Abstract
The insular cortex is associated with neuropsychiatric symptoms, changes in cardiovascular and autonomic control, and mortality in Alzheimer’s dementia. However, the insular cortex does not provide information on the contribution of the other cortices to cognitive decline. We hypothesized that the factors that affect to atrophy in insular cortex are different from other cortical regions. A total of 42 patients with probable Alzheimer’s dementia were included in the analyses. The manual drawing of regions of interest was used to detect insular cortex located in the deep gray matter and to avoid coatrophy. Covariates, which could affect to the atrophy of the cerebral cortex, were selected based on previous studies. Any of the demographic factors, vascular risk factors, and the severity scales of dementia was not associated with any insular volume ratio. We suggest that the pathomechanisms of atrophy in insular cortex are different from those of other cortex regions in Alzheimer’s disease.
Keywords: Alzheimer’s disease, insular cortex, magnetic resonance imaging, cortical thickness, atrophy
Introduction
As Alzheimer’s disease (AD) progresses, description of brain change including hippocampal atrophy, cortical atrophy in widespread regions, and expansions of the ventricular spaces are considered important imaging findings observed on structural magnetic resonance imaging (MRI). Theses alterations are believed to be promising imaging markers for AD progression and are attributed to the combined effects of neuronal death and intracortical myelin reduction. 1
Insular cortex is in the medial temporal lobe and connected to the hippocampus and entorhinal cortex. Anatomically, the insular cortex is divided by the central insular sulcus (CIS) into 2 parts: the larger anterior insula and the smaller posterior insula. 2 Many studies have reported that the insular cortex is related to variety of processing events, including interoceptive awareness, multimodal sensory integration, emotional processing, and regulation. 2,3 In AD, the insular cortex is associated with neuropsychiatric symptoms (NPS) and distinct changes in cardiovascular and autonomic control and mortality. 4 However, the insular cortex does not provide information on the contribution of the other cortices to cognitive decline, which is a representative manifestation of AD. A prior voxel-based morphometry (VBM) study revealed that gray matter loss in the parietal and cingulated cortices, not the insular cortex, is a feature of AD. 5
We hypothesized that the factors that affect to atrophy in insular cortex are different from other cortical regions in AD, including hippocampus and global cortex. The VBM has advantages in allowing volume analysis across the entire brain; however, VBM cannot comprehensively differentiate changes in tissue content from local misregistration of images and cannot rule out the role of coatrophy. 6,7 The coatrophy in some regions including insular cortex could be a quite influential factor confusing the result of the VBM. Moreover, the insular cortex is situated in deep brain region, where is hard to detect the gray matter. Therefore, the present study was specifically designed to focus in investigating factors that related to the atrophy of insular cortex in patients with AD.
Methods
Patients
We reviewed medical records of patients with dementia from the Konkuk dementia registry between March 2006 and October 2009. The data included all available information such as basic demographic characteristics, other medical conditions (including history of vascular risk factors), results of laboratory tests (including hemoglobin, white cell count, serum electrolytes, glucose, urea, creatinine, liver function tests, thyroid-stimulating hormone, lipid battery [total cholesterol, triglyceride, HDL, and LDL cholesterol], and free thyroid hormone and vitamins B 1, B 6 and B 12), global cognitive assessment (Mini-Mental State Examination [MMSE], Geriatric Depression Scale [GDepS], and Clinical Dementia Rating scale [CDR]), apolipoprotein E (ApoE) genotyping, and brain imaging. Comprehensive neuropsychological assessments were also included, which consisted of the modified Korean version of the Hopkins Verbal Learning Test, the Digit Span Forward and Backward, the Rey-Osterrieth Complex Figure Test, the Korean version of the Boston Naming Test, the Stroop Test and the Word Fluency Test, and the Rey-Osterrieth Complex Figure copy test.
