Abstract
Introduction:
This study investigated the effects of testosterone (T) treatment on cognition, mood, and quality of life in men with mild cognitive impairment (MCI) and low serum T levels.
Methods:
A total of 351 community-dwelling men were screened, and 37 men evidenced both MCI and low T of whom 27 agreed for further screening. Twenty-two met all the study inclusion/exclusion criteria and enrolled in a 6-month randomized, double-blind, placebo-controlled study.
Results:
Total T levels significantly increased in the T treatment group. No significant changes were observed in measures of cognition, mood, or quality of life other than improvement in 1 objective measure of verbal memory (P < .05) and decreased depression symptoms (P < .02) in the treatment group.
Conclusions:
Testosterone treatment may modestly improve verbal memory and depression symptoms in men with both MCI and low T.
Keywords: mild cognitive impairment, testosterone treatment, verbal memory, cognition, quality of life, low serum testosterone
Introduction
Age-related decline in serum testosterone (T) is associated with decrements in cognitive abilities independent of health status. 1 Additionally, low bioavailable T levels have been correlated with a higher risk of cognitive decrements including mild cognitive impairment (MCI) and Alzheimer’s disease (AD). 2 -5
These findings suggest that men with low T levels are most at risk of age-related cognitive decline and AD and might benefit from T treatment to prevent the development of AD or age-associated cognitive decline. Studies in our laboratory support the fact that older men who are eugonadal or with low T levels demonstrate cognitive and mood improvements within short periods (6-12 weeks) of injectable T treatment when assessed at peak or at supraphysiological T levels. 6 Other studies have found improvements in visuospatial function with 20 weeks of T oral treatment. 7 However, not all studies have found an improvement in cognition from T supplementation. 8,9
Although epidemiological studies suggest that future studies of T supplementation should target men with hypogonadism, few clinical studies to date have directly examined cognitive response in men with hypogonadism having MCI who are at risk of further cognitive decline. Mild cognitive impairment is often considered a prodromal state to developing AD, and recent findings on the pathophysiology of AD suggest that the process begins many years before clinical manifestation, and therefore therapeutic trials should attempt to enroll patients in the earliest phases of the disease process when treatment may have maximal impact.
This study examined cognitive response from T treatment in a sample of older men with low T levels at risk of further decline due to MCI. The intervention was over a longer period of time (6 months) compared to previous studies and studied the effect of adjusting serum T levels to the normal physiologic range using a transdermal T gel formulation that provided a relatively steady physiologic replacement levels, rather than peak or supraphysiological levels achieved in previous studies using T injections.
Methods
This study was approved by the Veterans Affairs Puget Sound Health Care System Institutional Review Board. Written informed consent was obtained for the screening visit, and if eligible and interested in participating, informed consent was obtained for the 6-month study.
Screening Assessment
Community-dwelling men were recruited through flyers or print advertisements and were self- or physician-referred based on self-reported memory difficulties and/or symptoms of low T. Participants were screened at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) in Seattle, Washington. Screening included a cognitive evaluation, physical examination, and laboratory evaluation to determine eligibility for study inclusion. Inclusion criteria included (1) age range from 60 to 90 years; (2) diagnosis of MCI (amnestic or multiple domain type) according to Peterson criteria. 10 (3) low or low-normal total T level below 300 ng/dL; (4) American Urological Association symptom score ≤19; and (5) body mass index <35 and stable weight in the previous year. Cognitive-enhancing medications (eg, cholinesterase inhibitors) were allowed if patients were on a stable dose for longer than 2 months and remained on the medications for the duration of the trial. Exclusion criteria included (1) hematocrit >50%, (2) known severe symptoms of benign prostatic hypertrophy (BPH) (including prostate-specific antigen [PSA] > 4.0), (3) significant liver disease (serum glutamic-oxaloacetic transaminase >3 times normal), or significant renal disease, limiting heart (unstable angina, heart failure) or peripheral vascular disease or severe cardio pulmonary disease or chronic obstructive pulmonary disease, (4) acute or significant major illness including unstable angina, recent myocardial infarction, recent history of congestive heart failure, or history of stroke, (5) significant history of alcohol abuse or current alcohol abuse (>3 drinks/day) or other substance abuse, (6) insulin-dependent diabetes mellitus, (7) obstructive sleep apnea that is clinically significant and not treated with continuous positive airway pressure (CPAP; use of CPAP was acceptable), (8) history of prostate or breast cancer; (9) uncontrolled hypertension (blood pressure >160/90), (10) history of, or current symptoms of, psychiatric disorder, neurological condition, or depression, and (11) limited sight or mobility that precluded obtaining informed consent or completing objective testing and assessment.
