Abstract
Mild cognitive impairment (MCI) is a dynamic state between normal cognition and dementia, where interventions can be taken to stop or delay the progression to dementia. It is broadly of 2 types—amnestic, where memory loss is the chief concern and nonamnestic, where it is not. One variant of nonamnestic, dysexecutive, being more prevalent is sometimes known as a separate subtype by itself. Diagnosis of MCI is mostly clinical and is aided by various scales and neuropsychological testing. Functional imaging studies help in early detection and is superior to biomarkers or structural magnetic resonance imaging. Although there is no evidence supporting any pharmacological intervention, cognitive rehabilitation, memory training, and caregiver support play a strong role in limiting and sometimes reversing the ongoing cognitive decline. As the spectrum of MCI is heterogeneous, making the right diagnosis can be a challenging; hence, we need a systematic yet cost-effective algorithm for the timely management of MCI.
Keywords: mild cognitive impairment, amnestic, nonamnestic, dysexecutive, functional imaging, neuropsychological testing
Introduction
Mild cognitive impairment (MCI) outlines dysfunction in several mental processes such as memory, learning, attention, language, executive control, and decision making. Over the years, many clinicians have postulated their ideas about cognitive dysfunctions related to MCI and dementia. Kral 1 in 1962 designated the term “senescent forgetfulness” to describe memory loss in the elderly population. More than 2 decades later, National Institute of Mental Health Work Group 2 associated “Age-Associated Memory Impairment” with healthy, nondemented, persons older than 50 years of age—who have been experiencing declining memory since they were young adults. International Psychogeriatric Association proposed “age-associated cognitive decline” in 1994 3 to refer to the pathology of multiple cognitive domains declining with normal aging. The Canadian Study of Health and Aging, 4 in 1997, used the term “cognitive impairment no dementia” to characterize an intermediate cognitive dysfunction that doesn’t constitute dementia.
The term MCI was coined by Reisberg and colleagues 5 in 1988 to describe a transitional state between normal cognition and dementia, which may turn to full blown dementia in the future. The MCI can hence serve as the clinical stage in which meaningful interventions can be initiated to avoid or delay the onset and/or severity of the impending dementia. In 1999, Petersen and colleagues 6 defined patients with MCI as nondemented individuals who have documented memory impairments that is inconsistent for their age and yet have normal performance in nonmemory cognitive territories and in the activities of daily living. These researchers also found that patients with MCI displayed deficits confined to poor performance on memory tests, while the patients with Alzheimer's Disease (AD) displayed a wider array of cognitive deficits. 6 The concept of MCI has undergone radical changes since then, and this article attempts to provide a comprehensive review on the same.
Clinical Criteria for MCI
The clinical criteria for MCI has been a subject of a great deal of study, validation, and criticism. In 2004, revised International Working Group criteria 7 expanded the preexisting concept of MCI to include objective and subjective impairments in any of the several cognitive domains. Later in 2004, Petersen revised his earlier criteria and redefined MCI as a state of decline in cognition, which is not dementia yet is not normal for the given age and does not amount to significant impairments in functional activities. 8 He further divided MCI into 2 clinical phenotypes of amnestic MCI (aMCI) and nonamnestic MCI (naMCI)—with the presence or absence of memory impairment. In 2009, Jak and colleagues 9 developed a “comprehensive neuropsychological criteria” for identification of multiple subtypes of MCI that required at least 2 performances within a cognitive domain to fall >1 standard deviations (SDs) below the established cutoff. This criteria revealed a better balance of sensitivity and specificity to detect impairment than the earlier ones. These newer practices are further discussed in the clinical subtype section of this article.
Epidemiology of MCI
The Mayo Clinic Study of Aging 10 in 2008 had estimated 15% prevalence of MCI in nondemented persons aged older than 70 years. They also reported that aMCI is twice as common as naMCI. However, latest research by Libon at al 11 suggests that patients with MCI presenting solely with primary anterograde amnesia or a dysexecutive state, that is, single domain MCI, constitute the minority of cases, while multiple-domain MCI (mxMCI) is the most prevalent presentation. The MCI prevalence in the population varies across various studies per the clinical definitions used, 12 –20 although the rates generally converge in the range of 14% to 18% for individuals aged 70 years or older. 20
Regardless of the definition, those who have MCI generally progress to dementia in higher proportions than the cognitively normal individuals, 13 –20 although there is still a degree of inconsistency regarding who is going to develop dementia. The MCI is also related to higher health-related resource utilization. Results of a recent study obtained from multivariate analyses of longitudinal data show that, after controlling for participant and informant characteristics, direct medical costs were 44% higher for participants with MCI than for those without. 21 Participants with MCI were almost 5 times as likely to use informal care as those without. 21
Various clinical studies, both cross-sectional and retrospective, have examined a wide number of putative risk factors for MCI. 6,22 –31 Male gender, 22 African American race, 23 increased age, 6,23 –25 lower education level, 22 –25 apolipoprotein E (APOE) ∊4 genotype, 22,23,25 lack of physical exercise, 26 smoking, 27 a diet high in processed food, 28 carbohydrate-rich diet, 29 and comorbidities like hypertension, 24 hypercholesterolemia, 30 diabetes, 24 chronic renal failure, 31 and depression 23 have been identified to be associated with MCI.