Patients having dementia with probable AD were included. The diagnosis of dementia and probable AD was based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer patients and Related Disorders Association (NINCDS-ADRDA), respectively. 8 All the patients provided written informed consent to use their clinical data obtained in this study for future studies, and this study was approved by the institutional review boards of the Konkuk University Hospital. Among 289 patients in the registry, 80 patients with probable AD were included. Among these patients, 38 patients with incomplete data were excluded. Finally, 42 patients with probable AD were included in the final analyses.
Magnetic Resonance Imaging Image Acquisition and Measurement of the Insula
The MR images were obtained at the Konkuk University Medical Center using a Signa HDx 3.0 T unit (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel high-resolution head coil. The routine MRI protocol included the following sequences: (1) axial and sagittal T1-weighted inversion recovery (repetition time [TR]/echo time [TE]/inversion time [TI], 2468/12/920 ms, respectively; section thickness, 5 mm; matrix, 512 × 224), (2) axial T2-weighted fast spin echo (TR/effective TE, 4000/106 ms; section thickness, 5 mm; matrix 384 × 384), (3) axial fluid-attenuated inversion recovery (FLAIR; TR/TE/TI, 11 000/105/2600 ms; section thickness, 5 mm; matrix, 384 × 224), and (4) axial T2-weighted gradient echo (TR/TE, 550/17 ms; section thickness, 5 mm; matrix, 384 × 224; flip angle, 15°). To analysis cortical volumes, we obtained additional T1-weighted volumetric spoiled gradient recalled echo (TR/TE, 7.3/2.7 ms; section thickness, 1.5 mm; matrix, 256 × 256; flip angle, 13°). The field of view was 230 × 230 mm2.
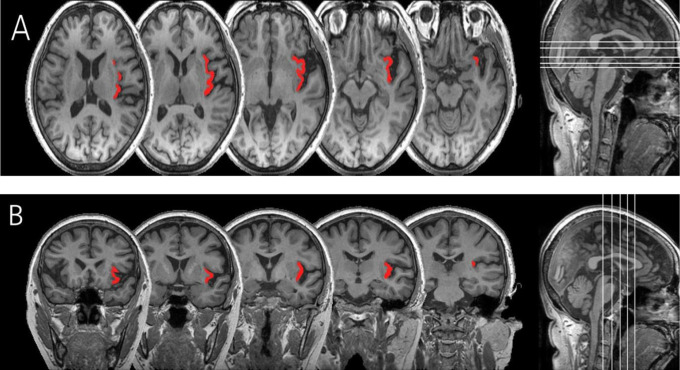
The insular cortex has been subdivided into 2 subregions bilaterally: the right insular cortex (RIC) and left insular cortex (LIC). The CIS was traced at the sagittal slices. The CIS has been well specified. 9 When the CIS was not marked, we used landmarks designating the anterior boundary of the posterior insular cortex as the most relevant lines on 1 slice caudal to mamillary bodies, as described previously. 10 The CIS was regarded as the inferior and superior boundaries of the anterior and posterior insula, respectively. The superior peri-insular sulcus (SPIS) and the anterior peri-insular sulcus (APIS) were outlined to demarcate the superior border and anterior border of insula, respectively. 11 The inferior peri-insular sulcus (IPIS) is the dorsal boundary of the insula, eventually the insula is enclosed by the SPIS, APIS, and IPIS. The fusion of the SPIS with the APIS, and the SPIS and the IPIS were defined as the most caudal and rostral point of the insula, respectively. The vertical plane of the junction of the internal capsule and external capsule in an axial image was considered to be the most anterior border of the insula, and the vertical plane of posterior commissure in a sagittal image demarcated the most posterior border of insula from the surrounding cortical areas. The drawing of regions of interest (ROIs) with each insular cortex was blindly performed without knowledge of the diagnosis and demographics. The ROIs were drawn manually on the consecutive coronal images of MRI following the same criteria described in previous neuroanatomical studies 9,11 using the MRIcro software package (Figure 1).
Figure 1.
Axial (A) and coronal (B) views of left insular cortex of an patient with Alzheimer’s dementia.
