Abstract
Background
Patients with cancer frequently require multidisciplinary teams for optimal cancer outcomes. Network analysis can capture relationships among cancer specialists, and we developed a novel physician linchpin score to characterize “linchpin” physicians whose peers have fewer ties to other physicians of the same oncologic specialty. Our study examined whether being treated by a linchpin physician was associated with worse survival.
Methods
In this cross-sectional study, we analyzed Surveillance, Epidemiology, and End Results–Medicare data for patients diagnosed with stage I to III non-small cell lung cancer or colorectal cancer (CRC) in 2016-2017. We assembled patient-sharing networks and calculated linchpin scores for medical oncologists, radiation oncologists, and surgeons. Physicians were considered linchpins if their linchpin score was within the top 15% for their specialty. We used Cox proportional hazards models to examine associations between being treated by a linchpin physician and survival, with a 2-year follow-up period.
Results
The study cohort included 10 081 patients with non-small cell lung cancer and 9036 patients with CRC. Patients with lung cancer treated by a linchpin radiation oncologist had a 17% (95% confidence interval = 1.04 to 1.32) greater hazard of mortality, and similar trends were observed for linchpin medical oncologists. Patients with CRC treated by a linchpin surgeon had a 22% (95% confidence interval = 1.03 to 1.43) greater hazard of mortality.
Conclusions
In an analysis of Medicare beneficiaries with nonmetastatic lung cancer or CRC, those treated by linchpin physicians often experienced worse survival. Efforts to improve outcomes can use network analysis to identify areas with reduced access to multidisciplinary specialists.
Improving access to health care is considered paramount to improving patient outcomes and achieving health equity (1-4). Assessing the spatial distribution of health-care professionals is a well-established method for determining access to primary and specialty health-care services (5,6). Workforce reports on the spatial distribution of oncologists from the American Society of Clinical Oncology have shown striking geographic disparities in oncologist density across the United States, with rural areas particularly at risk of oncology workforce shortages (7). Prior research has found elevated cancer mortality rates in regions characterized by low oncologist density and greater travel burden to specialty care (8,9). Because medical, surgical, and radiation oncologists practice within complex systems to coordinate the delivery of multidisciplinary treatment plans, however, measuring access to the physician oncology workforce presents unique challenges (10). Care coordination measures for multidisciplinary cancer care are absent or limited, challenging our ability to assess ways in which the oncology workforce functions across health-care professionals to serve patients with cancer (11).
We hypothesized that integrating characteristics of physician patient-sharing networks into measurement of the oncology physician workforce would better capture the structure of the multidisciplinary relationships linking physicians and patient outcomes. Relationships among physicians represent an aspect of health-care access that could affect both potential and realized access to care as well as outcomes—that is, a cancer physician’s patient outcomes may be intertwined with that physician’s multidisciplinary patient-sharing network and experience coordinating care with peers. We have developed a network measure that identifies physicians who are vital for bringing their specialty’s expertise to the region—in other words, a “linchpin” (12). For example, a medical oncologist’s linchpin score assesses the extent to which their peers (physicians from other specialties with whom they share patients) are connected to other medical oncologists. Being a linchpin oncologist could be reflective of a health-care delivery system that has reduced specialist access, fewer resources, lower competition among specialists, and geographic or other barriers to establishing interdisciplinary relationships between clinicians.
In our prior work examining linchpin oncologists in a cancer patient-sharing network assembled from nationwide Medicare claims, we found that linchpin oncologists were more likely to practice in nonmetropolitan areas than oncologists who were not characterized as linchpins (13). We also found that hospital referral regions with a higher-than-expected proportion of linchpin oncologists tracked with indicators of socioeconomic disadvantage and lower rates of radiation therapy receipt. A better understanding of how physician relationships are associated with overall survival is critical to guide efforts aimed at reducing the disparities in cancer mortality that have been observed across race, rurality, and socioeconomic status (3,14-17).
The objective of this study was to assess the extent to which being treated by linchpin medical, surgical, or radiation oncologists (herein referred to as oncologists) is associated with survival in patients with cancer. In this study, we focused on outcomes for patients with nonmetastatic non-small cell lung cancer (NSCLC) or colorectal cancer (CRC) because these diseases often require multidisciplinary care and have higher mortality rates than other common cancers, such as breast and prostate cancer. Using Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data from patients diagnosed with NSCLC or CRC in 2016-2017, we assembled patient-sharing networks to examine whether being treated by a linchpin oncologist was associated with risk of mortality following diagnosis.
Methods
Data source and study cohort
The SEER-Medicare database links SEER cancer registry demographic and tumor characteristics with Medicare claims for health services. Our study cohorts included patients diagnosed with incident CRC or NSCLC between January 1, 2016, and December 31, 2017. We excluded patients who were younger than 66 or older than 99 years of age at the time of diagnosis, had a missing or non-US residential zip code, or were not continuously enrolled in fee-for-service Medicare in the 12 months before diagnosis and until the sooner of death or 12 months following diagnosis (Supplementary Figures 1 and 2, available online). We excluded patients with a death date missing from the Medicare Beneficiary Summary File or whose death dates did not match between SEER and Medicare Beneficiary Summary File. Finally, we limited our study cohort to patients with American Joint Committee on Cancer (AJCC) stage I to III cancers because access to surgery, radiation therapy, and chemotherapy is expected to prolong survival for these patients. The study was approved by the institutional review board at Dartmouth College.
Network assembly
We identified the physicians who had encounters with the patients in our cohorts in the 3 months before and 12 months following their cancer diagnosis in the Medicare Carrier files. From these encounters, physicians were connected if they had encounters with common patients to form a “patient-sharing network.” In this network, the relationships, or “edges,” between physicians were quantified by the number of shared patients. We analyzed separate patient-sharing networks for patients with lung cancer and colorectal cancer. Physician age, sex, and specialty were identified from the Medicare Data on Provider Practice and Specialty (MD-PPAS) file. We identified physicians with specialties of medical oncology, radiation oncology, and surgery. For CRC surgeons, we included those with a specialty of surgical oncology and general surgery; for lung cancer surgeons, we included those with a specialty of thoracic surgery, surgical oncology, and general surgery.
