Abstract
Montreal Cognitive Assessment (MoCA) test has been shown to be a reliable tool to detect mild cognitive impairment (MCI), however, no Georgian language version exists. The goal of this study is to determine the validity, reliability, and accuracy of Georgian version of MoCA in the evaluation of amnestic MCI (aMCI) and Alzheimer’s disease (AD). Montreal Cognitive Assessment was translated into Georgian language and was administered to healthy participants (HP) and patients with aMCI and AD. We studied 46 HS, 20 patients with aMCI, and 20 patients with AD. There was significant difference in MoCA scores between HP, patients with aMCI, and patients with AD (P = 0.04). The area under the receiver operating characteristic curve for the aMCI and AD groups by MoCA was 0.88 and 0.95, respectively, compared to 0.43 and 0.67 by Mini-Mental State Examination (MMSE). The Georgian version of MoCA is a valid, reliable, and sensitive screening tool to detect aMCI and AD in Georgian-speaking population and is superior to MMSE.
Keywords: MoCA, mild cognitive impairment, Alzheimer’s disease, Georgian language
Introduction
The prevalence of dementia is increasing in both developing and developed countries. 1 Over the past 2 decades, development of screening tools for the identification of early stages of cognitive impairment became an important goal for health-care providers as timely initiation of preventive interventions may potentially delay the development of clinically overt degenerative dementia. 2 Several definitions have been used to determine a threshold and differences between normal age-associated minor cognitive changes and dementia. The term “mild cognitive impairment” (MCI) was introduced to define this state and has been extensively studied in the last 2 decades. 2,3
Neuropsychological testing may be helpful to distinguish MCI from normal aging, but thus far there is no single test to diagnose this condition. 3 Mini-Mental State Examination (MMSE) has been used worldwide including Georgia as a brief screening test that quantitatively assesses the severity of cognitive impairment and documents cognitive changes occurring over time. 4 -6 However, MMSE is often insensitive to detect MCI. 2,3 Montreal Cognitive Assessment (MoCA) test was introduced about 20 years ago and showed higher specificity and sensitivity to detect MCI compared to MMSE. 7 -9 In addition, MoCA compared to MMSE is more sensitive to detect executive dysfunction. 9 Although MoCA is translated and adapted into 55 languages and dialects, no Georgian version exists. 7
Georgia is a country bounded to the west by the Black Sea, to the north by Russia, to the south by Turkey and Armenia, and to the southeast by Azerbaijan. Georgian is a Kartvelian language spoken by Georgians and is the most pervasive of the family of Kartvelian languages. Georgian is written in its own Georgian scripts that is unique in their appearance and consists of a 33-letter alphabet.
Given the lack of a reliable tool to screen for MCI and dementia, we translated and adopted MoCA into Georgian language. The goal of this study is to evaluate psychometric validity of Georgian version of MoCA.
Methods
Georgian MoCA
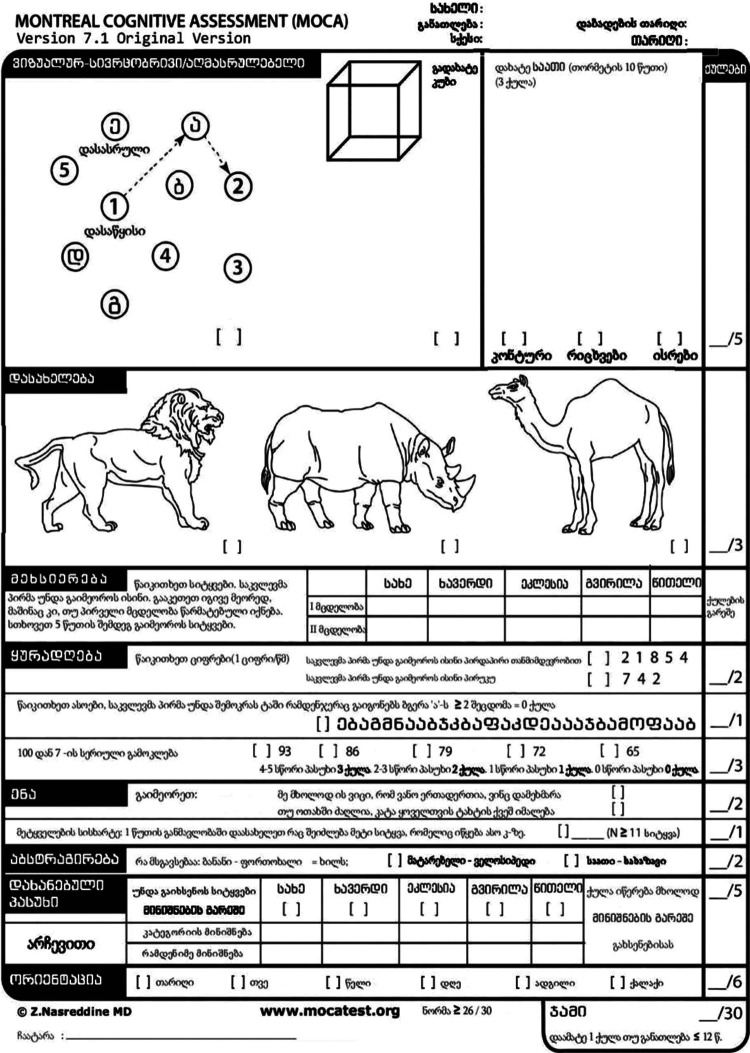