Clinical Subtypes of MCI
Conventional Criteria—aMCI and naMCI
Amnestic MCI
It is the most common subtype of MCI 9 and is often thought as a precursor to AD. These patients have episodic memory score more than 1.5 SDs below than that of age-appropriate normal individuals. 8
Single domain—these patients have only memory complaints and objective memory impairment with preserved general cognitive function. They neither have problems with activities of daily life nor do they qualify for dementia.
Multiple domain—these persons have main complaint of memory loss but they also have additional subtle impairments in other cognitive functions, which may be impaired in the range of 0.5 to 1 SD below than that of age- and education-matched controls. 8 These other impairments may be revealed with careful neuropsychological (NP) testing. Such patients may have subtle problems with activities of daily living but do not qualify for dementia.
Nonamnestic MCI
Single domain—these patients do not have a memory complaint and have isolated impairment in a nonmemory domain such as execution, language, visuospatial skills, and so forth. Depending on the domain, these patients can progress to frontotemporal dementia, primary progressive aphasia, dementia with Lewy body disease, or vascular dementia, and so forth.
Multiple domain—these patients have subtle impairments in multiple nonmemory domains of cognition.
Comprehensive NP Classification
The conventional classification system had its flaws. The dichotomous “amnestic” or “nonamnestic” scheme often obscured groups with unique yet important patterns of impairment and cannot adequately capture the heterogeneity of MCI. Also, findings from a recent study 32 illustrated that one of the subtypes produced by the conventional criteria, single domain naMCI, mostly performed within normal limits, suggesting its susceptibility to false-positive diagnostic errors. Hence in 2009, a comprehensive NP classification system was introduced, 9 which further provided 4 neuropsychologically distinct MCI clusters 32 mentioned subsequently:
Amnestic —impairments on measures of recall and recognition.
Mixed —impairments on multiple measures of recall and recognition, language, executive function, and visuospatial functioning.
Dysexecutive —impaired attention, executive functioning (including verbal fluency), and visuospatial functions, but intact memory performance.
Visuospatial—impairment on 1 measure of visual construction (block design).
Dysexecutive MCI
Over the years, another distinct variety of MCI has cropped up, known as the dysexecutive MCI (dMCI), which is essentially a naMCI having predominant executive dysfunction. 11,33 Studies have shown that dMCI is less likely to involve other areas of cognition over time or progress to dementia. 34 Also, patients with mxMCI with executive dysfunction who progressed to dementia were less likely to have an Alzheimer's-type dementia and more likely to experience a stroke, than patients with MCI without executive dysfunction. 34 Compared to the patients with stable dMCI, the patients with dMCI who progress to dementia showed brain atrophy in the bilateral insula and left lateral temporal lobe on magnetic resonance imaging (MRI). 35 Patients with dMCI converting to dementia are also older, have lower baseline performance on category fluency and a spatial location task, and report fewer symptoms. 35
The dMCI is classically differentiated from aMCI with the help of NP testing and imaging modalities. The dMCI, when compared with control participants, has significantly lower scores on the majority of executive function tests and has increased behavioral symptoms and left prefrontal cortex atrophy on MRI. 11 In contrast, aMCI has significantly lower scores on tests of memory and a pattern of atrophy including bilateral hippocampi and entorhinal cortex, right inferior parietal cortex, and posterior cingulate gyrus when compared with control participants. 11 A recent study has even implied that electroencephalography
alone can be a much better parameter than NP in distinguishing dMCI from mx-aMCI. 36
Mild Cognitive Impairment Symptoms and Clinical Diagnosis
Symptoms experienced by patients with MCI can be broadly classified as cognitive and neuropsychiatric. While some degree of cognitive decline is part of normal aging, patients with MCI exhibit pronounced decline in processing speed and executive control tasks 37 and significant memory impairment. Sometimes the subjective memory complains can predict the overall cognitive decline accurately 38,39 while at other times it comes forward as a nonspecific criterion. 40 Patients with MCI unlike their AD counterparts are more aware of their symptoms and are also more troubled by them. 41 Patients with MCI progressing to dementia report lesser perception of symptoms 42 and hence this can be used as an indicator to identify worsening disease.
Mood and behavioral symptoms too are common in MCI. 43 Depression is probably the most common neuropsychiatric symptom followed by irritability, anxiety, aggression, and apathy. 44,45 Patients with these behavioral symptoms often have more severe cognitive impairment than those without it 43,45 and patients with depression having MCI progress to AD at a higher rate than those without depression. 46 However compared to patients with MCI, patients with AD have both higher frequency and severity of neuropsychiatric symptoms. 47
A variety of methods are used to diagnose MCI including the Clinical Dementia Rating (CDR) scale. 48 This is a semistructured interview with the patient and the caregiver that assesses memory, along with orientation, judgement, and problem solving, knowledge of community affairs, home and hobbies, and personal care. Global Deterioration Scale (GDS) is another scale 49 that is occasionally used for MCI but commonly used for patients with AD. Patients with MCI fit into GDS stage 2 or 3 and a CDR of 0 or 0.5. 6
A brief mental status examination, such as the Mini-Mental State Examination (MMSE), is often insensitive to the detection of early impairment 50 ; more useful measures include the Short Test of Mental Status and the Montreal Cognitive Assessment. Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) is also increasingly been used for MCI. It consists of 11 tasks measuring the disturbances of memory, language, praxis, attention, and other cognitive abilities. Other tests include functional assessments such as Alzheimer's disease Cooperative Study-activities of daily living (ADCS-ADL) and Disability Assessment for Dementia and global cognition assessments such as the ADCS-Clinical Global Impression of Change.