Attribution of oncologists to patients
Because patients will typically encounter their oncologists after cancer diagnosis, we used a 90-day look-forward window starting on day 1 of their month of diagnosis to assign patients to a medical oncologist, radiation oncologist, or surgeon. Patients who had encounters with multiple oncologists of the same specialty type were assigned to the physician with whom they had the plurality of encounters within the 90-day time frame. This attribution approach is expected to capture the oncologists who were involved in treatment planning and initiation.
Independent variable of interest
The independent variable of interest in this study was oncologist linchpin score. The linchpin score was calculated for each oncologist in the lung cancer and CRC patient-sharing networks (12). In Figure 1, we demonstrate the linchpin score calculation for medical oncologist i () by summing edges with peers who lack ties to other medical oncologists, and then dividing by the sum of all shared ties. In this example, medical oncologist i shares patients with 4 other physicians (, and the values along each edge represent the number of shared patients. Only 1 of those physicians has an established tie with another medical oncologist. The linchpin score is calculated by summing the edges that medical oncologist i has with physicians who are not connected to another medical oncologist (in this example, , and then dividing by the sum of all edges. Each oncologist is assigned a score that ranges from 0 (eg, if all peers in Figure 1 shared patients with at least 1 other medical oncologist) to 1 (eg, if none of the peers in Figure 1 shared patients with another medical oncologist). We considered an oncologist to be a linchpin if the linchpin score was in the top 15% of the distribution of linchpin scores for their specialty; alternative thresholds were explored in sensitivity analyses.
Figure 1.
Illustration of linchpin score calculation. Numbers adjacent to edge lines represent shared patients.
Outcome variable
The primary outcome variable was overall survival in months within a 2-year follow-up observation period. Because of the 90-day look-forward window used to assign oncologists to patients, we started measuring a patient’s survival time 3 calendar months after diagnosis and followed the patient through to their month of death or the end of follow-up. As a result, patients who survived less than 3 months after cancer diagnosis were not included in these analyses. Patients who were alive at the end of the 2-year follow-up period were censored.
Covariate measures
Patient age in years at diagnosis, sex, race, ethnicity, AJCC tumor stage, US Census Bureau tract-level poverty, county-level rurality, and SEER region were obtained from the SEER Cancer File. For patient race, we used the SEER Race Recode variable (White/Black/Other), where “Other” is inclusive of American Indian/Alaska Native and Asian/Pacific Islander. The Charlson Comorbidity Index was calculated using the 12 months of claims preceding the month of cancer diagnosis (18). Poverty was measured using the Yost Index, which was developed using US-based quintiles of a composite socioeconomic status score from US Census tract-level American Community Survey 5-year estimates (19,20). We calculated node strength to determine the overall prominence, or centrality, of each oncologist in the network. Physician node strength is the sum of each physician’s patient-sharing ties and is highly correlated with patient volume. We dichotomized node strength using a threshold of the lowest 15th percentile of node strength for each cancer specialty to capture the least connected oncologists in the patient-sharing networks. Physician age, sex, and practice setting (metropolitan vs nonmetropolitan) were obtained from the MD-PPAS file. SEER categorizes the geographic location of each physician in the dataset by core-based statistical areas, which denote metropolitan, micropolitan, and non–core-based statistical areas. We considered oncologists to practice in a metropolitan area if they were categorized as metropolitan; otherwise, they were categorized as nonmetropolitan.
Statistical analyses
We examined bivariate associations between oncologist characteristics and linchpin status using χ2 and Wilcoxon rank sum tests for categorical and continuous variables, respectively. We assessed bivariate associations between being treated by a linchpin oncologist and patient race, ethnicity, rurality, and socioeconomic status using χ2 tests. We estimated a series of Cox proportional hazards models to examine the associations between patient characteristics and oncologist linchpin score and survival for each cancer type. First, we estimated adjusted Cox proportional hazards models, including all patient characteristics (patient age in years at diagnosis, sex, race, ethnicity, AJCC stage, Charlson Comorbidity Index, US Census tract-level poverty, county-level rurality, and SEER region), oncologist rurality, and oncologist node strength. An indicator variable for whether the patient was assigned an oncologist of each specialty type was included to account for patients who did not see all 3 types of specialists. We then estimated the models, including oncologist linchpin score. We repeated the models with group frailty for patient county (the survival analysis analogy of a random effect in a mixed effect regression model) to better distinguish the effect of a linchpin from geographic location. Finally, we explored potential effect modification of oncologist rurality and oncologist node strength on associations between oncologist linchpin score and survival with interaction terms.
Results
Our study included patients with a lung or colorectal cancer diagnosis in 2016-2017 within 16 SEER regions (Table 1). Regarding race and ethinicity, of the 10 081 patients diagnosed with lung cancer, 729 (7.2%) were Black, 348 (3.5%) were Hispanic, and 8960 (88.9%) were White. Patients residing in metropolitan areas accounted for 82.0% of the cohort (n = 8262), and those residing in US Census tracts in the highest quintile for socioeconomic status accounted for 23.7% of the cohort (n = 2387) compared with 16.8% (n = 1696) residing in US Census tracts in the lowest socioeconomic status quintile. Similarly, of the 9036 patients diagnosed with CRC, 7949 (88.0%) were White, 609 (6.7%) were Black, and 541 (6.0%) were Hispanic. Patients residing in metropolitan areas accounted for 82.5% of the cohort (n = 7453), and those residing in US Census tracts in the highest quintile for socioeconomic status accounted for 26.0% of the cohort (n = 2345) compared with 15.5% (n = 1398) residing in US Census tracts in the lowest socioeconomic status quintile.
Table 1.