Study Design
Following screening visits and informed consent, eligible participants were enrolled and randomized to receive either placebo or T gel. Participants were assigned to T gel or placebo gel by the study pharmacist from a predetermined randomization list created by the study statistician at the beginning of the study. Participants and study staff were blind to treatment condition with the exception of 1 study staff member who did not have direct contact with study participants and was unblinded for dosage adjustments and to complete safety checks of clinical blood draws. All other study personnel responsible for administering the cognitive tests and questionnaires, participants, and investigators were blind to the treatment condition. Testosterone or placebo gel was dosed at 50 to 100 mg/d, with a target total T level of 500 to 900 ng/dL in the treatment group. Clinical blood samples were taken at day 7, day 14, months 1, and 3 to test for serum T levels, PSA, and hematocrit in the clinical laboratory of the VAPSHCS, and, if necessary, T dose adjustments were made by the single study staff member. Cognitive testing and questionnaires were given at screening, baseline, months 3, and 6. Additional procedures such as prostate examination (digital rectal examination of the prostate [DRE]) were performed at screening, months 3, and 6. Blood for serum T levels that were measured in the research laboratory (see subsequently) were also taken at the study visits (baseline, months 3, and 6) and then aliquoted and frozen for serum assay at the study conclusion. Electrocardiogram measures were taken at screening and month 3. Blood at baseline visit was assessed for apolipoprotein E (APOE) genotype using a buffy coat separation 11 (see Table 1 for an overview of study procedures).
Table 1.
Procedures Performed Throughout 6-Month Study.a
| Screening | Baseline | Day 7 | Day 14 | Month 1 | Month 3 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Cognitive testing | × | × | × | × | |||
| Questionnaires | × | × | × | × | |||
| PSA | × | × | × | × | × | × | |
| Hematocrit | × | × | × | × | × | × | |
| Total T | × | × | × | × | × | × | |
| Physical with DRE | × | × | × | ||||
| ECG | × | × |
Abbreviations: PSA, prostate-specific antigen; Total T, total testosterone; DRE, digital rectal examination of the prostate; ECG, electrocardiogram.
aPhysicals with DRE, PSA, testosterone and hematocrit tests, and ECGs were reviewed by a physician to ensure the safety of participants.
Cognitive Assessment Measures
The cognitive battery consisted of tests of spatial, verbal, visual and working memory, language fluency, and selective attention validated on numerous previous studies of aging and AD and sensitive to changes in cognition from metabolic function or hormone manipulation. Use of a sensitive neuropsychological battery was necessary as the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) was designed for patients with dementia and therefore not sufficiently sensitive for MCI. 12 Equal alternate versions of tests were used on subsequent visits where available to help reduce practice effects. In addition, the cognitive tests were given at the screening visit in addition to the baseline visit, with only the baseline visit used in the analysis. This procedure was followed in order to help reduce practice effects, as the majority of practice effects occur between the first and the second session. 13
Verbal memory measures included a prose or story recall task modeled on the Wechsler story recall task 14 and a list learning task that was an adapted version of the Rey Auditory Verbal Learning Test (RAVLT) with only 3 learning trials. 15 The Story Recall task involved listening to a short story and recalling the story immediately after hearing it and again after a 20-minute delay. For the list learning task (RAVLT), participants listened to a word list of 15 unrelated nouns and were asked to recall as many words as possible from the same list in 3 trials followed by a delay.
Visual and spatial memory measures included the Route Test 16 and a modified version of the Visual Spatial Learning Test Revised (VSLT). 17 For the Route Test, participants were asked to navigate a designated route on a 6 × 24 mat with a grid pattern. Participants had to reproduce the route after each learning trial and after a delay. For VSLT, tokens with different symbols were placed on a board with a grid. After a short study period, participants were asked to recreate the same pattern and then asked to recall it again after a 30-minute delay.
Visual reasoning ability was measured using a task similar to block design. 18 Participants were asked to construct designs from their component parts using 3-dimensional blocks. Time to complete the designs was recorded. Letter/Number Sequencing (LNS) from the Wechsler Adult Intelligence Scale III was used to assess working memory. 19 The participants were given a list of numbers and letters and asked to recall the list ordering the numbers first from lowest to highest and then ordering the letters in alphabetical order. Verbal ability was measured with a 2 trial verbal fluency task similar to Controlled Oral Word Association Letter and Category Fluency. 20 Participants were asked to verbally generate as many words as possible beginning with a specific letter in 1 minute. Two trials were completed with 2 different letters. For Category Fluency, participants were asked to generate as many words as possible that fit into a certain category (eg, Boys Names) in 1 minute.
Mood and Quality-of-life Measures
Participants completed questionnaires at screening, baseline, months 3, and 6 to assess mood and quality of life. In the Profile of Mood States (POMS), participants were asked how they had been feeling in the past 2 weeks by rating several adjectives from “not at all” to “extremely.” Scoring included comparison to a normative group with T-scores for 5 mood subscales. 21 For the Activities of Daily Living Questionnaire (ADLQ), common tasks were presented, and participants were asked to rate their performance on each task as a lot of difficulty, a little more difficulty, or without changes from usual performance. For ADLQ, a lower score indicates a higher level of functioning. 22 Multi Function Memory Questionnaire (MMQ) examined feelings, mistakes, and strategies used with regard to memory, and a higher score indicates a higher level of functioning. 23 The Geriatric Depression Scale (GDS) asked 30 questions regarding common symptoms of depression and higher scores indicate more depressed mood. 24 The Short Form Health Survey (SF-36) inquired about general, physical, and emotional health, limitation of activities, energy, and social activities. A higher score indicates a higher level of functioning in each subscale. 25
Hormone Assays
Blood was drawn at baseline, months 3, and 6, and serum was frozen at −70°C until the conclusion of the study when all samples were assayed in an endocrine research laboratory. Serum sex hormone-binding globulin (SHBG) was measured by time-resolved fluoroimmunoassay from PerkinElmer (Norton, Ohio). Testosterone was measured by liquid chromatography tandem mass spectrometry 26 and free T calculated using formula of Vermeulen. 27 Mean intra-assay and interassay coefficients of variation are 4.9% and 7.1% for total T and 5.7% and 6.3% for SHBG, respectively.