Neuropsychological Evaluation
Making a distinction between MCI and normal aging can be a challenge, as subtle forgetfulness and having difficulty recalling common words may also be a part of normal aging. Distinguishing other functional deficits associated with MCI can be even more taxing, as some patients won’t be even aware of them. Hence, NP is used to distinguish the borderline MCI cases from normal aging, for example, a recent trial identified early aMCI groups without having objective memory symptoms with the help of discrepancy of scores between Verbal IQ and General Memory tests. 51 Even in a pre-MCI population, decline in NP or a score lower than a threshold may be a predictive of patients likely to progress to MCI. Although higher proportion of patients with MCI can be identified by the CDR criteria than NP; patients with MCI identified by NP have a higher rate of conversion to dementia. 52 These findings suggest that the CDR is sensitive to subtle changes in cognition not identified by the NP algorithm but is also sensitive to demographic and clinical factors probably leading to a greater number of false positives.
Neuropsychological testinggenerally involves testing general intellectual function, memory, construction, language, attention, and executive functions. Intellectual functioning is commonly verified by the Wechsler Adult Intelligence Scale-IV or the National Adult Reading Test-American Version or Raven's Coloured Progressive Matrices. Verbal memory is tested by California Verbal Learning Test-II, while visual memory and construction is tested by 24-point modified Rey Osterrieth figure. One recently published study successfully predicted the conversion to MCI in patients with normal cognition and conversion to AD in patients with MCI, by a visual paired comparison task. 53 The task assessed memory function, by determining a preference for a novel picture compared to a previously viewed picture, measured by viewing time using infrared eye tracking. Language function has been assessed with semantic fluency tests (“animal” naming test) and the Boston Naming Test, whereas attention and executive function are assessed by with digit span tests and the Stroop Color-Word Interference Test. Depression is screened by the GDS, which is a 30-item self-reported assessment scale and has a higher sensitivity for patients with MCI than their demented counterparts. 54
Computerized cognitive testing is becoming increasingly popular, given its greater precision and efficiency in measuring cognitive function. Tests such as Mindstreams by NeuroTrax Corp (New York; formerly known as “Mild Impairment Battery”) or CogState Brief Battery by Cogstate Ltd (Australia) detects MCI superior to that of traditional NP tests. 55,56 It consists of custom software installed on the testing computer that serves as a platform for interactive cognitive tests that produce precise accuracy and reaction time. 57 The complete Global Assessment Battery (administration time: approximately 45 minutes) samples a wide range of cognitive domains, including memory (verbal and nonverbal), executive function, visual spatial skills, verbal fluency, attention, information processing, and motor skills. It successfully detects MCI and mild dementia even in the presence of depression. 58
Role of Other Diagnostic Modalities
Neuroimaging
In AD, the earliest signs of cerebral atrophy are found in the medial temporal lobe, 59 hence structural neuroimaging studies focusing on these areas could predict conversion from MCI to AD. 60 These changes are generally appreciated 1 to 2 years prior to cognitive decline and are better predictors of future AD than CSF biomarkers 60,61 ; 1 recent study has even reported that local vascular damage is also associated with increased brain atrophy in patients with MCI. 62 But unfortunately in multiple-domain naMCI such changes can rarely be perceived 63 ; however, 1 study has hinted that cognitive impairment in both aMCI and dMCI is related to cerebral hypoperfusion. 64 Some studies have also associated increased white matter hyperintensities and cortical infarction in MRI also with MCI 65,66 Theoretically, alterations in neuronal activity precede neuronal loss, so hypometabolism should precede hippocampal atrophy. 67 This might be one of the reasons that almost all studies have reported that functional imaging is superior to structural imaging in early detection of MCI as well as in predicting the chances of conversion of MCI to AD or general cognitive decline. 68 –72
Fluorodeoxyglucose positron emission tomography is a specialized imaging technique that may be useful for early prediction of the chances of conversion of MCI to AD, particularly in the presence of APOE ∊4 allele. 73 Patients with impaired episodic memory having PET abnormality have very high chances of converting to AD in near future. 74 Also, patients with higher 11C-labeled Pittsburgh compound-B amyloid tracer retention have higher rate of progression to dementia than those who do not. 75 –77 Patients with aMCI also exhibit temporomesial and temporoparietal hypoperfusion on single-photon emission computed tomography (SPECT). 78 It has been found in functional MRI studies that greater memory-related hippocampal activation also successfully predicts future cognitive decline. 79
Diffusion tensor imaging (DTI) is an MRI technique that enables the measurement of the restricted diffusion of water in tissue in order to produce neural tract image and has increasingly been used in dementia trials in the recent time. In a recent DTI study of patients with MCI, 80 better paired associate learning is found to be selectively associated with lower axial diffusion but not with lower radial diffusion (DR), mean diffusivity (MD), or fractional anisotropy (FA) of the temporal stems. Conversely, better paired associate learning is associated with lower DR, higher FA, and lower MD in the fornix. 80 One recent study on DTI has testified that microstructural degradation of the fornix precedes hippocampal atrophy in aMCI, and hence it may serve as a novel imaging marker. 81 Another recent study revealed that DTI tractography enables anatomical definition of region of interest for correlation of behavioral parameters with diffusion indices, and functionality can be correlated with white matter integrity. 82
CSF and Plasma Biomarkers
In 2007, Ray and colleagues discovered 18 signaling proteins in the plasma, which successfully predicted the onset of AD in patients with MCI but failed to foresee non-AD dementias. 83 Further studies on CSF biomarkers have revealed a low ratio of CSF Aβ42–tau in majority of patients with MCI. 84 This low ratio, which is generally existent in AD, is found in patients with both aMCI and naMCI. 84
Genetic Testing
There has been a lot of controversy regarding whether APOE ∊4 genotype is a strong risk factor for conversion of MCI to AD; while several studies have supported the association, 85 –88 there are others who have negated it. 89,90 A new study has reported deletion variant of a2b-adrenergic receptor to be associated with decreased risk of MCI. 91 Another recent study identified certain gene expression signatures that recognized patients with aMCI that progressed to AD within 2 years with a prediction accuracy of 74% to 77%. 92 But still there is paucity of substantial evidences to justify a routine genetic testing in patients with MCI.
