer rates rebounded after dropping sharply, incidence rates neither fully rebounded nor surpassed prepandemic levels. Given the large decrease in incidence rates from March to May 2020, we would expect a compensating surge in rates after May to catch up on diagnoses, but there was no such surge through the end of the 2020 calendar year. Unlike screen-detected cancers, thyroid cancer had the biggest decrease. The decline in thyroid cancer incidence may be primarily attributed to opportunistic detection—where individuals seek general health care and undergo imaging tests for reasons unrelated to cancer—revealing unexpected thyroid cancer cases that are likely to be indolent.
The main strength of the study is the high-quality, population-based cancer registry data representing approximately half of the US population. The main limitation is the use of Vintage 2020 population based on the 2010 census as the denominator for rate calculations, which may not represent the most up-to-date population size for accurately reflecting the COVID-19 pandemic’s impact on population estimates. Although a Vintage 2022 dataset is available, it is not the population dataset that is required to calculate SEER rates. The census creates a sex-, age-, and county-level population dataset for the National Cancer Institute that is race bridged (each individual is assigned a single race based on a modeling effort). This race-bridged population is usually available before each April release; however, this year 2023 there was a delay. Nonetheless, for a sensitivity analysis, we obtained the Vintage 2021 (based on 2020 census) population data to show the impact of using this population on rates as compared with Vintage 2020 (based on 2010 census)—even though the SEER Program is not planning to use the Vintage 2021 data for rate calculations. In 2020, the Vintage 2021 population data (derived from the 2020 census) shows a slight increase to 331 511 512 compared with the Vintage 2020 population of 329 484 123 based on the 2010 census (ie, originally used in our analysis). This difference of 2 027 389 individuals represents 0.6% of the total US population in the Vintage 2021 data. The populations by 5-year ages were similar between these vintages (data not shown). Thus, we expect the impact of using Vintage 2021 to be small; although some variability in rates could appear in smaller geographic areas such as a single county, our analyses do not show rates in such small geographic locations.
We did not adjust for seasonality or reporting delay in the monthly rate calculations. Reporting delay accounts for cases not yet reported by cancer registries. For all cancers combined, reporting delay for cancer incidence tends to be small: approximately 3% of cases based on data from previous years (18). To understand the delay in 2019 monthly rates, we analyzed the current submission of SEER data to the previous submission. The current submission included 2020 rates, whereas we anticipated that the 2019 rates would be more complete based on the previous year’s submission than the current year. However, we found that for 2019 rates, the overall delay was minor and clinically insignificant between the current and previous year’s submissions. Thus, even if we accounted for the delay in monthly rates, it would not explain the clinically significant decrease in incidence rates that we observed from March to May 2020. Despite these limitations, this is the first study to analyze the pandemic’s impact on monthly cancer incidence rates by a broad range of factors.
The COVID-19 pandemic had a clinically significant impact on cancer incidence rates in 2020. The reasons for the declines in cancer incidence rates are not entirely clear, but potential factors include delays in cancer screening and diagnosis because of changes in health-care utilization. The changes in health-care utilization during the pandemic can be attributed to 2 factors: limitations on the health-care system’s capacity to provide cancer care and shifts in individuals’ behavior when seeking medical attention. These results underscore the need for continued efforts to monitor and mitigate the effects of the pandemic on cancer prevention, diagnosis, and care, particularly for vulnerable populations and regions. Further research is needed to understand the long-term consequences of the pandemic on cancer incidence, shifts in stage at diagnosis, and mortality trends.
Acknowledgements
We acknowledge the Surveillance Research Program, Division of Cancer Control and Population Sciences of the National Cancer Institute registries for the cancer registry data. The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript and decision to submit it for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Contributor Information
Nadia Howlader, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Manami Bhattacharya, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Steve Scoppa, Information Management Services, Calverton, MD, USA.
Daniel Miller, Information Management Services, Calverton, MD, USA.
Anne-Michelle Noone, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Serban Negoita, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Kathy Cronin, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Angela Mariotto, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Data availability
Data and code used in this project cannot be directly shared by authors. The data and statistical software used are publicly available through the National Cancer Institute’s SEER Program.
Author contributions
Nadia Howlader, PhD, MS (Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Supervision; Validation; Writing—original draft; Writing—review & editing), Manami Bhattacharya, PhD, MS (Formal analysis; Writing—original draft; Writing—review & editing), Steve Scoppa, BS (Formal analysis), Daniel Miller, BA (Formal analysis), Anne-Michelle Noone, PhD, MS (Formal analysis; Writing—original draft; Writing—review & editing), Serban Negoita, MD DrPH (Validation; Writing—original draft; Writing—review & editing), Kathy Cronin, PhD, MPH (Conceptualization; Data curation; Supervision; Validation; Writing—original draft; Writing—review & editing), and Angela Mariotto, PhD (Conceptualization; Data curation; Supervision; Validation; Writing—original draft; Writing—review & editing).