Data analysis
Differences in baseline demographics between treatment groups were assessed using the 2-sample t test. Summary statistics for serum hormone measures (total T, free T, and SHBG) were computed for each study visit and treatment group, and for each treatment group, paired t tests were performed to determine whether change from baseline visit was significantly different from 0 for the months 3 and 6 visits.
Data from the cognitive tests, standard scores for questionnaires, and hormone assay results were analyzed using a linear mixed effects regression model with treatment group, study visit and treatment group by study visit interaction as fixed effects, and study participant as a random effect. Study visit was modeled as categorical (with 2 dummy variables, for 3-month visit and 6-month visit) with baseline visit as the reference category. This model tested whether the overall pattern of change from baseline at months 3 and 6 in repeated serum hormone, cognitive, and mood measures differed between the T- and placebo-treated groups. The significance of the interaction term would suggest that the pattern of change in the outcome variable over the course of study differed by treatment group. Hypothesis testing was carried out using the likelihood ratio test. Significance was set at P < .05. All analyses were carried out using R version 3.0.2 (R Core Team, 2013) and the Imer package version 1.1-6.
Results
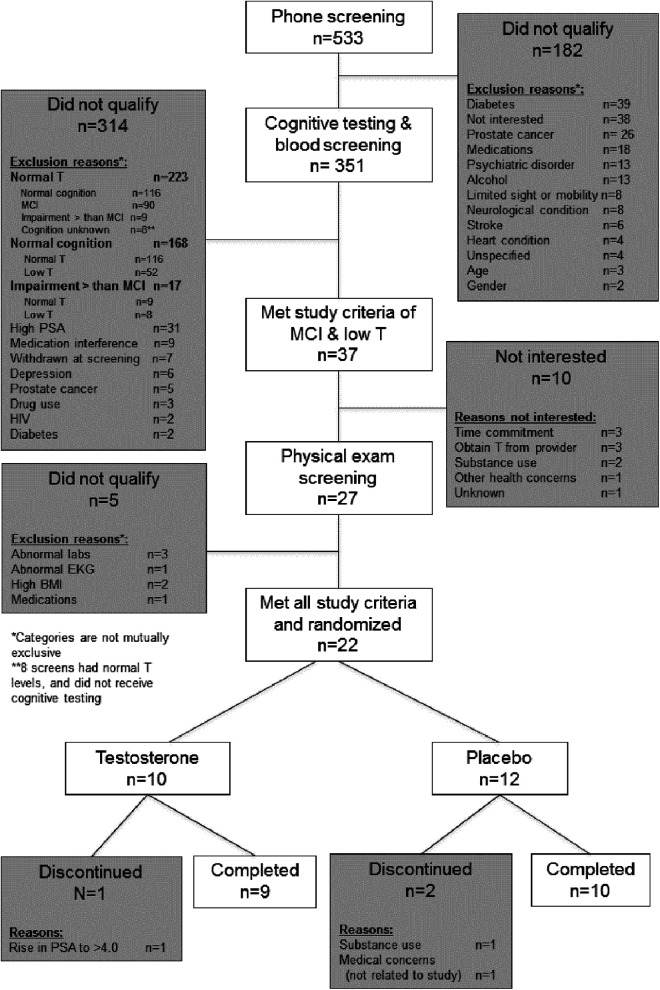
Of 351 men who met criteria for an initial screening visit, 37 (17.7%) men met both MCI and low T and were eligible to progress to the second part of the screening visit (physical examination) of which 22 (59%) met all study criteria and elected to participate in the study. Ten men who were eligible for the second part of the screening examination dropped for the following reasons: ability to obtain T gel from their physician without participating in a study that included possibility of placebo, concern about the time commitment, and 1 participant who had a recurrence of substance use following screening visit and prior to start of study medication (see Figure 1).
Figure 1.
CONSORT flow diagram indicating number of study participants and reasons for drop outs at each stage of screening, enrollment, and study completion.
The 22 enrolled participants had a mean age of 70.5 ± 8.2 years (range 60-88), mean years of education of 16.1 ± 2.4 (range 12-20), and mean Modified Mini-Mental Status Examination (3MSE) score of 92.5 ± 6.7 (range 72-100). Of the 22 men who were randomized, 12 were randomized to placebo and 10 to T and 19 completed the 6-month study. There were no significant differences between the treatment and the placebo groups in demographics.
Serum Total T
Table 2 shows summary statistics for serum hormone levels by study visit and treatment group. Based on simple paired t tests, for the T-treated group, total T levels increased from baseline in the T-treated group by a mean of 339 ng/dL at month 3 and 294 ng/dL at month 6, and free T in the T-treated group increased by a mean of 7.9 ng/dL at month 3 and 6.5 ng/dL at month 6. Change values for hormone data are shown in Table 3. Total and free T levels in the placebo-treated group did not significantly change at month 3 or 6. The fitted values for the serum hormone levels at baseline as well as the fitted change from baseline at months 3 and 6 for each treatment group based on the linear mixed effects models are presented at the bottom of Table 3. No significant differences were found between the groups for baseline measures of serum hormone levels. For total T and free T, likelihood ratio test revealed a significant interaction between visit and treatment group (P < .0001) and a post hoc pairwise comparison demonstrated significant differences in both months 3 and 6 changes by the treatment group (P < . 0001 for both) compared to the placebo group. The SHBG level did not differ significantly in either group throughout the 6-month study (Table 3).