Therapeutic Interventions
Pharmacological — at present, there is no single medication approved by the Food and Drug Administration for the treatment of MCI. The class of drugs that are most commonly used for the management of patients with MCI are listed subsequently.
Acetylcholinesterase inhibitors—several large clinical trials have assessed the efficacy of donepezil, 93 –95 galantamine, 96 and rivastigmin 97 in patients with MCI, but none of the drugs were found successful in treating the long-term clinical cognitive dysfunction and stopping or lowering the progression to AD. Galantamine-treated patients also reported higher deaths, mostly from vascular causes. 98 However, the ADCS drug trial in patients with aMCI revealed that treatment with donepezil delayed progression to AD among patients with depression. 47
Antioxidants and anti-inflammatory agents—antioxidants such as high daily dosage of vitamin E 93 –99 and nonsteroidal anti-inflammatory drugssuch as rofecoxib 100 also do not help in reducing the risk of progression to AD in patients with MCI. Trials evaluating the effects of Ginkgo biloba in patients with MCI also found no benefit for treatment in preventing cognitive decline 101 or the imminent dementia. 102 However, in a study in Italy, elderly patients with MCI had significant improvements in several measures of cognitive function when supplemented with an oily emulsion of docosahexaenoic acid–phospholipids containing melatonin and tryptophan for 12 weeks, compared with the placebo. 103 Another study having patients treated with melatonin tablets at night for at least 15 months also exhibited significantly better performance in MMSE and ADAS-cog. 104
There are some ongoing trials with few newer antioxidants or anti-inflammatory agents in patients with MCI—like a study with a formulation containing N-acetyl cysteine added to folic acid, vitamin B12, vitamin E, S-adenosylmethionine, and acetyl-l-carnitine to evaluate the effect on the behavioral status (clinicaltrials.gov NCT00903695) and to determine whether this formulation can delay MCI conversion to AD (clinicaltrials.gov NCT01320527). Other such novel clinical trials in MCI include—evaluating the effect of resveratrol on ADAS-cog score (clinicaltrials.gov NCT01219244) and assessing the impact of curcumin on amyloid deposition in the brain (clinicaltrials.gov NCT01383161).
Insulin —A pilot clinical trial on intranasal insulin treatment has shown improved memory, ADAS-Cog score, and functional abilities in patients with both aMCI and AD. 105
Antidepressants —As discussed earlier, patients with MCI having depression experienced greater deficits in cognitive functioning and are more prone to developing AD, than their nondepressed counterparts. 45,46,48 Thus, identifying and treating depression in individuals with MCI may improve their memory and cognitive functioning. 106
Cognitive (brain training)— One study revealed that postcognitive rehabilitation, there is significantly improved performance on memory tasks in patients with MCI. 107 Although a literature review on patients with aMCI receiving cognitive therapy revealed significant improvements on 44% of objective measures of memory and 12% of objective measures of cognition other than memory. 108 Some other studies have also confirmed that the effect of a cognitive rehabilitation program results in significant improvement in multiple cognitive domains as measured by the global cognitive scales or NP evaluation,and the effects are preserved over extended follow-up periods, thereby concluding that early cognitive intervention may delay conversion to AD. 109,110
Other promising research came with a recent randomized controlled clinical trial having 8 memory training sessions for patients with MCI suggesting evidences of cognitive plasticity. 111 The training sessions involved learning mnemonic strategies based on ecological tasks, completion of tasks, which recruited attention and executive functions and educational content provision on memory and aging; and at the end of the sessions the patients with MCI exhibited cognitive performance typical of individuals without cognitive impairment.