Funding
The work was funded by the Surveillance Research Program, Division of Cancer Control and Population Sciences of the National Cancer Institute.
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1. Yabroff KR, Wu XC, Negoita S, et al. Association of the COVID-19 Pandemic With Patterns of Statewide Cancer Services. J Natl Cancer Inst. 2022;114(6):907-909. doi: 10.1093/jnci/djab122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Negoita S, Chen HS, Sanchez PV, et al. Annual report to the nation on the status of cancer, part 2: early assessment of the COVID-19 pandemic’s impact on cancer diagnosis [published online ahead of print, September 27, 2023]. Cancer. 2023;10.1002/cncr.35026. doi: 10.1002/cncr.35026. [DOI] [PMC free article] [PubMed]
- 3. Nogueira LM, Palis B, Boffa D, Lum S, Yabroff KR, Nelson H.. Evaluation of the impact of the COVID-19 pandemic on reliability of cancer surveillance data in the national cancer database. Ann Surg Oncol. 2023;30(4):2087-2093. doi: 10.1245/s10434-022-12935-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chtourou A, Sanchez PV, Golden T,. et al. Impact on the volume of pathology reports before and during the COVID-19 pandemic in SEER cancer registries. Cancer Epidemiol Biomarkers Prev. 2023;32(11):1591-1598. doi: 10.1158/1055-9965.EPI-23-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenbaum L. The untold toll — the pandemic’s effects on patients without covid-19. N Engl J Med. 2020;382(24):2368-2371. doi: 10.1056/NEJMms2009984 [DOI] [PubMed] [Google Scholar]
- 6. SEER. SEER Incidence Database - SEER Data & Software. https://seer.cancer.gov/data/index.html. Accessed March 27, 2023.
- 7. SEER. County/Tract Attributes - SEER Datasets.https://seer.cancer.gov/seerstat/variables/countyattribs/index.html. Accessed May 3, 2023.
- 8. SEER. U.S. Population Data - SEER Population Data. https://seer.cancer.gov/popdata/index.html. Accessed March 8, 2023.
- 9. SEER. SEERStat Software.https://seer.cancer.gov/seerstat/index.html. Accessed March 16, 2023.
- 10. Mariotto AB, Feuer EJ, Howlader N, Chen HS, Negoita S, Cronin K.. Interpreting cancer incidence trends: challenges due to the COVID-19 pandemic. J Natl Cancer Inst. 2023;115(9):1109-1111. doi: 10.1093/jnci/djad086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh GK, Siahpush M.. Widening rural–urban disparities in all-cause mortality and mortality from major causes of death in the USA, 1969–2009. J Urban Health. 2014;91(2):272-292. doi: 10.1007/s11524-013-9847-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelbrecht K, Roy S, Capkun G, Kahler K, Olson M.. Impact of the COVID-19 pandemic on healthcare resource utilization across selected disease areas in the USA. J Comp Eff Res. 2022;11(11):815-828. doi: 10.2217/cer-2022-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The New York Times. New York Coronavirus Map and Case Count. The New York Times. https://www.nytimes.com/interactive/2023/us/new-york-covid-cases.html. Published April 1, 2020. Accessed May 3, 2023.
- 14. Ferguson W, Furticella J, Hinderaker A, Howard S, Newman A, Rogers K. Two Years of the Pandemic in New York, Step by Awful Step. The New York Times. https://www.nytimes.com/interactive/2022/nyregion/nyc-covid-timeline.html. Published March 17, 2022. Accessed May 15, 2023.
- 15. Mervosh S, Lu D, Swales V.. See Which States and Cities Have Told Residents to Stay at Home. The New York Times. https://www.nytimes.com/interactive/2020/us/coronavirus-stay-at-home-order.html. Published March 24, 2020. Accessed May 3, 2023.
- 16. Fedewa SA, Star J, Bandi P, et al. Changes in cancer screening in the US during the COVID-19 pandemic. JAMA Netw Open. 2022;5(6):e2215490. doi: 10.1001/jamanetworkopen.2022.15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Star J, Bandi P, Siegel RL, et al. Cancer screening in the United States during the second year of the COVID-19 pandemic. J Clin Oncol. 2023;41(27):4352-4359. doi: 10.1200/JClinOncol.22.02170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF.. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537-1545. doi: 10.1093/jnci/94.20.1537 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code used in this project cannot be directly shared by authors. The data and statistical software used are publicly available through the National Cancer Institute’s SEER Program.