Table 2.
Mean (Standard Deviation) Serum Hormone Levels by Treatment Group and Study Visit.a
| Testosterone-Treated Group | Placebo-Treated Group | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | Month 3 | Month 6 | Baseline | Month 3 | Month 6 | |
| Total testosterone, ng/dL | 308.2 (92.1) | 649.6 (133.4) | 600.7 (228.3) | 284.2 (76.5) | 294.3 (77.5) | 278.2 (104.9) |
| SHBG, nmol/L | 48.0 (18.1) | 44.8 (19.8) | 46.8 (17.5) | 39.8 (16.0) | 36.5 (15.3) | 37.5 (13.6) |
| Free testosterone, ng/dL | 4.8 (1.2) | 12.8 (4.9) | 11.1 (5.2) | 5.0 (1.4) | 5.6 (1.4) | 5.1 (1.7) |
Abbreviations: SD, standard deviation; SHBG, sex hormone-binding globulin.
aBold values indicate a significant change from baseline, based on the paired t test (P < .01 for all significant changes).
Table 3.
Fitted Baseline Mean (SE) and Change Values (SE) for Neurocognitive, Mood, and Hormone Measures by Treatment Group and Study Visit.
| Testosterone-Treated Group | Placebo-Treated Group | P Valuea | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | Month 3 Change From Baseline | Month 6 Change From Baseline | Baseline | Month 3 Change From Baseline | Month 6 Change From Baseline | ||
| Number of participants | 10 | 10 | 9 | 12 | 10 | 10 | |
| Cognitive measures | |||||||
| RAVLT | |||||||
| Immediate (total score, 4 trials) | 44.7 (2.7) | 0.6 (2.2) | −0.1 (2.2) | 37.4 (2.5) | 0.6 (2.1) | 2.0 (2.1) | .71 |
| Short delay (total score) | 7.5 (1.1) | 1.2 (1.0) | 0.7 (1.0) | 6.0 (1.0) | −0.7 (0.9) | 1.4 (1.0) | .15 |
| Long delay (total score) | 6.2 (1.1) | 2.3 (0.9) | 0.9 (0.9) | 4.9 (1.0) | 0.0 (0.8) | 1.5 (0.8) | .05 |
| Story Recall | |||||||
| Immediate (total words) | 22.1 (2.3) | 4.2 (2.4) | 0.8 (2.4) | 22.0 (2.1) | −2.1 (2.3) | −1.7 (2.3) | .15 |
| Delay (total words) | 16.4 (2.8) | 5.8 (2.9) | 2.7 (2.9) | 16.5 (2.5) | −2.7 (2.8) | −0.6 (2.8) | .10 |
| Visual Spatial Learning Test | |||||||
| Immediate (correct design and position, 5 trials) | 14.5 (2.0) | −3.6 (1.6) | 0.9 (1.6) | 10.7 (1.9) | 0.1 (1.7) | 5.0 (1.7) | .14 |
| Delay (correct design and position) | 3.8 (0.7) | −1.2 (0.7) | 0.4 (0.7) | 3.5 (0.6) | −0.6 (0.7) | 0.7 (0.7) | .79 |
| Letter-Number Sequencing | |||||||
| Span | 5.3 (0.4) | −0.1 (0.3) | −0.5 (0.3) | 5.1 (0.3) | −0.1 (0.3) | −0.1 (0.3) | .55 |
| Total score | 10.5 (0.9) | 0.3 (0.6) | −1.4 (0.6) | 9.3 (0.8) | 0.8 (0.6) | 0.3 (0.6) | .11 |
| Computerized Simple Reaction Time | |||||||
| 2-second intertrial interval, milliseconds | 335 (66) | 34 (94) | 30 (97) | 375 (63) | −59 (92) | 118 (92) | .37 |
| 5-second Intertrial Interval (milliseconds) | 316 (23) | 23 (24) | 21 (25) | 360 (22) | −29 (24) | −31 (24) | .20 |
| Computerized Choice Reaction Time | |||||||
| 2-second intertrial interval, milliseconds | 494 (19) | 14 (26) | 17 (27) | 533 (18) | 5 (26) | 7 (26) | .95 |
| 5-second intertrial interval, milliseconds | 523 (27) | 73 (34) | 3 (35) | 549 (26) | 22 (34) | 11 (34) | .39 |
| Route Test | |||||||
| Immediate (total score, 3 trials) | 30.7 (2.6) | −4.5 (2.8) | −0.1 (2.9) | 26.7 (2.3) | −2.9 (2.7) | −0.6 (2.7) | .84 |
| Delay (total score) | 11.2 (1.0) | −0.1 (1.2) | −0.7 (1.3) | 10.3 (1.0) | −2.3 (1.2) | −0.5 (1.2) | .28 |
| Complex Design Construction, seconds | 64.2 (10.2) | −7.3 (5.5) | −5.0 (5.5) | 52.1 (9.3) | −8.7 (5.3) | 0.5 (5.3) | .62 |
| Verbal Fluency (total score, 2 trials) | 25.6 (2.5) | 1.0 (1.9) | 0.8 (1.9) | 24.6 (2.2) | 1.0 (1.8) | −2.3 (1.8) | .37 |
| Mental Rotation (total score) | 10.5 (1.1) | 2.3 (0.9) | 2.1 (0.9) | 11.7 (1.0) | 2.8 (0.9) | 2.1 (0.9) | .91 |
| Mood and other measures | |||||||
| Activities of Daily Living Questionnaire | 4.3 (1.2) | −0.5 (1.1) | −1.6 (1.2) | 3.5 (1.1) | 1.2 (1.0) | 0.5 (1.0) | .31 |
| Geriatric Depression Scale | 7.2 (1.3) | 0.7 (1.5) | −2.8 (1.5) | 4.1 (1.2) | 2.3 (1.3) | 2.7 (1.3) | .02 |
| Profile of Mood States | |||||||