Lifestyle modification — Moderate degree of physical exercise, like regular brisk walking, improves cognition, and delays further decline, 112,113 improves verbal and spatial memory 114 and significantly enhances the quality of life. 115 People on Mediterranean diet also pose a lower risk of developing MCI and conversion of MCI to dementia. 116 Prolonged daily computer use in the elderly patients 24 is also found to be associated with lower odds of having MCI.
Newer therapies — A recent multicenter randomized controlled trial in China in patients with MCI involving electroacupuncture intervention revealed improvement in general cognition, memory, and visual-space skills. 117
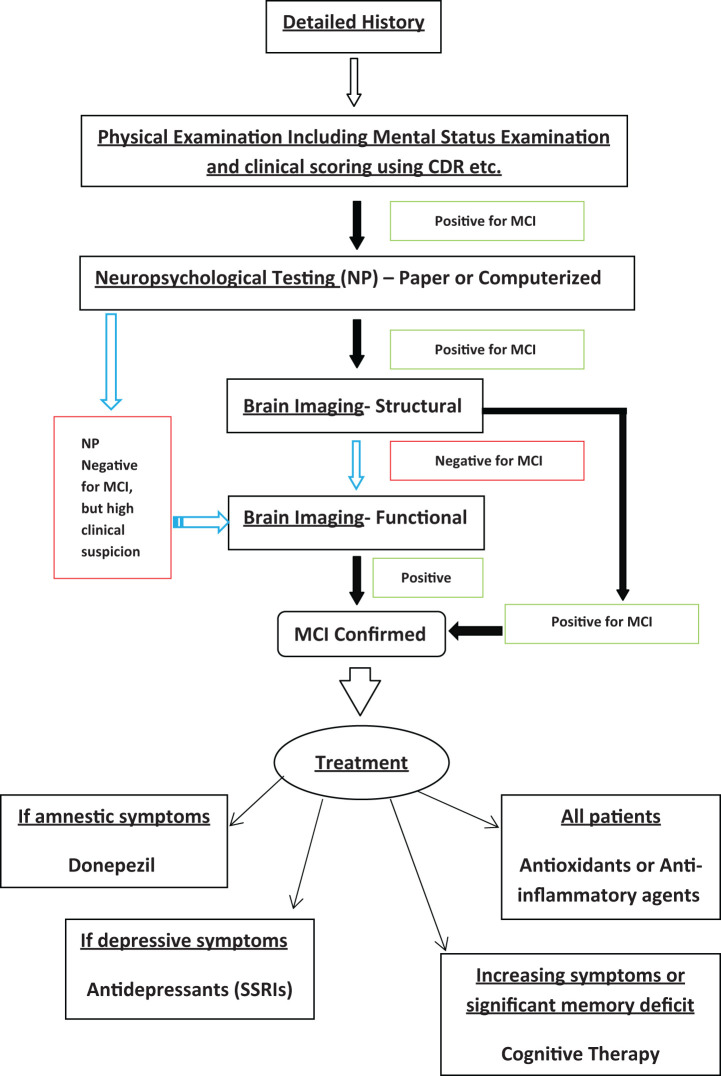
Suggested Clinical Algorithm
Figure 1 summarizes an algorithm that can be used to approach any patients with suspected MCI. A descriptive history followed by a detailed physical examination including clinical cognitive assessment can often be sufficient to suspect MCI. Suspected cases should be further confirmed by NP testing and imaging. High clinical suspicion of MCI, but a negative test result on NP may be an early case of MCI or even pre-MCI; such cases should skip structural imaging and straight go to functional imaging modalities. Other patients should be referred first for structural MRI to assess the extent of the disease, and if the results are negative, then only should be referred to functional imaging. This would result in an early diagnosis yet judicious utilization of the resources. Relevant treatment modalities of patients with MCI per their clinical signs and symptoms are also suggested in the algorithm.
Figure 1.
Proposed algorithm for diagnosis and management of a patient with suspected mild cognitive impairment (MCI).
Caring for the Caregivers
Like all other dementias, the role of caregivers in patients with MCI can be challenging; apart from the usual difficulties, there is always a degree of uncertainty in the minds of the caregiver about the ultimate fate of the patient. This may give rise to ambiguous feelings, distress, and false interpretations of the changes observed. 118 –121 In several studies, both patients with MCI and their partners expressed a need for more information and support, 122 –125 indicating the call for a psychosocial intervention aimed at information, support, and effective coping.
A recently published study has examined the efficacy of a comprehensive group program aimed at caring partners of patients with MCI, which comprised elements of psychoeducation, cognitive rehabilitation, and cognitive behavioral therapy. Qualitative results at program completion suggested that the caregivers reported gains in knowledge, insight, acceptance, and coping skills 123 ; however, the group intervention was not proven to be effective.