Study cohort patient characteristics
| Characteristic | Lung cancer | Colorectal cancer |
|---|---|---|
| n = 10 081 | n = 9036 | |
| Age at diagnosis, No. (%), y | ||
| 66-69 | 1898 (18.8) | 1607 (17.8) |
| 70-74 | 2894 (28.7) | 1907 (21.1) |
| 75-79 | 2478 (24.6) | 2022 (22.4) |
| 80-84 | 1720 (17.1) | 1718 (19.0) |
| ≥85 | 1091 (10.8) | 1782 (19.7) |
| Female sex, No. (%) | 5126 (50.8) | 4819 (53.3) |
| Race, No. (%) | ||
| Black | 729 (7.2) | 609 (6.7) |
| White | 8960 (88.9) | 7949 (88.0) |
| Other | 392 (3.9) | 478 (5.3) |
| Hispanic ethnicity, No. (%) | 348 (3.5) | 541 (6.0) |
| American Joint Committee on Cancer stage, No. (%) | ||
| I | 5158 (51.2) | 2346 (26.0) |
| II | 1514 (15.0) | 3483 (38.6) |
| III | 3409 (33.8) | 3207 (35.5) |
| Charlson Comorbidity Index, mean (SD) | 2.05 (1.9) | 1.52 (1.8) |
| Rurality, No. (%) | ||
| Metropolitan | 8262 (82.0) | 7453 (82.5) |
| Nonmetropolitan | 1819 (18.0) | 1583 (17.5) |
| Yost Index quintile, No. (%) | ||
| 1 (lowest socioeconomic status) | 1696 (16.8) | 1398 (15.5) |
| 2 | 1846 (18.3) | 1537 (17.0) |
| 3 | 1983 (19.7) | 1791 (19.8) |
| 4 | 2169 (21.5) | 1965 (21.7) |
| 5 (highest socioeconomic status) | 2387 (23.7) | 2345 (26.0) |
| Surveillance, Epidemiology, and End Results registry, No. (%) | ||
| San Francisco-Oakland | 328 (3.3) | 351 (3.9) |
| Connecticut | 553 (5.5) | 445 (4.9) |
| Metropolitan Detroit | 591 (5.9) | 478 (5.3) |
| Iowa | 626 (6.2) | 694 (7.7) |
| New Mexico | 162 (1.6) | 209 (2.3) |
| Seattle (Puget Sound) | 617 (6.1) | 494 (5.5) |
| Utah | 121 (1.2) | 165 (1.8) |
| Metropolitan Atlanta | 300 (3.0) | 230 (2.5) |
| San Jose-Monterey | 213 (2.1) | 207 (2.3) |
| Los Angeles | 440 (4.4) | 550 (6.1) |
| Rural Georgia | 21 (0.2) | 25 (0.3) |
| Greater California | 1858 (18.4) | 1655 (18.3) |
| Kentucky | 1098 (10.9) | 730 (8.1) |
| Louisiana | 693 (6.9) | 636 (7.0) |
| New Jersey | 1395 (13.8) | 1398 (15.5) |
| Greater Georgia | 1065 (10.6) | 769 (8.5) |
We next compared oncologists who were linchpins with those who were not by their centrality in the patient-sharing networks, here captured by node strength, their practice location, and their demographics (age and sex) (Table 2). Linchpin medical oncologists and radiation oncologists were more likely to be in the bottom 15% of node strength (ie, were more isolated) and were more likely to practice in a nonmetropolitan location than nonlinchpin comparison physicians in both the lung cancer and CRC patient-sharing networks. Linchpin surgeons were also less central in the networks than nonlinchpin surgeons (P < .001), but they were not statistically significantly more likely to practice in a nonmetropolitan area in either the lung or CRC patient-sharing network (P = .454 and P = .164, respectively). Radiation oncologists who were linchpins were marginally older for both lung and CRC treating physicians (median age, 51 vs 49 years P = .014, and median age, 55 vs 50 years, P = .018, respectively). Linchpin oncologists were slightly more likely to be male among lung cancer radiation oncologists (P = .022) and CRC surgeons (P = .019).
Table 2.
Characteristics of linchpin oncologistsa
| Lung cancer |
Colorectal cancer |
|||||
|---|---|---|---|---|---|---|
| Not a linchpin | Linchpin | P b | Not a linchpin | Linchpin | P b | |
| Medical oncology | ||||||
| Node strength, No. (%) | <.001 | <.001 | ||||
| Connected (top 85%) | 1513 (98.1) | 281 (83.1) | 1441 (96.3) | 245 (78.3) | ||
| Isolated (bottom 15%) | 29 (1.9) | 57 (16.9) | 55 (3.7) | 68 (21.7) | ||
| Practice setting, No. (%) | <.001 | <.001 | ||||
| Metropolitan | 1461 (94.7) | 291 (86.1) | 1416 (94.7) | 279 (89.1) | ||
| Nonmetropolitan | 81 (5.3) | 47 (13.9) | 80 (5.3) | 34 (10.9) | ||
| Age, median (IQR), y | 49 (42-59) | 50 (42-60) | .648 | 49 (42-59) | 50 (41-60) | .399 |
| Sex, No. (%) | .330 | .371 | ||||
| Female | 459 (29.8) | 91 (26.9) | 449 (30.0) | 102 (32.8) | ||
| Male | 1083 (70.2) | 247 (73.1) | 1046 (70.0) | 209 (67.2) | ||
| Radiation oncology | ||||||
| Node strength, No. (%) | <.001 | <.001 | ||||
| Connected (top 85%) | 812 (97.1) | 120 (78.9) | 430 (93.1) | 74 (79.6) | ||
| Isolated (bottom 15%) | 24 (2.9) | 32 (21.1) | 32 (6.9) | 19 (20.4) | ||
| Practice setting, No. (%) | <.001 | .011 | ||||
| Metropolitan | 796 (95.2) | 130 (85.5) | 435 (94.2) | 80 (86.0) | ||
| Nonmetropolitan | 40 (4.8) | 22 (14.5) | 27 (5.8) | 13 (14.0) | ||
| Age, median (IQR), y | 49 (41-58) | 51 (43-60) | .014 | 50 (42-58) | 55 (43-60) | .018 |
| Sex, No. (%) | .022 | .419 | ||||
| Female | 213 (25.5) | 25 (16.4) | 108 (23.4) | 26 (28.0) | ||
| Male | 622 (74.5) | 127 (83.6) | 354 (76.6) | 67 (72.0) | ||
| Surgery | ||||||
| Node strength, No. (%) | .004 | <.001 | ||||
| Connected (top 85%) | 748 (94.4) | 108 (87.1) | 1899 (93.4) | 281 (69.6) | ||
| Isolated (bottom 15%) | 44 (5.6) | 16 (12.9) | 135 (6.6) | 123 (30.4) | ||
| Practice setting, No. (%) | .454 | .164 | ||||
| Metropolitan | 686 (86.6) | 111 (89.5) | 1800 (88.5) | 347 (85.9) | ||
| Nonmetropolitan | 106 (13.4) | 13 (10.5) | 234 (11.5) | 57 (14.1) | ||
| Age, median (IQR), y | 53 (45-60) | 53 (47-60) | .549 | 51 (42-60) | 53 (44-61) | .024 |
| Sex, No. (%) | >.1 | .019 | ||||
| Female | 83 (10.5) | <11 | 311 (15.3) | 43 (10.6) | ||
| Male | 710 (89.5) | >112 | 1722 (84.7) | 361 (89.4) | ||
Counts of physicians between 0 and 11 are suppressed to adhere to the data use agreement with the National Cancer Institute. IQR = interquartile range.