Two raters devised training sessions to standardize and unify the boundaries of insular cortex. The drawing of the ROIs was made after obtaining consent about the boundaries of insular cortex using a few selected images. The drawing of the ROIs of each insular cortex was manually investigated by the first author (rater A). An experienced neuroradiologist with an extensive background in neuroanatomy participated as the other rater (rater B) and conducted the same drawing process while also blinded to diagnosis, gender, age, and severity in cases of AD. Ten cases were randomly selected to determine the inter-rater reliability of the measurements. Intraclass correlation coefficients (ICCs) were calculated for the RIC, and PIC from a subset of 10 cases. There was a very good inter-rater reliability, although the sample size was small. For the subregions of the insula in patients with AD, ICCs were 0.93 (95% confidence interval [CI]: 0.81-0.97) on RIC and 0.97 (95% CI: 0.92-0.98) on LIC.
Assessment of Covariates
Intracranial volume (ICV) was calculated to correct for differences in head size. Images were resampled to 1.0 mm3 isovoxels and spatially realigned based on the axis of the anterior–posterior commissural line. After individual segmentation of gray matter, white matter, and cerebrospinal fluid volumes, the segmented subtotals were summed. Image analysis was performed using Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology, University College, London, United Kingdom) in Matlab 7.5 student version (Mathworks, Sherborn, Massachusetts).
Covariates that could affect to the atrophy of the cerebral cortex were selected based on previous studies. 12 Demographic factors include age, sex, handedness, years of education, smoking and drinking status, and body mass index (BMI). The smoking status was assessed as follows: past smokers who had quit smoking for at least 6 months, current smokers, and nonsmokers. The drinking status was classified as daily drinking, weekly drinking, monthly drinking, or never drinking based on previous studies. 12,13 The BMI was calculated as weight in kilograms divided by the square of height in meters with considering age. Vascular factors included hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, myocardial infarction, cerebral infarcts, and white matter hyperintensities (WMHs). Hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and myocardial infarction were based on self-report, medical record, and electrocardiography. Cerebral infarcts included lacunar infarcts and were defined as any cortical infarcts and subcortical infarcts regardless of major dimension. To confirm an infarct, only the lesions that have high signal on T2-weighted and FLAIR images and additional low signal on T1-weighted images were included. The modified rating scale of the WMH proposed by the Clinical REsearch for Dementia Of South Korea (CREDOS) study was applied to evaluate the severity of WMH. The WMH scale is classified into minimal, moderate, and severe according to the periventricular WMH and deep WMH ratings. The T1-weighted axial, T2-weighted axial, and FLAIR images are used for assessing. The rating mechanism for the periventricular WMH and deep WMH is described in elsewhere. 14
Data Analyses
Ratios of bilateral insular cortical volume were cacluated as follows: insular volume/ICV) × 100. Correlation analysis, Mann-Whitney test, and Kruskall Wallis test were used to deduce factors that affected the volume of the insular subregions. SPSS (version 17.0; SPSS Inc, Chicago, Illinois) was used and P value <.05 was considered as the threshold of significance.
Results
Demographic and Clinical Characteristics of Patients
Females were dominant (76.2%), and the mean CDR score was 1.0, indicating that most of the patients were in the mild stage. More than half of the patients reported hypertension (52.4%). The ApoE genotyping was performed in 90.5% of all patients; the results showed that 57.9% of these patients possessed the ApoE ∊4 allele (Table 1). Because only a few patients existed in the following category, we excluded the following covariates to avoid bias: atrial fibrillation, myocardial infarction, current smoking, and daily drinking.
Table 1.
Demographics and Vascular Characteristics.
| Category | Age, Years | Education, Years | MMSE | CDR | CDRSOB | GDepS | BMI |
|---|---|---|---|---|---|---|---|
| Mean (SD) | 71.8 (7.04) | 6.9 (4.95) | 17.2 (6.77) | 1.0 (0.56) | 5.7 (3.20) | 13.3 (6.02) | 24.5 (2.61) |
| Category | Female | Right handed | HTN | DM | HyperL | Cerebral infarcts | SWMH (mild) |
| N (%) | 32 (76.2) | 39 (92.9) | 22 (52.4) | 8 (19.0) | 7 (16.7) | 8 (19.0) | 35 (83.3) |
Abbreviations: MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating scale; CDRSOB, Clinical Dementia Rating scale Sum of Boxes; GDepS, Geriatric Depression Scale; BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; HyperL, hyperlipidemia; SWMH, scale of white matter hyperintensity; SD, standard deviation.