Conclusion
Much of the challenges regarding successful management of MCI lie in the vagueness of its clinical definition, for example, the degree of cognitive impairment in nonmemory cognitive domains, the degree of functional impairment, and its heterogeneous presentations. Apart from a systematic pharmacological and nonpharmacological therapeutic strategy, we are also in dire need of a simple algorithm for the diagnosis of MCI—which might have a combination of entities from history, clinical scores, to NP, to imaging results; but at the same time also be on the right side of the cost–benefit ratio. Such an algorithm is suggested here. Furthermore, caregiver support and general awareness regarding MCI in the population can be 2 other aspects that may prove to be a cornerstone in the management of MCI.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86(6):257–260. [PMC free article] [PubMed] [Google Scholar]
- 2. Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age associated memory impairment: proposed diagnostic criteria and measures of clinical change-report of a national institute of mental health work group. Dev Neuropsychol.1986;2(4):261–276. [Google Scholar]
- 3. Levy R. Aging-associated cognitive decline. Int Psychogeriatr. 1994;6(1):63–68. [PubMed] [Google Scholar]
- 4. Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–1796. [DOI] [PubMed] [Google Scholar]
- 5. Reisberg B, Ferris S, de Leon MJ. Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the Alzheimer type. Drug Dev Res. 1988;15(2-3):101–114. [Google Scholar]
- 6. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. [DOI] [PubMed] [Google Scholar]
- 8. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 9. Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts RO, Geda YE, Knopman D, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libon DJ, Xie SX, Eppig J, et al. The heterogeneity of mild cognitive impairment: a neuropsychological analysis. J Int Neuropsychol Soc. 2010;16(1):84–93. [DOI] [PubMed] [Google Scholar]
- 12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment depend on definition: a population study. Arch Neurol. 2011;68(6):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saxton J, Snitz BE, Lopez OL, et al. Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry. 2009;80(7):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C. Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56(8):1424–1433. [DOI] [PubMed] [Google Scholar]
- 16. Visser PJ, Verhey FR. Mild cognitive impairment as a predictor for Alzheimer’s disease in clinical practice: effect of age and diagnostic criteria. Psychol Med. 2008;38(1):113–122. [DOI] [PubMed] [Google Scholar]
- 17. Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(7):603–611. [DOI] [PubMed] [Google Scholar]
- 18. Bruscoli M, Lovestone S. Is MCI really just dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129–140. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell AJ, Feshki-Shiri M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252–265. [DOI] [PubMed] [Google Scholar]
- 20. Petersen RC, Rosebud RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-related resource use and costs in elderly adults with and without mild cognitive impairment. J Am Geriatr Soc. 2013;61(3):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60(10):1394–1399. [DOI] [PubMed] [Google Scholar]
- 24. Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007;68(23):2019–2026. [DOI] [PubMed] [Google Scholar]
- 25. Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66(6):828–832. [DOI] [PubMed] [Google Scholar]
- 26. Geda YE, Silber TC, Roberts RO, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: a population-based study. Mayo Clin Proc. 2012;87(5):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Impact of cardiovascular risk factors on cognitive function: the Tromso study. Eur J Neurol. 2011;18(5):737–743. [DOI] [PubMed] [Google Scholar]
- 28. Torres SJ, Lautenschlager NT, Wattanapenpaiboon N, et al. Dietary patterns are associated with cognition among older people with mild cognitive impairment. Nutrients. 2012;4(11):1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts RO, Roberts LA, Geda YE, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis. 2012;32(2):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sparks DL, Kryscio RJ, Connor DJ, et al. Cholesterol and cognitive performance in normal controls and the influence of elective statin use after conversion to mild cognitive impairment: results in a clinical trial cohort. Neurodegener Dis. 2010;7(1-3):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buchman AS, Tanne D, Boyle PA, et al. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73(12):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark LR, Delano-Wood L, Libon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc. 2013;19(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zola SM, Manzanares CM, Clopton P, Lah JJ, Levey AI. A behavioral task predicts conversion to mild cognitive impairment and Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2013;28(2):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pa J, Boxer A, Chao LL, et al. Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol. 2009;65(4):414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huey ED, Manly JJ, Tang MX, et al. Course and etiology of dysexecutive MCI in a community sample. Alzheimers Dement. 2013;9(6):632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson JK, Pa J, Boxer AL, et al. Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;30(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Missonnier P, Herrmann FR, Richiardi J, et al. Attention-related potentials allow for a highly accurate discrimination of mild cognitive impairment subtypes. Neurodegener Dis. 2013;12(2):59–70. [DOI] [PubMed] [Google Scholar]
- 38. Ballesteros S, Mayas J, Reales JM. Cognitive function in normal aging and in older adults with mild cognitive impairment. Psicothema. 2013;25(1):18–24. [DOI] [PubMed] [Google Scholar]
- 39. Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25(4):779–786. [DOI] [PubMed] [Google Scholar]
- 40. Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156(4):531–537. [DOI] [PubMed] [Google Scholar]