P values were calculated using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
In bivariate analyses presented in Table 3, we found that patient race, ethnicity, rurality, and socioeconomic status were associated with the likelihood of being treated by a linchpin oncologist. Among the patients with NSCLC, 22.7% of nonmetropolitan patients (vs 12.3% of metropolitan patients, P < .001), and 17.8% of patients in the lowest socioeconomic status quintile (vs 10.2% of patients in the highest socioeconomic status quintile, P < .001) were treated by a linchpin medical oncologist; similar trends were observed for linchpin radiation oncologists. There were no statistically significant associations between patient race, ethnicity, rurality, or socioeconomic status and seeing a linchpin lung cancer surgeon. Among patients with CRC, 22.2% of nonmetropolitan (vs 12.2% of metropolitan, P < .001) and 17.9% of patients in the lowest socioeconomic status quintile (vs 8.7% of those in the highest socioeconomic status quintile, P < .001) were treated by a linchpin medical oncologist. Similar trends were observed for patients treated by linchpin radiation oncologists and linchpin surgeons. Non-White patients and Hispanic patients with CRC were also more likely to see linchpin radiation oncologists (P = .009 and P = .046, respectively) and linchpin surgeons (P = .004 and P = .001, respectively) than White patients with CRC.
Table 3.
Associations between patient race, ethnicity, rurality, and socioeconomic status and being treated by a linchpin oncologist
| Medical oncology |
Radiation oncology |
Surgery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Not treated by linchpin, % | Treated by linchpin, % | P a | Not treated by linchpin, % | Treated by linchpin, % | P a | Not treated by linchpin, % | Treated by linchpin, % | P a | |
| Lung cancer cohort | n = 5339 | n = 882 | n = 4218 | n = 670 | n = 3883 | n = 346 | ||||
| Race | .036 | .202 | .801 | |||||||
| Black | 85.3 | 14.7 | 86.1 | 13.9 | 92.9 | 7.1 | ||||
| White | 86.1 | 13.9 | 86.5 | 13.5 | 91.8 | 8.2 | ||||
| Other | 80.3 | 19.7 | 81.6 | 18.4 | 91.5 | 8.5 | ||||
| Ethnicity | .127 | .101 | .077 | |||||||
| Hispanic | 82.2 | 17.8 | 81.8 | 18.2 | 87.8 | 12.2 | ||||
| Non-Hispanic | 85.9 | 14.1 | 86.4 | 13.6 | 92.0 | 8.0 | ||||
| Rurality | <.001 | <.001 | .205 | |||||||
| Metropolitan | 87.7 | 12.3 | 88.2 | 11.8 | 92.1 | 7.9 | ||||
| Nonmetropolitan | 77.3 | 22.7 | 77.9 | 22.1 | 90.6 | 9.4 | ||||
| Yost Index quintile | <.001 | <.001 | .482 | |||||||
| 1 (lowest socioeconomic status) | 82.2 | 17.8 | 80.9 | 19.1 | 92.5 | 7.5 | ||||
| 2 | 82.5 | 17.5 | 82.1 | 17.9 | 91.7 | 8.3 | ||||
| 3 | 84.8 | 15.2 | 86.2 | 13.8 | 91.5 | 8.5 | ||||
| 4 | 88.2 | 11.8 | 88.8 | 11.2 | 90.6 | 9.4 | ||||
| 5 (highest socioeconomic status) | 89.8 | 10.2 | 91.5 | 8.5 | 92.7 | 7.3 | ||||
| Colorectal cancer cohort | n = 4724 | n = 758 | n = 898 | n = 161 | n = 6936 | n = 918 | ||||
| Race | .307 | .009 | .004 | |||||||
| Black | 83.7 | 16.3 | 76.2 | 23.8 | 83.7 | 16.3 | ||||
| White | 86.4 | 13.6 | 86.1 | 13.9 | 88.6 | 11.4 | ||||
| Other | 85.2 | 14.8 | 75.0 | 25.0 | 89.4 | 10.6 | ||||
| Ethnicity | .079 | .046 | .001 | |||||||
| Hispanic | 82.9 | 17.1 | 76.7 | 23.3 | 83.4 | 16.6 | ||||
| Non-Hispanic | 86.4 | 13.6 | 85.4 | 14.6 | 88.6 | 11.4 | ||||
| Rurality | <.001 | .001 | <.001 | |||||||
| Metropolitan | 87.8 | 12.2 | 86.5 | 13.5 | 89.1 | 10.9 | ||||
| Nonmetropolitan | 77.8 | 22.2 | 77.4 | 22.6 | 84.6 | 15.4 | ||||
| Yost Index quintile | <.001 | .001 | <.001 | |||||||
| 1 (lowest socioeconomic status) | 82.1 | 17.9 | 79.0 | 21.0 | 83.9 | 16.1 | ||||
| 2 | 82.3 | 17.7 | 77.8 | 22.2 | 87.6 | 12.4 | ||||
| 3 | 83.3 | 16.7 | 86.4 | 13.6 | 86.7 | 13.3 | ||||
| 4 | 88.2 | 11.8 | 90.6 | 9.4 | 90.2 | 9.8 | ||||
| 5 (highest socioeconomic status) | 91.3 | 8.7 | 86.6 | 13.4 | 91.0 | 9.0 | ||||
P values were calculated using χ2 tests.