Volume Ratio of Insular Cortex
Total insular cortical volume was 8.37 ± 0.71. Any demographic factor was not associated with ratio of insular cortex. There was no difference in the volume ratio of insular cortex between groups with and without vascular risk factors, including presence of APOE ∊4. The MMSE, CDR, and GDepS scores were not associated with any insular volume ratio (Tables 2 and 3).
Table 2.
Demographic Factors That Affect to the Ratios of the Insular Cortex in Patients With Alzheimer’s Dementia.a,b
| Age | Edu | BMI | MMSE | CDRSOB | GDepS | |
|---|---|---|---|---|---|---|
| RIC | −.245 | .184 | −.289 | .124 | −.199 | .111 |
| LIC | −.100 | .096 | −.153 | .041 | −.170 | .053 |
| Total IC | −.178 | .145 | −.227 | .085 | −.193 | .086 |
Abbreviations: BMI, body mass index; MMSE, Mini-Mental State Examination; CDRSOB, Clinical Dementia Rating scale Sum of Boxes; GDepS, Geriatric Depression Scale; Edu, years of education; RIC, right insular cortex; LIC, left insular cortex; IC, insular cortex.
aValues are Pearson's correlation coefficient (r).
bNone of factors are P < .05.
Table 3.
Difference of insular cortical volume ratio between groups with and without vascular risk factors.a
| Sex | HTN | DM | Hyper lipidemia | Cerebral Infarcts | WMHb | ApoE3 | |
|---|---|---|---|---|---|---|---|
| RIC | .224 | .279 | .386 | .792 | .320 | .370 | .549 |
| LIC | .919 | .102 | .671 | .466 | .403 | .741 | .781 |
| Total IC | .423 | .137 | .765 | .895 | .368 | .574 | .672 |
Abbreviations: HTN, hypertension; DM, diabetes mellitus; WMH, white matter hyperintensity; RIC, right insular cortex; LIC, left insular cortex; IC, insular cortex.
aValues are P value.
bMild WMH versus moderate and severe WMH.
Discussion
Factors Affecting the Volume Ratio of the Insular Cortex
Although the covariates were selected based on previous studies, which could affect to the atrophy of the cerebral cortex, 12 there was no factor affecting the volume ratio of insular cortex. Age is a critical and an independent factor of hippocampus and global cortical thinning. 15,16 Previous studies reported age-related gray matter volume loss mainly located in the frontal lobe, superior temporal, hippocampus, insula, and superior parietal cortices. 16,17 The present study showed negative correlation with age and insular cortical volume; however, there was no significance. Education may increase regional cortical thickness in healthy controls, leading to increased brain reserve. 18 In AD, high levels of education are correlated with cortical thinning in the frontal and temporoparietal association cortices. 15 Volume of hippocampus and global cortex was also associated with levels of education. This supports the cognitive reserve theory, which posits a link between education and the concept of reserve, which enables people with more education to cope better with brain pathology or age-related changes. However, a study about association between insular cortical volume and education reported a negative result, 15 and our study revealed the same results. The most studies about cognitive reserve theory have focused on the specific region, especially the hippocampus. The present findings do not support the brain reserve theory as being applicable to the insular cortex.