- 41. Mitchell AJ. Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age Ageing. 2008;37(5):497–499. [DOI] [PubMed] [Google Scholar]
- 42. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 43. Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. [DOI] [PubMed] [Google Scholar]
- 44. Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–1602. [DOI] [PubMed] [Google Scholar]
- 45. Feldman H, Scheltens P, Scarpini E, et al. Behavioral symptoms in mild cognitive impairment. Neurology. 2004;62(7):1199–1201. [DOI] [PubMed] [Google Scholar]
- 46. Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc. 2010;58(2):330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu PH, Edland SD, Teng E, et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology. 2009;72(24):2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11(1):65–71. [DOI] [PubMed] [Google Scholar]
- 49. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 50. Reisberg B, Ferris SH, DeLeon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 51. Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. [DOI] [PubMed] [Google Scholar]
- 52. Murayama N, Tagaya H, Ota K, et al. Neuropsychological detection of the early stage of amnestic mild cognitive impairment without objective memory impairment. Dement Geriatr Cogn Disord. 2013;35(1-2):98–105. [DOI] [PubMed] [Google Scholar]
- 53. Saxton J, Snitz BE, Lopez OL, et al. Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry. 2009;80(7):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Debruyne H, Van Buggenhout M, Le Bastard N, et al. Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry. 2009;24(6):556–562. [DOI] [PubMed] [Google Scholar]
- 55. Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of the mindstreams computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Jager CA, Schrijnemaekers AC, Honey TE, Budge MM. Detection of MCI in the clinic: evaluation of the sensitivity and specificity of a computerised test battery, the Hopkins Verbal Learning Test and the MMSE. Age Ageing. 2009;38(4):455–460. [DOI] [PubMed] [Google Scholar]
- 57. Woodard JL. Geriatric neuropsychological assessment. In: Lichtenberg PA, ed. Handbook of Assessment in Clinical Gerontology. 2nd ed. Burlington, MA: Academic Press; 2011. [Google Scholar]
- 58. Doniger GM, Dwolatzky T, Zucker DM, et al. Computerized cognitive testing battery identifies mild cognitive impairment and mild dementia even in the presence of depressive symptoms. Am J Alzheimers Dis Other Demen. 2006;21(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leung KK, Bartlett JW, Barnes J, Manning EN, Ourselin S, Fox NC. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: rates and acceleration. Neurology. 2013;80(7):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clerx L, van Rossum IA, Burns L, et al. Measurements of medial temporal lobe atrophy for prediction of Alzheimer's disease in subjects with mild cognitive impairment. Neurobiol Aging. 2013;34(8):2003–2013. [DOI] [PubMed] [Google Scholar]
- 61. Becker JT, Davis SW, Hayashi KM, et al. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63(1):97–101. [DOI] [PubMed] [Google Scholar]
- 62. Barnes J, Carmichael OT, Leung KK, et al. Vascular and Alzheimer's disease markers independently predict brain atrophy rate in Alzheimer's Disease Neuroimaging Initiative controls. Neurobiol Aging. 2013;34(8):1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73(4):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chao LL, Pa J, Duarte A, et al. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis Assoc Disord. 2009;23(3):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65(1):94–100. [DOI] [PubMed] [Google Scholar]
- 66. Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lippa CF, Chetelat G. Tarot decks and PET scans predicting the future of MCI. Neurology. 2010;75(3):204–205. [DOI] [PubMed] [Google Scholar]
- 68. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fellgiebel A, Scheurich A, Bartenstein P, Muller MJ. FDG-PET and CSF phospho-tau for prediction of cognitive decline in mild cognitive impairment. Psychiatry Res. 2007;155(2):167–171. [DOI] [PubMed] [Google Scholar]
- 70. Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46(10):1625–1632. [PubMed] [Google Scholar]
- 71. Chetelat G, Eustache F, Viader F, et al. FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase. 2005;11(1):14–25. [DOI] [PubMed] [Google Scholar]
- 72. De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22(4):529–539. [DOI] [PubMed] [Google Scholar]
- 73. Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63(12):2332–2340. [DOI] [PubMed] [Google Scholar]
- 74. Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koivunen J, Scheinin N, Virta JR, et al. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology. 2011;76(12):1085–1090. [DOI] [PubMed] [Google Scholar]
- 76. Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guedj E, Barbeau EJ, Didic M, et al. Identification of subgroups in amnestic mild cognitive impairment. Neurology. 2006;67(2):356–358. [DOI] [PubMed] [Google Scholar]
- 79. Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79(6):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boespflug EL, Storrs J, Sadat-Hossieny S, et al. Full diffusion characterization implicates regionally disparate neuropathology in mild cognitive impairment [published online January 24, 2013]. Brain Struct Funct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8(3):e58887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 2013;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signalling proteins. Nat Med. 2007;13(11):1359–1362. [DOI] [PubMed] [Google Scholar]
- 84. Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8(7):619–627. [DOI] [PubMed] [Google Scholar]
- 85. Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68(19):1588–1595. [DOI] [PubMed] [Google Scholar]
- 86. Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67(2):229–234. [DOI] [PubMed] [Google Scholar]
- 87. Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- 88. Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE ∊4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Devanand DP, Pelton GH, Zamora D, et al. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62(6):975–980. [DOI] [PubMed] [Google Scholar]