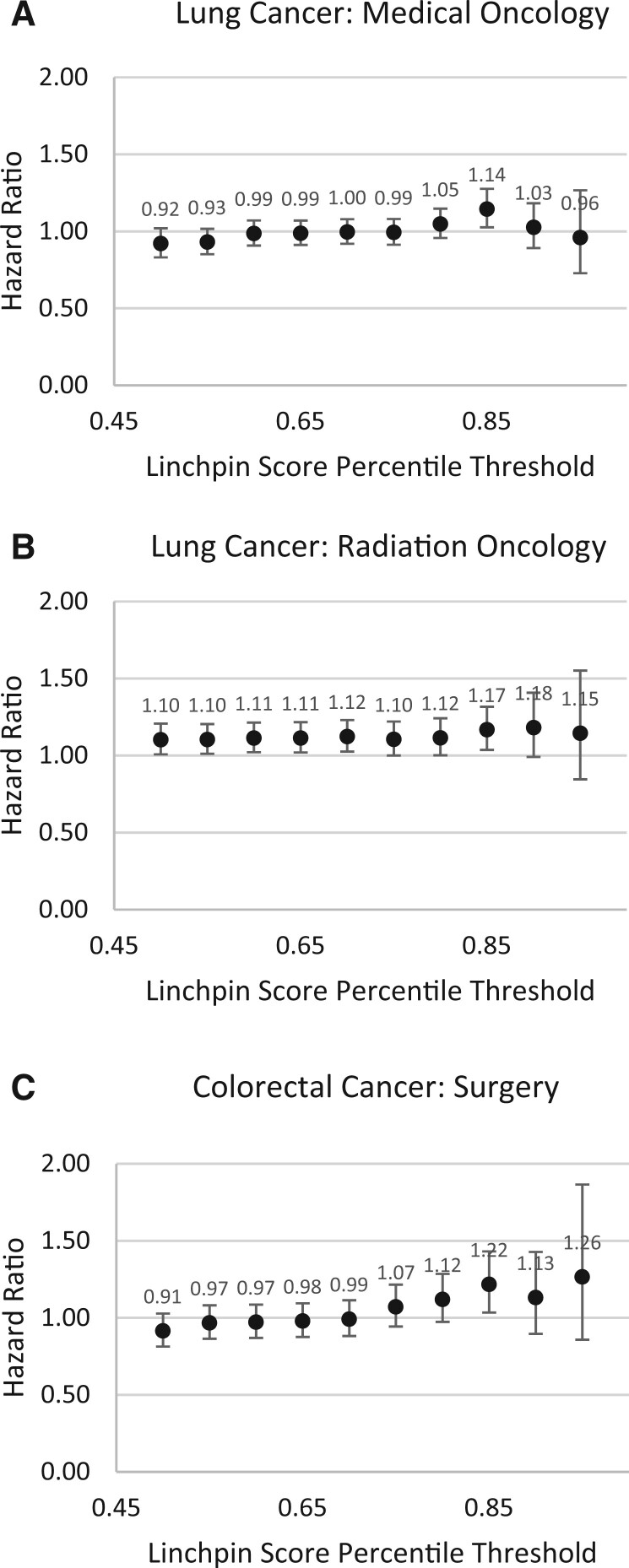
We assessed the extent to which being treated by a linchpin oncologist was associated with survival in both patient cohorts (Table 4). The observed mortality was approximately 38% (n = 3778) for the NSCLC cohort and 19% (n = 1667) for the CRC cohort. For lung cancer, being treated by a linchpin medical oncologist or radiation oncologist was associated with worse survival. Of patients with NSCLC who saw a medical oncologist, those treated by a linchpin medical oncologist had a greater hazard of mortality (adjusted hazard ratio [HR] = 1.14, 95% confidence interval [CI] = 1.03 to 1.28, P = .015). Similar estimates were observed for linchpin radiation oncologists (adjusted HR = 1.17, 95% CI = 1.04 to 1.32, P = .011). For patients with CRC, the association of oncologist linchpin status with survival was greatest for surgeons. Of patients with CRC who saw a surgeon, patients treated by a linchpin surgeon had a greater hazard of mortality (adjusted HR = 1.22, 95% CI = 1.03 to 1.43, P = .018). These results were comparable when including group frailty for patient county. Sensitivity analyses exploring a range of alternative thresholds for linchpin score demonstrated that the strongest associations with mortality were typically for linchpin score thresholds between the 80th and 95th percentiles (Figure 2). The overall associations between physician node strength and rurality were not statistically significant, the exception being patients treated by a rural lung cancer surgeon, who had a greater hazard of mortality (adjusted HR = 1.27, 95% CI = 1.02 to 1.58, P = .034).
Table 4.