In the present study, the severities of AD or depression were not associated with cortical atrophy of insula. As the insular cortex is not the key lesion of memory or cognition, the gray matter atrophy other than the insular cortex could be a feature of AD. 5
Many studies have proven that vascular risk factors are related to the hippocampal or global cortical volume. These factors include hypertension, diabetes mellitus, hyperlipidemia, cardiovascular events, and WMH on MRI scans. 12,19 –23 However, the insular cortex was never been selected as a related region to our knowledge. In the present study, vascular risk factors did not correlate with the volume of the insular cortex. Several explanations are possible to explain why the insular cortex is different from other cortex regions. First, there is the possibility that the dynamics of insular atrophy are already complete in stage of early AD. According to the “Braak and Braak staging” system, the destructive process begins in the entorhinal cortex, thereafter invading other portions of the cerebral cortex. 24 The insular cortex and medial temporal region are the structures involved at early stage. 25 As atrophy of the insular cortex has already occurred in the asymptomatic period, the vascular factors would not affect the insular atrophy in AD. Second, the insular cortex could be a region that is resistant to vascular insults. However, there is no evidence that the integrity of the blood–brain barrier is much stronger than other regions. Although the insular cortex is supplied by multiple branches (superior trunk, inferior trunk, prefrontal, precentral, central, and angular arteries of cortical branch) of MCA, 26 there is no evidence that the insular cortex has appreciably more abundant vascular supply. Third, the number of patients with vascular risk factors in the patient group may have been too small, except for hypertension, so the statistical analysis could be incomplete. However, the result was not different in case of hypertension, which included 52.4% of patients.
The ApoE ∊4 allele is a risk factor for AD, but its affect on brain volumes is controversial. In most volumetric studies, the presence of ApoE ∊4 allele has been associated with smaller brain volume. 27,28 Recent VBM studies reported that the effect of ApoE ∊4 allele on brain structures is not limited to the hippocampus, although many discrepancies remain. 29,30 However, several studies have shown no effect of ApoE ∊4 allele on whole brain volume. 31,32 We showed that the effect of ApoE ∊4 allele was not related to the volume ratio of any insular cortex. As sex could be a modulate factor to the regional cortical volume, 33 we did subanalyses according to sex, but the results did not reveal any differences.
The present study has some limitations that the number of normal controls was relatively small compared to the number of patients. However, the normal controls were verified as normal distribution. This research revealed the negative data, which could be an another limitation. Nevertheless, this finding means that the insular cortex might be an early imaging marker not influenced by multiple factors.
In conclusion, our study shows that the pathomechanisms of atrophy in insular cortex are different from other cortex regions in AD, including hippocampus and global cortex. Demographic factors, vascular risk factors, the presence of ApoE ∊4 allele, and the severity of AD did not affect to insular atrophy as much as to other cortices.
Acknowledgments
The authors thank Hui Jin Ryu, MA, Min Young Kim, MA, Chung-Hwan Kang, RT, and the Clinical Research Center for Konkuk University Medical Center Memory Clinic for their support and guidance in the neuropsychological evaluation of patients and imaging data acquisition and management of this study. Most importantly, the authors thank all those who participated in the study for their dedication to helping research in dementia.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ewers M, Frisoni GB, Teipel SJ, et al. Staging Alzheimer's disease progression with multimodality neuroimaging. Prog Neurobiol. 2011;95(4):535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457(2):66–70. [DOI] [PubMed] [Google Scholar]
- 3. Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur Psychiatry. 2007;22(6):387–394. [DOI] [PubMed] [Google Scholar]
- 4. Royall DR. Insular Alzheimer disease pathology and the psychometric correlates of mortality. Cleve Clin J Med. 2008;75(suppl 2):S97–S99. [DOI] [PubMed] [Google Scholar]
- 5. Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23(2):708–716. [DOI] [PubMed] [Google Scholar]