| Tension-Anxiety | 48.1 (1.7) | −1.7 (1.9) | −1.1 (1.8) | 46.0 (1.5) | −0.3 (1.7) | −3.5 (1.7) | .33 |
| Depression | 46.8 (1.2) | −2.7 (1.4) | −1.6 (1.4) | 48.5 (1.1) | −2.8 (1.2) | −3.1 (1.3) | .65 |
| Anger-Hostility | 47.4 (1.9) | −2.2 (2.6) | 0.0 (2.5) | 48.3 (1.7) | −1.0 (2.3) | 0.1 (2.4) | .92 |
| Vigor-Activity | 57.7 (2.2) | −4.5 (2.8) | 1.9 (2.7) | 55.5 (2.0) | −0.1 (2.4) | −0.5 (2.5) | .19 |
| Fatigue | 49.4 (2.1) | −1.1 (2.7) | −1.8 (2.6) | 49.6 (2.0) | 0.3 (2.4) | −0.6 (2.4) | .90 |
| Serum hormone values | |||||||
| Total testosterone, ng/dL | 308 (40) | 339 (52) | 294 (52) | 280 (40) | 12 (51) | −4 (51) | <.01 |
| SHBG, nmol/L | 48.0 (5.2) | −3.2 (2.7) | −3.0 (2.7) | 39.0 (5.0) | −3.3 (2.7) | −2.2 (2.7) | .97 |
| Free testosterone, ng/dL | 4.8 (1.0) | 7.9 (1.2) | 6.5 (1.2) | 5.0 (1.0) | 0.5 (1.2) | 0.1 (1.2) | <.01 |
Abbreviations: Free testosterone, non-SHBG bound testosterone; RAVLT, Rey Auditory Verbal Learning Test; SE, standard error; SHBG, sex hormone binding globulin.
a P value for likelihood ratio test of the significance of the study visit by treatment group interaction term in the linear mixed effects regression model; see text for details. GDS was lower (less depression) in the treatment group at month 6 compared to baseline, including treatment by group interaction p < .02. RAVLT was higher (improved) compared to baseline in the treatment group p < .05. Rey Auditory Verbal Learning Test, Story Recall, Visual Spatial Learning Test, Letter-Number Sequencing, Route Test, Verbal Fluency, Mental Rotation—higher score indicates higher level of functioning. Complex Design Construction, Computerized Simple and Choice Reaction Time—lower score indicates faster time and thus higher level of functioning. Activities of Daily Living Questionnaire (ADLQ)—lower score indicates a higher level of functioning; Geriatric Depression Scale (GDS)—higher score indicates a more depressed mood; Profile of Mood States (POMS)—T-scores (mean of 50 standard deviation of ten) compare participants to published norms and higher score indicates elevated emotion in subscale.
Mood and Quality-of-Life Measures
Table 3 shows the fitted values for the mood and quality-of-life measures at baseline as well as the fitted change from baseline at months 3 and 6 for each treatment group based on the linear mixed effects model. The T-treated group endorsed significantly fewer depression symptoms at month 6 as measured by GDS at month 6 compared to baseline, while the placebo group rated their depression increasingly higher throughout the study (study visit by treatment group interaction P < .02, month 6 by treatment group interaction P < .005). The additional mood measures that were collected during the study including MMQ, ADLQ, and POMS are not reported here due to large percentage of missing observations. Participants with time constraints were allowed to complete questionnaires at home to help reduce burden of study visit duration, and the return rate was not as favorable compared to those that were administered by a study assistant during the study visit.
Cognitive Measures
No significant changes were observed in the computerized tasks of attention or spatial abilities, the Route spatial memory test, the visual memory test (VSLT), verbal fluency, or working memory task (LNS; see Table 3). For the RAVLT verbal memory (long delay recall), the pattern of change from baseline differed by treatment group (study visit by treatment group interaction P < .05). However, this change was mostly driven by improvements at 3 months for the T group compared to placebo that were not sustained at 6 months. Although not significant, there was a trend toward improvement in the T-treated group versus placebo-treated group on the other verbal memory task of Story Recall at 3 months (P = .10).