Hazard ratios of mortality among patients with lung and colorectal cancer, with and without oncologist linchpin scorea
| Lung cancer adjusted hazard ratio (95% confidence interval) | Colorectal cancer adjusted hazard ratio (95% confidence interval) | |||
|---|---|---|---|---|
| (3774 events/10 056 patients) |
(1667 events/8994 patients) |
|||
| Characteristic | Model 1 | Model 2 (with oncologist linchpin score) | Model 1 | Model 2 (with oncologist linchpin score) |
| Rural | 1.03 (0.93 to 1.14) | 1.03 (0.93 to 1.14) | 1.05 (0.89 to 1.24) | 1.05 (0.89 to 1.25) |
| Hispanic | 1.09 (0.92 to 1.3) | 1.08 (0.91 to 1.29) | 0.82 (0.65 to 1.03) | 0.81 (0.64 to 1.02) |
| Race | ||||
| White | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Black | 1.15 (1.02 to 1.3)b | 1.15 (1.01 to 1.3)b | 1.13 (0.93 to 1.37) | 1.12 (0.92 to 1.36) |
| Other | 0.79 (0.66 to 0.95)b | 0.79 (0.66 to 0.95)b | 0.89 (0.71 to 1.13) | 0.90 (0.71 to 1.13) |
| Yost Index quintile | ||||
| 1 (lowest socioeconomic status) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 2 | 0.98 (0.88 to 1.09) | 0.98 (0.88 to 1.08) | 1.02 (0.86 to 1.20) | 1.03 (0.87 to 1.21) |
| 3 | 0.91 (0.82 to 1.02) | 0.91 (0.82 to 1.02) | 0.88 (0.74 to 1.04) | 0.88 (0.74 to 1.05) |
| 4 | 0.89 (0.79 to 0.99)b | 0.89 (0.79 to 1.00)b | 0.92 (0.77 to 1.09) | 0.92 (0.77 to 1.10) |
| 5 (highest socioeconomic status) | 0.80 (0.71 to 0.91)d | 0.81 (0.71 to 0.91)d | 0.77 (0.64 to 0.93)c | 0.78 (0.64 to 0.93)c |
| Age group, y | ||||
| 66-69 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 70-74 | 1.16 (1.05 to 1.29)c | 1.16 (1.05 to 1.29)c | 1.03 (0.85 to 1.25)d | 1.03 (0.85 to 1.26)d |
| 75-79 | 1.32 (1.19 to 1.47)d | 1.32 (1.19 to 1.47)d | 1.46 (1.22 to 1.76)d | 1.47 (1.22 to 1.76)d |
| 80-84 | 1.52 (1.35 to 1.69)d | 1.52 (1.36 to 1.7)d | 2.03 (1.7 to 2.43)d | 2.03 (1.70 to 2.43)d |
| ≥85 | 2.10 (1.86 to 2.37)d | 2.11 (1.87 to 2.38)d | 3.07 (2.59 to 3.65)d | 3.08 (2.59 to 3.66)d |
| Female sex | 0.80 (0.75 to 0.86)d | 0.80 (0.75 to 0.86)d | 0.88 (0.8 to 0.97)b | 0.88 (0.8 to 0.97)b |
| American Joint Committee on Cancer stage | ||||
| I | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| II | 1.98 (1.79 to 2.18)d | 1.98 (1.79 to 2.18)d | 1.26 (1.09 to 1.45)c | 1.26 (1.09 to 1.45)c |
| III | 2.73 (2.53 to 2.95)d | 2.73 (2.53 to 2.95)d | 2.32 (2.02 to 2.67)d | 2.32 (2.02 to 2.67)d |
| Charlson Comorbidity Index score | 1.12 (1.1 to 1.14)d | 1.12 (1.1 to 1.14)d | 1.19 (1.16 to 1.22)d | 1.19 (1.16 to 1.22) |
| Rural medical oncologist | 1.00 (0.83 to 1.2) | 0.95 (0.79 to 1.15) | 1.02 (0.77 to 1.36) | 1.03 (0.77 to 1.37) |
| Rural radiation oncologist | 1.18 (0.97 to 1.43) | 1.16 (0.96 to 1.41) | 1.16 (0.72 to 1.88) | 1.20 (0.74 to 1.96) |
| Rural surgeon | 1.28 (1.03 to 1.59)b | 1.27 (1.02 to 1.58)b | 1.14 (0.91 to 1.42) | 1.13 (0.91 to 1.41) |
| Medical oncologist node strength, bottom 15% | 1.15 (0.82 to 1.63) | 1.06 (0.75 to 1.5) | 1.15 (0.77 to 1.71) | 1.15 (0.76 to 1.72) |
| Radiation oncologist node strength, bottom 15% | 0.87 (0.58 to 1.3) | 0.82 (0.55 to 1.23) | 0.8 (0.42 to 1.51) | 0.81 (0.43 to 1.54) |
| Surgeon node strength, bottom 15% | 1.00 (0.58 to 1.74) | 0.95 (0.55 to 1.66) | 0.86 (0.61 to 1.2) | 0.81 (0.57 to 1.13) |
| Linchpin medical oncologist | N/A | 1.14 (1.03 to 1.28)b | N/A | 0.94 (0.78 to 1.13) |
| Linchpin radiation oncologist | N/A | 1.17 (1.04 to 1.32)b | N/A | 0.93 (0.63 to 1.36) |
| Linchpin surgeon | N/A | 1.1 (0.9 to 1.35) | N/A | 1.22 (1.03 to 1.43)b |
Model also included fixed effects for Surveillance, Epidemiology, and End Results registry and whether the patient saw a medical oncologist, radiation oncologist, or surgeon. N/A = not applicable.
P < .05;
P < .01;
P < .001.
Figure 2.
Sensitivity analyses for a range of linchpin score thresholds for A) lung cancer medical oncologists, B) lung cancer radiation oncologists, and C) colorectal cancer surgeons.
Finally, we used interaction terms to assess whether the effect of linchpin oncologists on patient outcomes depended on whether the oncologist practiced in a nonmetropolitan setting or were peripheral in the patient-sharing network, as captured by low node strength (Supplementary Table 1, available online). We found that risk of mortality among patients with NSCLC treated by a linchpin radiation oncologist is greater among nonmetropolitan (vs metropolitan) radiation oncologists (adjusted HR = 1.43, 95% CI = 1.03 to 1.98, P = .03). No other statistically significant interactions were observed between linchpin status and practice setting or node strength.