- 6. Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128 (pt 11):2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitwell JL. Voxel-based morphometry: An automated technique for assessing structural changes in the brain. J Neurosci. 2009;29(31):9661–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 9. Naidich TP, Kang E, Fatterpekar GM, et al. The insula: Anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol. 2004;25(2):222–232. [PMC free article] [PubMed] [Google Scholar]
- 10. Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60(11):1069–1077. [DOI] [PubMed] [Google Scholar]
- 11. Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83 (2–3):155–171. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76(17):1485–1491. [DOI] [PubMed] [Google Scholar]
- 13. Zhou H, Deng J, Li J, Wang Y, Zhang M, He H. Study of the relationship between cigarette smoking, alcohol drinking and cognitive impairment among elderly people in china. Age Ageing. 2003;32(2):205–210. [DOI] [PubMed] [Google Scholar]
- 14. Noh Y, Lee Y, Seo SW, et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J Stroke Cerebrovasc Dis. 2014;23(4):636–642. [DOI] [PubMed] [Google Scholar]
- 15. Seo SW, Im K, Lee JM, et al. Effects of demographic factors on cortical thickness in Alzheimer's disease. Neurobiol Aging. 2011;32(2):200–209. [DOI] [PubMed] [Google Scholar]
- 16. Taki Y, Thyreau B, Kinomura S, et al. A longitudinal study of age- and gender-related annual rate of volume changes in regional gray matter in healthy adults. Hum Brain Mapp. 2013;34(9):2292–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalpouzos G, Chetelat G, Baron JC, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30(1):112–124. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Julkunen V, Paajanen T, et al. Education increases reserve against Alzheimer's disease-evidence from structural MRI analysis. Neuroradiology. 2012;54(9):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ. Serum lipids and hippocampal volume: The link to Alzheimer's disease? Ann Neurol. 2004;56(5):745–748. [DOI] [PubMed] [Google Scholar]
- 20. Seo SW, Lee JM, Im K, et al. Cardiovascular risk factors cause cortical thinning in cognitively impaired patients: Relationships among cardiovascular risk factors, white matter hyperintensities, and cortical atrophy. Alzheimer Dis Assoc Disord. 2012;26(2):106–112. [DOI] [PubMed] [Google Scholar]
- 21. den Heijer T, Schuit SC, Pols HA, et al. Variations in estrogen receptor alpha gene and risk of dementia, and brain volumes on MRI. Mol Psychiatry. 2004;9(12):1129–1135. [DOI] [PubMed] [Google Scholar]
- 22. den Heijer T, Launer LJ, Prins ND, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64(12):263–267. [DOI] [PubMed] [Google Scholar]
- 23. Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55(11):1449–1455. [DOI] [PubMed] [Google Scholar]
- 24. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 25. Ye BS, Seo SW, Yang JJ, et al. Comparison of cortical thickness in patients with early-stage versus late-stage amnestic mild cognitive impairment. Eur J Neurol. 2014;21(1):86–92. [DOI] [PubMed] [Google Scholar]
- 26. Mavridis I, Boviatsis E, Anagnostopoulou S. Exploring the neurosurgical anatomy of the human insula: a combined and comparative anatomic-radiologic study. Surg Radiol Anat. 2011;33(4):319–328. [DOI] [PubMed] [Google Scholar]
- 27. DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30(8):1548–1553. [DOI] [PubMed] [Google Scholar]
- 28. Wahlund LO, Julin P, Lannfelt L, Lindqvist J, Svensson L. Inheritance of the ApoE epsilon4 allele increases the rate of brain atrophy in dementia patients. Dement Geriatr Cogn Disord. 1999;10(4):262–268. [DOI] [PubMed] [Google Scholar]
- 29. Lemaitre H, Crivello F, Dufouil C, et al. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24(4):1205–1213. [DOI] [PubMed] [Google Scholar]
- 30. Pievani M, Rasser PE, Galluzzi S, et al. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer's disease in vivo. Neuroimage. 2009;45(4):1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drzezga A, Grimmer T, Henriksen G, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72(17):1487–1494. [DOI] [PubMed] [Google Scholar]
- 32. Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: Six-year follow-up of normal subjects. Neurology. 2005;64(10):1704–1711. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Paajanen T, Westman E, et al. Effect of APOE epsilon4 allele on cortical thicknesses and volumes: The AddNeuroMed study. J Alzheimers Dis. 2010;21(3):947–966. [DOI] [PubMed] [Google Scholar]