Translation and adaptation of MoCA was performed in full concordance with International Test Commission Guidelines. 10 Figure 1 shows the MoCA translated into the Georgian language. In the Georgian version of the test, only 2 minor changes were made: (1) In the first sentence of the first item of verbal fluency domain, the English name John was replaced by the common Georgian name Vano, which is Georgian version of Ivan or Johan or John. (2) In the second point of verbal fluency domain, where a study subject has to name as many words beginning with “F” as he/she can during 1 minute, we changed “F” into “K.” We chose this letter because the number of words beginning with “K” in Georgian is equivalent to the number of words beginning with “F” in English.
Figure 1.
Georgian MoCA. MoCA indicates Montreal Cognitive Assessment.
Subjects
The patients and healthy participants (HP) were recruited from the outpatient clinic of Department of Neurology, Khechinashvili University Hospital, Tbilisi, Georgia, from March 1, 2014, to March 1, 2015. Local ethics committee of the Khechinashvili University Hospital approved the study protocol and informed consent form. All study participants or caregivers (in case of dementia) signed approved consent form.
Interview, neurological examination, and neuropsychological testing were conducted by board-certified neurologists. Amnestic MCI (aMCI) was diagnosed based on Petersen diagnostic criteria, 11 and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria was used for the diagnosis of Alzheimer’s disease (AD). 12
Healthy participants were recruited from the random sample of the family members accompanied by patients visiting the aforementioned outpatient clinic who agreed to participate and signed informed consent. No attempt was made to match for age, except recruiting subjects who were older than 50 years. All HP were interviewed for medical history, and those with a known history of cardiovascular disease (hypertension, coronary artery disease, and atrial fibrillation), diabetes mellitus, and neurological and psychiatric disorders were excluded. No medical assessment of HP was performed.
As per original MoCA description, 1 point was added to MoCA’s total score for the persons with education ≤12 years. 7 To establish retesting reliability, n = 30 subjects (n = 10 from each group including HP, aMCI, and AD) were repeatedly tested after 3 weeks from the first investigation using MoCA and MMSE. Sensitivity and specificity were calculated to assess the diagnostic accuracy of the MoCA based on the recommended cutoff score of <26 points for MCI and <24 for dementia. 7
Statistical Analysis
Values were reported as mean (±standard deviation [SD]), percentage, or median with interquartile range (25-75 percentile), and nonparametric tests (Mann-Whitney U or χ2) were performed to compare the distributions or classifications of variables as appropriate. Cronbach α was computed to measure internal consistency of MoCA test. Receiver operating characteristic (ROC) analysis was used to assess the ability of the Georgian MoCA to compare with the MMSE. Larger area under curves indicated better diagnostic performance. Sensitivities, specificities, positive predictive values, and negative predictive values were measured at cutoff scores. P < .05 was considered statistically significant throughout the analysis. SPSS version 22 was used for statistical analysis.