Discussion
We found that a network-based measure identifying linchpin oncologists, whose peers lack ties to other oncologists of the same specialty, was often associated with worse survival for patients diagnosed with nonmetastatic NSCLC or CRC in 2016-2017. The associations between linchpin oncologists and survival varied by cancer type and specialty, which may reflect differences in the centralization of care and organization of referral patterns across these dimensions. Examining the extent to which being treated by a linchpin oncologist is associated with clinical outcomes other than survival could uncover potential mechanisms underlying these observations. This work adds to theory-based research on utilization of health-care services from an ecological perspective, which posits that access to health-care resources is influenced by the spatial distribution of individual, environmental, and health system factors (21).
We found that linchpin oncologists (vs nonlinchpin oncologists) were more likely to practice in nonmetropolitan settings, were slightly older, and were peripheral in the network. We also found that linchpin oncologists were more likely to treat patients who were from minority racial and ethnic groups, resided in rural areas, and were socioeconomically disadvantaged, supporting the notion that linchpin status of oncologists may be indicative of limited access to multidisciplinary care because of geographic or other barriers. Our work adds to studies that found that patients with cancer living in areas characterized by lower oncologist density experienced worse outcomes (8,9). Our findings also extend prior work investigating associations between oncologist characteristics and outcomes for patients with cancer, which have often examined surgeon case volume, specialization, experience, and rurality (22,23). It is possible that patient-sharing networks measures, such as linchpin score, interact with practice rurality as well as other oncologist and workforce measures to associate with patient outcomes by varying amounts, depending on their combined levels (eg, the association involving 1 of these variables varies across levels of the other variables). Our study found that nonmetropolitan practice setting can influence the association between linchpin oncologists and patient outcomes. Future work examining factors associated with being a linchpin oncologist in different settings may lead to refinement in how linchpin oncologists are defined (eg, because of scarcity vs other reasons) and a more nuanced understanding of the causes and consequences of oncologists becoming locally unique in their networks.
Our approach—using network analysis to understand access to cancer specialists—has several strengths over existing approaches. Oncologist density is an established measure of the oncology workforce and is often calculated within small areas, such as counties or hospital service areas. Many sparsely populated areas often have no oncologists, however, which is understandable because the alternative would likely lead to oversupply and inefficiency. To address these limitations and innovate how the oncology workforce is measured, linchpin score examines the patient-sharing ties for each physician across a patient-sharing network that is not constrained by small areas. Advantages to our network-based approach for measuring access to care are 1) linchpin score does not require partitioning patients and physicians into specific geographic units, which may not reflect referral patterns nor resources available in neighboring geographic units, and 2) linchpin score does not depend on the geographic units being perfectly nested within the geographic region represented in the data, which may not be feasible in all data sources.
Our study has several limitations, as well. First, our data were limited to fee-for-service Medicare beneficiaries aged 66 years and older in SEER regions, and our results may not generalize to other populations. Second, we are unable to observe all patient-sharing relationships among physicians, but considering that the median age at diagnosis for lung cancer is 71 years and for CRC is 67 years, our patient-sharing networks likely captured most oncologists involved in treating patients with these cancers and the relationships among them. Third, fee-for-service Medicare is characterized by relatively few administrative constraints on access to health-care professionals compared with private insurance and the Medicare Advantage program, suggesting that physician linchpin scores could have greater implications within different coverage settings (eg, settings characterized by more “narrow networks”) (24). Fourth, we were limited to studying a shorter-term survival outcome with the years of data obtained for this study; however, analyses presented both here and in the Supplementary Material (available online) accounted for censoring by the end of follow-up. Fifth, unobserved confounding from unmeasured patient and physician factors is a limitation of this work because of the observational study design. Sixth, the 3 out of 6 associations between linchpin status and mortality that were statistically significant at P < .05 do not remain statistically significant after adjusting for multiplicity. Finally, there is no established threshold by which an oncologist should be considered a linchpin; however, in sensitivity analyses, we considered a range of thresholds, and the strongest associations with survival time tended to occur when linchpins were defined as being within the top 10th to 20th percentiles of linchpin scores for their specialty. This finding suggests that there may be a point toward the upper end of the distribution of linchpin scores that leads to associations with worse outcomes because of particularly sparse patient-sharing networks that is not observed at lower ranges of the distribution.
In conclusion, our findings have important implications for health-care professionals and policymakers working to address anticipated workforce shortages in cancer care. Our investigation of a novel physician-level network measure found that oncologist linchpin score was often associated with worse survival among patients diagnosed with nonmetastatic NSCLC or CRC. Nationwide or regional coordinated efforts to address uneven geographic distributions of specialty care through creating referral pattern guidelines could use network analysis to identify areas particularly vulnerable to workforce shortages or other barriers related to access to multidisciplinary cancer care. For example, in New Zealand, a national gynecologic cancer steering group oversees care coordination and hub-and-spoke referral guidelines to facilitate connections between major comprehensive cancer centers and smaller satellite hospitals (25). Overall, our results suggest that efforts to improve cancer health outcomes through increasing access to cancer care would benefit from considering the underlying structure of patient-sharing networks to ensure that efforts are focused on health systems and physicians practicing in more vulnerable networks.
Supplementary Material
Acknowledgements
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; Information Management Services, Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s National Program of Cancer Registries under cooperative agreement 1NU58DP007156; the National Cancer Institute’s SEER program under contract HHSN261201800032I (awarded to the University of California, San Francisco), contract HHSN261201800015I (awarded to the University of Southern California), and contract HHSN261201800009I (awarded to the Public Health Institute). The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the state of California, Department of Public Health, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors.
Contributor Information
Erika L Moen, Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA; The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA; Dartmouth Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA.
Rachel O Schmidt, The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA.
Tracy Onega, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Department of Population Health Science, University of Utah, Salt Lake City, UT, USA.
Gabriel A Brooks, The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA; Dartmouth Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA; Department of Medicine, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA.