Adverse Events and Dropouts
While taking study medication, 1 participant went to the emergency department (ED) for chest pains, upper arm pain, and dizziness. This participant was cleared from the ED within hours and continued on study medication which after unblinding was revealed to be active medication—T gel. Another participant went to the ED for confusion and disorientation. The participant was also cleared from the ED within hours and continued on study medication which after unblinding was found to be placebo. One participant in the T-treated group had a rise in PSA above 4.0 ng/mL and discontinued study medication per study protocol.
Additional Measures
Genotyping for APOE on the sample revealed 3 participants with APOE 4 allele (all 3 were 3/4), 2 of whom were randomly assigned to the T-treated group and 1 to the placebo-treated group. Inclusion of APOE status as binary factor (yes for 3/4 and no for other allele combinations) in the model did not modify the effect of treatment on the outcome.
At the completion of the study, participants responded to questions about their impression of overall change (a clinical global impression of change, CGIC) on a Likert-type scale, with 6 representing markedly improved, 3 representing no change, and 0 representing markedly worse. Average CGIC in the placebo-treated group was 2.5 and T-treated group was 2.7, a nonsignificant difference. Participants also indicated their belief regarding study assignment (ie, active study medication or placebo). Most participants were not able to discern whether they had received T gel versus placebo, as 50% of the placebo-treated group indicated they had received active T gel and 44% of the T-treated group indicated they had received T gel.
Discussion
This clinical trial examined cognitive and mood changes with 6 months of T versus placebo treatment in older men with low T levels and MCI. We observed changes in response to treatment on some but not all measures of mood and cognition. A significant improvement in a single measure of verbal memory was observed. This modest benefit of T supplementation on verbal memory was observed at 3 months but not sustained at 6 months. This is consistent with Fukai et al who found that in a study of 11 men with MCI, 2 separate global cognitive measures improved after 6 months of oral T treatment, although this study was not placebo controlled. 28 Other studies have also demonstrated a beneficial change in verbal memory or working memory from T supplementation in men with low T levels but without a diagnosis of MCI. 29,30 However, prior studies of T supplementation in patients with MCI 9 or healthy older men 8 have observed no discernable change in cognition.
Findings from our previous studies in men with low to low-normal T levels and AD found improvements in spatial abilities and spatial memory 31 with similar changes in men without baseline memory impairments. 32 We did not observe a similar change in spatial memory in this sample.
Animal models provide support for an important role of gonadal steroids in the central nervous system and in particular hippocampal plasticity in addition to evidence of a functional role in cognition and in particular spatial cognition. 33
It has been increasingly recognized that the clinic-pathological process of AD involves a lengthy pathological process with a long prodromal period prior to the emergence of clinical symptoms. This has resulted in recommendations to include patients with MCI or mild memory difficulties in therapeutic clinical trials with proxy study end points such as neuroimaging of β-amyloid, a protein involved in the pathophysiology of AD. It is possible that therapeutic efforts in the preclinical stages may have a greater impact on the disease process compared to interventions targeted at well-characterized patients with AD having clinical symptoms who may already have well-developed pathology.
Studies of T supplementation in patients with AD have been mixed with 2 studies reporting a beneficial effect on cognition 31,34 and 1 study finding no improvement after 24 weeks of T gel. 35 In 2 different animal models of AD, T has shown a beneficial impact on disease markers as well as cognitive function. 36
Testosterone may have an impact on particular aspects of AD pathophysiology such as β-amyloid and τ. Plasma levels of Aβ40 are inversely correlated with endogenous T levels in men with AD/MCI and significantly increase in response to complete depletion of T. 37 Animal studies indicate brain increases of Aβ40,42 from gonadectomy are reversed with androgens. 38 Thus, T supplementation may also have an impact on pathophysiological processes in addition to cognition and quality of life (see review, Pike et al). 39
In addition to a change in verbal memory, we observed a reduction in depression symptoms as measured by the GDS. The baseline scores on the GDS were not in the range that could be considered depression. Rather the range of scores would be considered within the normal to mildly depressed range at baseline. Both cognitive impairments and depression symptoms are common in older men with low T levels, 3,4,40 with some indication of specific depression or dysthymic (mild depression) symptoms that are evident in tandem with T levels. Depression symptoms and quality-of-life factors are both predictive of future cognitive decline in older adults suggesting that when left untreated, depression can put patients at risk of continued decline and impairment. 41,42
Several studies have examined T supplementation in men with depression or dysthymia (subclinical depression symptoms). In general, studies indicate that in older men with low T and dysthymia (a persistent form of mild depression), depression symptoms improve with T supplementation. 43 However, T treatment of more significant depression symptoms reaching clinical depression range may not be beneficial (see review, Siedman et al). 44
Occurrences of adverse effects in all participants (T and placebo) included incidents of chest pain, confusion, dizziness as well as a rise in PSA for 1 participant. Beyond the rise in PSA for 1 participant who received T gel, we did not observe other known side effects of T replacement such as acne, gynecomastia (breast tenderness, breast enlargement), hair loss and/or hair growth, or worsening of sleep apnea. Two participants reported preexisting mild sleep apnea for which they regularly used a CPAP machine, and they reported no change during treatment.