Results
We studied 86 subjects: 46 HP without cognitive impairment, 20 patients with aMCI, and 20 patients with AD. Table 1 shows demographic and neuropsychological characteristics of these 3 groups. Healthy participants were significantly younger compared to patients with aMCI and AD (P = .04). Although there was female predominance in the control group, this difference was not statistically significant (P = .9). There was no difference in years of education between the 3 groups (P = .5).
Table 1.
Demographical and Neuropsychological Characteristics of Study Cohort.
| Variables | Controls (n = 46) | aMCI (n = 20) | AD (n = 20) | P |
|---|---|---|---|---|
| Age, years | 57.7 ± 10.8 | 62.8 ± 11.5 | 70.5 ± 6.5 | .04 |
| Female, % (n) | 67.4 (31) | 25.0 (5) | 45 (9) | .9 |
| Education, years | 11.5 ± 0.5 | 11.6 ± 0.5 | 11.7 ± 0.5 | .5 |
| MoCA | 26.3 ± 2.5 | 19.2 ± 1.8 | 11.7 ± 3.9 | .04 |
| MMSE | 29.6 ± 0.8 | 26.8 ± 1.9 | 18.4 ± 6.3 | .04 |
Abbreviations: AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment.
There was statistically significant (P = .04) difference in MoCA scores between HP (26.3 ± 2.5), patients with aMCI (19.2 ± 1.8), and patients with AD (11.7 ± 3.9). Observed difference remained significant after adjustment for age (P = .043), as well as after adjustment for age, gender, and level of education (P = .045).
Mini-Mental State Examination scores were significantly (P = .041) different between the groups, showing the same trend with highest scores documented in HP (29.6 ± 0.8) followed by aMCI (26.8 ± 1.9) and AD groups (18.4 ± 6.3). This difference remained significant after adjustment for age (P = .041), as well as after adjustment for age, gender, and level of education (P = .041).
Test–retest reliability data were collected from a subsample of HP (n = 10) and patients with aMCI and AD (n = 10). Statistical analysis revealed good internal consistency of repeated MoCA testing in all 3 groups including HP (Cronbach α 0.86), patients with aMCI (Cronbach α 0.92), and patients with AD (Cronbach α 0.89).
Area under the ROC curve for the aMCI and AD groups by MoCA was 0.88 and 0.95 (Figures 2 and 3), while corresponding values by MMSE were 0.43 and 0.67, respectively (Figures 2 and 3). Table 2 shows the sensitivities, specificities, positive predictive values, and negative predictive values of the Georgian MoCA at different cutoff values. The recommended MoCA cutoff value of 26 points showed excellent sensitivity (100%) to detect aMCI, however, specificity of this cutoff was low (44%). The optimal cutoff value for the Georgian MoCA appears to be 22, as the sensitivity of the Georgian MoCA for aMCI screening is 100% and specificity 69%.
Figure 2.
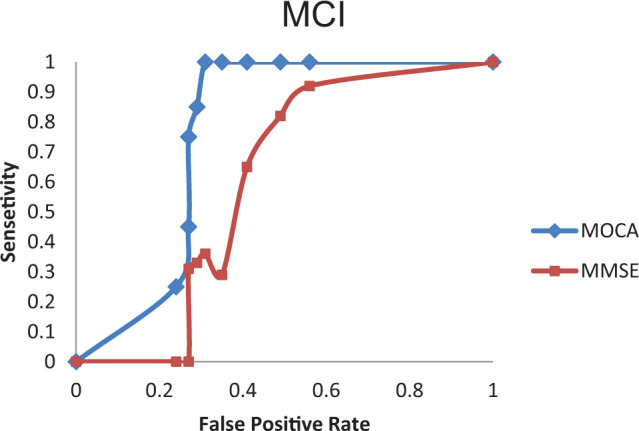
Receiver operating characteristic (ROC) curve analysis of the Mini-Mental State Examination (MMSE) and the Georgian version of the Montreal Cognitive Assessment (MoCA) to detect mild cognitive impairment (MCI).
Figure 3.