A James O’Malley, Department of Biomedical Data Science, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA; The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA; Dartmouth Cancer Center, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA.
Data availability
The SEER-Medicare linked data underlying this article cannot be shared because of provisions outlined in the Data Use Agreement between the study principal investigator (E.L.M.) and the National Cancer Institute. Researchers interested in obtaining these data can submit a project-specific data request to the National Cancer Institute.
Author contributions
Erika L Moen, PhD, MS (Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Writing—original draft), Rachel O. Schmidt, MS (Formal analysis; Methodology; Writing—review & editing), Tracy Onega, PhD, MPAS, MS (Conceptualization; Methodology; Writing—review & editing), Gabriel A. Brooks, MD (Conceptualization; Methodology; Writing—review & editing), A. James O’Malley, PhD (Conceptualization; Methodology; Writing—review & editing).
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant No. R37CA263936 to E.L.M).
Conflicts of interest
The authors report no conflicts of interest.
References
- 1. Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ.. Unlimited access to care: Effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):25-31. doi: 10.1158/1055-9965.EPI-05-0537 [DOI] [PubMed] [Google Scholar]
- 2. Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL.. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Keefe EB, Meltzer JP, Bethea TN.. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health. 2015;3(51):1-15. doi: 10.3389/fpubh.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel MI, Lopez AM, Blackstock W, et al. Cancer disparities and health equity: a policy statement from the American society of clinical oncology. J Clin Oncol. 2020;38(29):3439-3448. doi: 10.1200/JClinOncol.20.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS.. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179(4):506-514. doi: 10.1001/jamainternmed.2018.7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machado SR, Jayawardana S, Mossialos E, Vaduganathan M.. Physician density by specialty type in urban and rural counties in the US, 2010 to 2017. JAMA Netw Open. 2021;4(1):e2033994.doi: 10.1001/jamanetworkopen.2020.33994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkwood MK, Bruinooge SS, Goldstein MA, Bajorin DF, Kosty MP.. Enhancing the American society of clinical oncology workforce information system with geographic distribution of oncologists and comparison of data sources for the number of practicing oncologists. J Oncol Pract. 2014;10(1):32-38. doi: 10.1200/JOP.2013.001311 [DOI] [PubMed] [Google Scholar]
- 8. Stewart SL, Cooney D, Hirsch S, et al. The effect of gynecologic oncologist availability on ovarian cancer mortality. World J Obstet Gynecol. 2014;3(2):71-77. doi: 10.5317/wjog.v3.i2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aneja S, Yu JB.. The impact of county-level radiation oncologist density on prostate cancer mortality in the United States. Prostate Cancer Prostatic Dis. 2012;15(4):391-396. doi: 10.1038/pcan.2012.28 [DOI] [PubMed] [Google Scholar]
- 10. Weaver SJ, Jacobsen PB.. Cancer care coordination: opportunities for healthcare delivery research. Transl Behav Med. 2018;8(3):503-508. doi: 10.1093/tbm/ibx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultz EM, Pineda N, Lonhart J, Davies SM, McDonald KM.. A systematic review of the care coordination measurement landscape. BMC Health Serv Res. 2013;13(1):119. doi: 10.1186/1472-6963-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nemesure MD, Schwedhelm TM, Sacerdote S, O'Malley AJ, Rozema LR, Moen EL.. A measure of local uniqueness to identify linchpins in a social network with node attributes. Appl Netw Sci. 2021;6(56):1-14. doi: 10.1007/s41109-021-00400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moen EL, Brooks GA, O'Malley AJ, Schaefer A, Carlos HA, Onega T.. Use of a novel network-based linchpin score to characterize accessibility to the oncology physician workforce in the United States. JAMA Netw Open. 2022;5(12):e2245995. doi: 10.1001/jamanetworkopen.2022.45995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLancey JOL, Thun MJ, Jemal A, Ward EM.. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2908-2912. doi: 10.1158/1055-9965.EPI-08-0131 [DOI] [PubMed] [Google Scholar]
- 15. Hashibe M, Kirchhoff AC, Kepka D, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med 2018;7(4):1490-1497. doi: 10.1002/cam4.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT.. Persistent poverty and cancer mortality rates: an analysis of county-level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949-1954. doi: 10.1158/1055-9965.EPI-20-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT.. Enduring cancer disparities by persistent poverty, rurality, and race: 1990-1992 to 2014-2018. J Natl Cancer Inst. 2022;114(6):829-836. Doi: 10.1093/jnci/djac038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. Doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19. Yost K, Perkins C, Cohen R, Morris C, Wright W.. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 20. Yu M, Tatalovich Z, Gibson JT, Cronin KA.. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81-92. doi: 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 21. Ryvicker M. A conceptual framework for examining healthcare access and navigation: a behavioral-ecological perspective. Soc Theory Health. 2018;16(3):224-240. doi: 10.1057/s41285-017-0053-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D.. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin. 2009;59(3):192-211. doi: 10.3322/caac.20018 [DOI] [PubMed] [Google Scholar]
- 23. Bilimoria KY, Phillips JD, Rock CE, Hayman A, Prystowsky JB, Bentrem DJ.. Effect of surgeon training, specialization, and experience on outcomes for cancer surgery: A systematic review of the literature. Ann Surg Oncol. 2009;16(7):1799-1808. doi: 10.1245/s10434-009-0467-8 [DOI] [PubMed] [Google Scholar]
- 24. Yasaitis L, Bekelman JE, Polsky D.. Relation between narrow networks and providers of cancer care. J Clin Oncol. 2017;35(27):3131-3135. doi: 10.1200/JClinOncol.2017.73.2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sykes P, Vaughan M, Chrystal K, et al. Providing care for women with gynaecological malignancy: The need for a coordinated national approach. N Z Med J 2012;125(1360):57-65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The SEER-Medicare linked data underlying this article cannot be shared because of provisions outlined in the Data Use Agreement between the study principal investigator (E.L.M.) and the National Cancer Institute. Researchers interested in obtaining these data can submit a project-specific data request to the National Cancer Institute.