A common side effect of concern for T supplementation can include adverse effects on prostate such as BPH. Although the relationship between BPH and prostate cancer and T is complex, support for increasing risk of cancer or worsening BPH with T is lacking. 26 Testosterone supplementation is contraindicated in men with existing prostate cancer. This study followed guidelines of including a baseline PSA evaluation along with DRE of prostate health. A recent study of T supplementation in men with mobility impairment with high baseline risk of cardiovascular disease (Testosterone in Older Men [TOM] trial) found a higher than expected incidence of self-reported cardiovascular adverse events in the T-treated group compared to placebo. 45 Thus, men with underlying cardiovascular disease considered for T treatment will need to be carefully evaluated before initiation of treatment.
Strengths and Limitations
Although the incidence of both low T levels and MCI increases with age, the final sample size of the current study was smaller than anticipated, and results may be underpowered. To be included in this study, participants were required to have both MCI and low T and they had to meet additional health requirements such as low PSA (<4.0) and lack of (severe) lower urinary tract symptoms. Thus, the study sample represents men with low T, MCI, and additional health criteria. Among men who qualified for the study, some drop outs were related to their choice of seeking treatment of their low T with a primary care provider rather than participate in a study with randomization to placebo.
A limitation of the findings relates to the variability in baseline T levels in the sample as well as some variability in T levels after 3 months of treatment (Supplemental data—Figure 4). The T levels were obtained at the time of screening and processed by the clinical laboratory and T measurements were performed by platform-based immunoassay. Only men with a serum T level below 300 ng/dL at the time of screening were enrolled. Following the screening visit, blood samples were obtained, processed, and frozen for batch analysis using a more accurate mass spectrometry-based T assay at each study visit, and these data are presented in Tables 2 and 3, which indicate that not all men had total T levels lower than 300 ng/dL at baseline study visit as measured by sensitive batch analysis methods. Both biological and assay variation contribute to variability in T levels in men and this is reflected in the variation of T levels in the placebo group (Table 3). This has led to some recommendations of obtaining 2 or more serum hormone levels to be confident of hypogonadal status 46 ; however, a decision to obtain 1 clinical sample at time of screening was chosen to reduce participant burden. 47,48
Variability in T levels in the T-treated group was observed over the course of the 6-month study, with some drop in T levels after month 3 (see supplemental information Figure 4). Variation in medication absorption and adherence may have influenced T levels and may also be related to our findings of improved verbal memory at month 3 when T levels were raised in all participants, and this improvement was not maintained at month 6 when T levels dropped for some participants. A recent review of a large market survey database of hypogonadal men using topical gel indicated high discontinuation rates over time as well as dose escalation in some men. 49 The present study did not directly obtain adherence information, which may have been useful in association with measured T levels over time.
Despite the small sample size, the study design has several strengths including randomization of study group assignment, dose adjustment to help achieve consistent hormone level, repeat administration of cognitive tests at baseline to help reduce practice effects as well as use of equal alternate test versions and inclusion of both cognition and mood end points.
Summary
This study investigated effects of T treatment on cognition, mood, and quality of life in men with MCI and low serum T levels. Findings indicate T treatment may modestly improve verbal memory and depression symptoms in men with both MCI and low T. As the condition of MCI can progress to more severe levels of cognitive impairment and/or development of AD, the maintenance of cognition may be considered a beneficial outcome. However, the potential beneficial effects need to be balanced against the potential risks and monitoring efforts on the part of the clinician and patient. It is also possible that T may impact the pathophysiology of AD; however, this would need to be addressed in future studies in humans.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by NIA # AG027156. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, WA as well as the University of Washington, Seattle, WA. The study was also supported by Abbott Laboratories who provided study medication, but did not participate in study design, analyzing data or preparing the manuscript. We would also like to acknowledge the administrative support of Christina Bradic and Ashley Holder.
Supplemental Material: The online supplemental figures are available at http://aja.sagepub.com/supplemental.