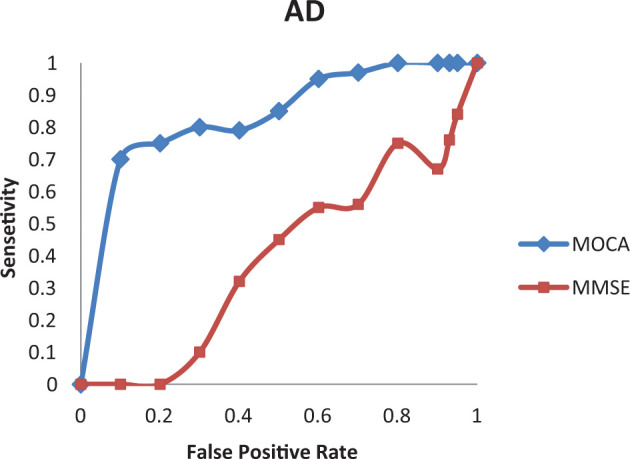
Receiver operating characteristic (ROC) curve analysis of the Mini-Mental State Examination (MMSE) and the Georgian version of the Montreal Cognitive Assessment (MoCA) to detect Alzheimer’s disease (AD).
Table 2.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of the Georgian Version of the Montreal Cognitive Assessment for Detection of Amnestic Mild Cognitive Impairment.
| Cutoff | Sens | Spec | PPV | NPV |
|---|---|---|---|---|
| 18 | 0.25 | 0.76 | 0.27 | 0.74 |
| 19 | 0.45 | 0.73 | 0.37 | 0.78 |
| 20 | 0.75 | 0.73 | 0.50 | 0.89 |
| 21 | 0.85 | 0.71 | 0.51 | 0.93 |
| 22 | 1.00 | 0.69 | 0.54 | 1.00 |
| 23 | 1.00 | 0.65 | 0.48 | 1.00 |
| 24 | 1.00 | 0.59 | 0.46 | 1.00 |
| 25 | 1.00 | 0.51 | 0.42 | 1.00 |
| 26 | 1.00 | 0.44 | 0.39 | 1.00 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Discussion
In this study, we have shown validity of MoCA as a screening tool to detect aMCI and AD in Georgian-speaking population. Translated Georgian version of MoCA will be 56th language adopting this test.
Our data show statistically significant difference in MoCA and MMSE scores between healthy individuals, patients with aMCI, and patients with AD, which is not unexpected finding and is compatible with previous reports. 7,13 Mini-Mental State Examination scores in Georgian population including healthy individuals and patients with aMCI were compatible to Trzepacz et al 13 ; however, patients with AD in our cohort had lower MMSE, probably reflecting more advance disease. 13 Although Georgian-speaking healthy individuals’ MoCA scores were similar to those reported by Trzepacz et al, 13 patients in the current study with aMCI showed about 3-point and those with AD about 5-point lower scores. 7,13 One possible explanation for this discrepancy is education, which was on average 5 years less in our patients with aMCI and AD. 13 Alternatively, lower MMSE scores in our patients with AD may indicate probably more advanced disease.
Georgian version of MoCA revealed good internal consistency (Cronbach α range 0.8-0.9) of repeated MoCA testing in all 3 groups including healthy persons, patients with aMCI, and patients with AD.
This study shows better performance of Georgian MoCA compared to MMSE to detect aMCI and AD. An area under the curve for the MCI and AD groups by MoCA was 0.88 and 0.95 compared to 0.43 and 0.67 by MMSE, respectively. This is in agreement with previous studies of English- and non-English-speaking populations confirming the advantage of MoCA for the screening of aMCI and dementia of different etiologies. 9,13,14,15
In this study, recommended MoCA cutoff score <26 to detect aMCI showed excellent sensitivity of 100%, which is similar to 90% originally reported by Nasreddine et al. 7 However, specificity was around 44%, which is much lower compared to originally reported 87%. 7 It is possible that this cutoff score <26 is not perfect as the data from the subsequent studies showed that Nasreddine et al’s original paper did not reflect a typical MCI population. 16 -19 We found that lower cutoff score of 22 was optimal to detect aMCI as it showed 100% sensitivity and 69% specificity. This is similar to reported MoCA results in Korean and US African American populations. 14,15 In addition to cultural differences, one possible explanation of this finding is the fact that in the current study, an average number of years in full-time education was 2 years lower compared to the cohort of Nasreddine et al. 7
One possible limitation of this study is relatively small sample size. However, even with this small sample size, we were able to show validity and reliability of Georgian MoCA for the screening of aMCI and AD in Georgian-speaking population in addition to its superiority compared to MMSE. Although these results are generalizable to Georgian-speaking patients with aMCI and AD, it may not be generalizable to the patients with vascular cognitive impairment or other types of degenerative dementias. Population-based study is in progress and we will have more normative data, which will allow better determination of cutoff values for the screening of MCI and dementia.