- 90. Aggarwal NT, Wilson RS, Beck TL, et al. The apolipoprotein E epsilon4 allele and incident Alzheimer's disease in persons with mild cognitive impairment. Neurocase. 2005;11(1):3–7. [DOI] [PubMed] [Google Scholar]
- 91. Koutroumani M, Daniilidou M, Giannakouros T, et al. The deletion variant of a2b-adrenergic receptor is associated with decreased risk in Alzheimer's disease and mild cognitive impairment. J Neurol Sci. 2013;328(1-2):19–23. [DOI] [PubMed] [Google Scholar]
- 92. Roed L, Grave G, Lindahl T, et al. Prediction of mild cognitive impairment that evolves into Alzheimer's disease dementia within two years using a gene expression signature in blood: a pilot study. J Alzheimers Dis. 2013;35(3):611–621. [DOI] [PubMed] [Google Scholar]
- 93. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. [DOI] [PubMed] [Google Scholar]
- 94. Salloway S, Ferris S, Kluger A, Goldman R, Griesing T. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. [DOI] [PubMed] [Google Scholar]
- 95. Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. [DOI] [PubMed] [Google Scholar]
- 96. Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. [DOI] [PubMed] [Google Scholar]
- 97. Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. [DOI] [PubMed] [Google Scholar]
- 98. Mayor S. Regulatory authorities review use of galantamine in mild cognitive impairment. BMJ. 2005;330(7486):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Farina N, Isaac MG, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2012;11:CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204–1215. [DOI] [PubMed] [Google Scholar]
- 101. Snitz BE, O'Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rondanelli M, Opizzi A, Faliva M, et al. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr Neurosci. 2012;15(2):46–54. [DOI] [PubMed] [Google Scholar]
- 104. Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI. Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegener Dis. 2012;1(3):280–291. [PMC free article] [PubMed] [Google Scholar]
- 105. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johnson LA, Mauer C, Jahn D, et al. Cognitive differences among depressed and non-depressed MCI participants: a project FRONTIER study. Int J Geriatr Psychiatry. 2013;28(4):377–382. [DOI] [PubMed] [Google Scholar]
- 107. Kinsella GJ, Mullaly E, Rand E, et al. Early intervention for mild cognitive impairment: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2009;80(7):730–736. [DOI] [PubMed] [Google Scholar]
- 108. Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–296. [DOI] [PubMed] [Google Scholar]
- 109. Rojas GJ, Villar V, Iturry M, et al. Efficacy of a cognitive intervention program in patients with mild cognitive impairment. Int Psychogeriatr. 2013;25(5):825–831. [DOI] [PubMed] [Google Scholar]
- 110. Buschert VC, Giegling I, Teipel SJ, et al. Long-term observation of a multicomponent cognitive intervention in mild cognitive impairment. J Clin Psychiatry. 2012;73(12):e1492–e1498. [DOI] [PubMed] [Google Scholar]
- 111. Olchik MR, Farina J, Steibel N, Teixeira AR, Yassuda MS. Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Arch Gerontol Geriatr. 2013;56(3):442–447. [DOI] [PubMed] [Google Scholar]
- 112. Denkinger MD, Nikolaus T, Denkinger C, Lukas A. Physical activity for the prevention of cognitive decline: current evidence from observational and controlled studies. Z Gerontol Geriatr. 2012;45(1):11–16. [DOI] [PubMed] [Google Scholar]
- 113. Netz Y, Dwolatzky T, Zinker Y, Argov E, Agmon R. Aerobic fitness and multidomain cognitive function in advanced age. Int Psychogeriatr. 2011;23(1):114–124. [DOI] [PubMed] [Google Scholar]
- 114. Nagamatsu LS, Chan A, Davis JC, et al. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. J Aging Res. 2013;2013:861–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Van Uffelen JG, Chin A Paw MJ, Hopman-Rock M, van Mechelen W. The effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: a randomized, controlled trial. Qual Life Res. 2007;16(7):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhao L, Zhang FW, Zhang H, et al. Mild cognitive impairment disease treated with electroacupuncture: a multi-center randomized controlled trial [in Chinese]. Zhongguo Zhen Jiu. 2012;32(9):779–784. [PubMed] [Google Scholar]
- 118. Wegierek AM. Taking care of a loved one who has Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27(7);463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blieszner R, Roberto KA, Wilcox KL, Barham EJ, Winston BL. Dimensions of ambigious loss in couples coping with mild cognitive impairment. Fam Relat. 2007;56(2):196–209. [Google Scholar]
- 120. Bruce JM, McQuiggan M, Williams V, Westervelt H, Tremont G. Burden among spousal and child caregivers of patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(4):385–390. [DOI] [PubMed] [Google Scholar]
- 121. Garand L, Dew MA, Eazor LR, Dekosky ST, Reynolds CF III. Caregiving burden and psychiatric morbidity in spouses of persons with mild cognitive impairment. Int J Geriatr Psychiatry. 2005;20(6):512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ryan KA, Weldon A, Huby NM, et al. Caregiver support service needs for patients with mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Davies HD, Newkirk LA, Pitts CB, et al. The impact of dementia and mild memory impairment (MMI) on intimacy and sexuality in spousal relationships. Int Psychogeriatr. 2010;22(4):618–628. [DOI] [PubMed] [Google Scholar]
- 124. McIlvane JM, Popa MA, Robinson B, Houseweart K, Haley WE. Perceptions of illness, coping, and well-being in persons with mild cognitive impairment and their care partners. Alzheimer Dis Assoc Disord. 2008;22(3):284–292. [DOI] [PubMed] [Google Scholar]
- 125. Joosten-Weyn Banningh LW, Vernooij-Dassen MJ, Vullings M, Prins JB, Olde Rikkert MG, Kessels RP. Learning to live with loved one with Mild Cognitive Impairment: effectiveness of waiting list controlled trial of a group intervention on significant others’ sense of competence and well-being. Am J Alzheimers Dis Other Demen. 2013;28(3):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]