References
- 1. Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004;39 (11-12):1633–1639. [DOI] [PubMed] [Google Scholar]
- 2. Moffat SD, Zonderman AB, Metter EJ, Smith AD. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62 (2):188–193. [DOI] [PubMed] [Google Scholar]
- 3. Chu LW, Tam S, Lee PW, et al. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol (Oxf). 2008;68 (4):589–598. [DOI] [PubMed] [Google Scholar]
- 4. Chu LW, Tam S, Wong RL, et al. Bioavailable testosterone predicts a lower risk of Alzheimer's disease in older men. J Alzheimers Dis. 2010;21 (4):1335–1345. [DOI] [PubMed] [Google Scholar]
- 5. Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: Progessive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor to 1 growth hormone. Proc Natl Acad Sci U S A. 1997;94 (14):7537–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherrier MM, Asthana S, Baker LD, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57 (1):80–88. [DOI] [PubMed] [Google Scholar]
- 7. Gray PB, Singh AB, Woodhouse LJ, et al. Dose-dependent effects of testosterone on sexual function, mood and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90 (7):3838–3846. [DOI] [PubMed] [Google Scholar]
- 8. Maki PM, Ernst M, London ED, et al. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92 (11):4107–4114. [DOI] [PubMed] [Google Scholar]
- 9. Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004, 59 (1):75–78. [DOI] [PubMed] [Google Scholar]
- 10. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256 (3):183–194. [DOI] [PubMed] [Google Scholar]
- 11. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43 (8):1467–1472. [DOI] [PubMed] [Google Scholar]
- 12. Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64 (9):1323–1329. [DOI] [PubMed] [Google Scholar]
- 13. Beglinger LJ, Gaydos B, Tangphao-Daniels O, et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20 (4):517–529. [DOI] [PubMed] [Google Scholar]
- 14. Sullivan K. Alternate forms of prose passages for the assessment of auditory-verbal memory. Arch Clin Neuropsychol. 2005;20 (6):745–753. [DOI] [PubMed] [Google Scholar]
- 15. Mitrushina M, Satz P, Chervinsky A, D’Elia L. Performance of four age groups of normal elderly on the Rey Auditory-Verbal Learning Test. J Clin Psychol. 1991;47 (3):351–357. [DOI] [PubMed] [Google Scholar]
- 16. Tiernan KN, Schenk K, Swadberg D, et al. Puget sound route learning test: examination of the validity and reliability of a novel route test in healthy older adults and Alzheimer's disease patients. Clin Psychol. 2004;8 (1):39–42. [Google Scholar]
- 17. Malec JF, Ivnik RJ, Smith GE, et al. Visual spatial learning test: normative data and further validation. Psychol Assess. 1992;4 (4):433–441. [Google Scholar]
- 18. Groth-Marnat G, Teal M. Block design as a measure of everyday spatial ability: a study of ecological validity. Percept Mot Skills. 2000;90 (2):522–526. [DOI] [PubMed] [Google Scholar]
- 19. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 20. Spreen O, Strauss E. A compendium of neuropsychological tests. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 21. McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 22. Galasko D, Bennett A, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzeheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (suppl 2):S33–S39. [PubMed] [Google Scholar]
- 23. Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57 (1):P19–P27. [DOI] [PubMed] [Google Scholar]
- 24. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24 (4):709–711. [PubMed] [Google Scholar]
- 25. Ware JE, Jr, Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 26. Page ST, Hirano L, Gilchriest J, et al. Dutasteride reduces prostate size and prostate specific antigen in older hypogonadal men with benign prostatic hyperplasia undergoing testosterone replacement therapy. J Urol. 2011;186 (1):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84 (10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 28. Fukai S, Akishita M, Yamada S, Toba K, Ouchi Y. Effects of testosterone in older men with mild-to-moderate cognitive impairment. J Am Geriatr Soc. 2010;58 (7):1419–1421. [DOI] [PubMed] [Google Scholar]
- 29. Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28 (6):875–8821. [DOI] [PubMed] [Google Scholar]
- 30. Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57 (5):M321–M325. [DOI] [PubMed] [Google Scholar]
- 31. Cherrier MM, Matsumoto AM, Amory JK, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64 (12):2063–2068. [DOI] [PubMed] [Google Scholar]
- 32. Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57 (1):80–88. [DOI] [PubMed] [Google Scholar]
- 33. Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63 (4):559–565. [DOI] [PubMed] [Google Scholar]
- 34. Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer's disease. Aging Male. 2003;6 (1):13–17. [PubMed] [Google Scholar]
- 35. Lu PH, Masterman DA, Mulnard R, et al. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63 (2):177–185. [DOI] [PubMed] [Google Scholar]
- 36. Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26 (51):13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gandy S, Almeida OP, Fonte J, et al. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285 (17):2195–2196. [DOI] [PubMed] [Google Scholar]
- 38. Ramsden M, Nyborg AC, Murphy MP, et al. Androgens modulate B-amyloid levels in male rat brain. J Neurochem. 2003;87 (4):1052–1055. [DOI] [PubMed] [Google Scholar]
- 39. Pike CJ, Rosario ER, Nguyen TV. Androgens, Aging, and Alzheimer's Disease. Endocrine. 2006;29 (2):233–241. [DOI] [PubMed] [Google Scholar]
- 40. Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87 (11):5001–5007. [DOI] [PubMed] [Google Scholar]
- 41. Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15 (5):406–415. [DOI] [PubMed] [Google Scholar]
- 42. Richard E, Schmand B, Eikelenboom P, et al. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer's disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33 (2-3):204–209. [DOI] [PubMed] [Google Scholar]
- 43. Shores MM, Moceri VM, Gruenewald DA, Brodkin KI, Matsumoto AM, Kivlahan DR. Low testosterone is associated with decreased function and increased mortality risk: a preliminary study of men in a geriatric rehabilitation unit. J Am Geriatr Soc. 2004;52 (12):2077–2081. [DOI] [PubMed] [Google Scholar]
- 44. Seidman SN, Weiser M. Testosterone and mood in aging men. Psychiatr Clin North Am. 2013;36 (1):177–182. [DOI] [PubMed] [Google Scholar]
- 45. Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68 (2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95 (6):2536–2559. [DOI] [PubMed] [Google Scholar]
- 47. Anawalt BD, Hotaling JM, Walsh TJ, Matsumoto AM. Performance of total testosterone measurement to predict free testosterone for the biochemical evaluation of male hypogonadism. J Urol. 2012;187 (4):1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67 (6):853–862. [DOI] [PubMed] [Google Scholar]
- 49. Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. J Sex Med. 2013;10 (5):1401–1409. [DOI] [PubMed] [Google Scholar]