In conclusion, Georgian MoCA is a valid and reliable test, which can be used in general practice for evaluation of aMCI and AD.
Footnotes
Authors’ Note: All authors made substantial contributions to the conception and design of the study and analysis and interpretation of data. Marina Janelidze contributed to study concept and design, drafting of the paper, and revising it critically for intellectual content. Nino Mikeladze contributed to data acquisition, analysis and interpretation of data, drafting of the paper, and revising it critically for intellectual content. N. Bochorishvili contributed to data acquisition, drafting of the paper, and revising it critically for intellectual content. A. Dzagnidze contributed to data acquisition, drafting of the paper, and revising it critically for intellectual content. M. Kapianidze contributed to data acquisition, drafting of the paper, and revising it critically for intellectual content. N. Mikava contributed to data acquisition, drafting of the paper, and revising it critically for intellectual content. I. Khatiashvili contributed to data acquisition, drafting of the paper, and revising it critically for intellectual content. D. Kakhiani contributed to data acquisition, drafting the paper, and revising it critically for intellectual content. E. Mirvelashvili contributed to study design and statistical analysis of the data. N. Shiukashvili contributed to statistical analysis of the data. Zurab Nadareishvili as a senior author contributed to study concept and design, analysis and interpretation of data, drafting of the paper and revising it critically for intellectual content, preparation of the manuscript, and final approval.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Rustaveli Georgian National Scientific Foundation, Grant N DI/16/8-313/13, Tbilisi, Georgia.
References
- 1. Kalaria RN, Maestre GE, Arizaga R, et al. ; World Federation of Neurology Dementia Research Group. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Portet F, Ousset PJ, Visser PJ, et al. ; MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC). Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006; 77(6):714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. [DOI] [PubMed] [Google Scholar]
- 4. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 5. Tsiskaridze A, Shakarishvili R, Janelidze M, Vashadze T, Chikhladze M. Cognitive correlates of leukoaraiosis in the early stages of Alzheimer’s disease. Funct Neurol. 1998;13(1):17–25. [PubMed] [Google Scholar]
- 6. Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. [DOI] [PubMed] [Google Scholar]
- 7. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 8. Tsoi KF, Chan JC, Hirai HW, Wong SS, Kwok TY. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450–1458. [DOI] [PubMed] [Google Scholar]
- 9. Dong Y, Sharma VK, Chan BP, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. 2010;299(1-2):15–18. [DOI] [PubMed] [Google Scholar]
- 10. International Test Commission. International Guidelines on Test Adaptation, 2005. www.intestcom.org. Accessed June, 2016. Updated September, 2016.
- 11. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 12. American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- 13. Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ; Alzheimer’s Disease Neuroimaging Initiative. Relationship between the Montreal Cognitive Assessment and Mini-Mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JY, Lee DW, Cho SJ, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21(2):104–110. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein FC, Ashley AV, Miller E, Alexeeva O, Zanders L, King V. Validity of the montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. J Geriatr Psychiatry Neurol. 2014;27(3):199–203. [DOI] [PubMed] [Google Scholar]
- 16. Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197–201. [DOI] [PubMed] [Google Scholar]
- 17. McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the Montreal Cognitive Assessment (MoCA) as a screening test for mild cognitive impairment (MCI) in a cardiovascular population. J Geriatr Psychiatry Neurol. 2011;24(1):33–38. [DOI] [PubMed] [Google Scholar]
- 18. Larner AJ. Screening utility of the Montreal Cognitive Assessment (MoCA): in place of—or as well as—the MMSE? Int Psychogeriatr. 2012;24(3):391–396. [DOI] [PubMed] [Google Scholar]
- 19. Freitas S, Simoes MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1): 37–43. [DOI] [PubMed] [Google Scholar]