Abstract
Alzheimer’s disease (AD) is the most frequent cause of dementia. This work aims to assess the effectiveness of reality orientation (RO), a traditional, extensively documented cognitive enhancement technique, when combined with acetylcholinesterase inhibitors in the treatment of AD. Fourteen patients with AD having mild to moderate dementia receiving standard treatment with donepezil were randomly assigned to control and treatment groups. Patients in the treatment group were submitted to weekly RO sessions for 6 months. Cognitive outcomes were assessed based on scores in the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery and the Clock Drawing Test (CDT). Mean CERAD neuropsychological battery, Mini-Mental State Examination (MMSE), and CDT scores improved in the treatment group and worsened in the control group. A number of CERAD neuropsychological battery and MMSE scores were statistically significant. Our findings suggest that RO is a valuable long-term complementary intervention for dementia in AD.
Keywords: Alzheimer’s disease, dementia, cognition, reality orientation
Introduction
Alzheimer’s disease (AD) is the most frequent cause of dementia. 1 The principal risk factor for the disease is age. 2 Given the recent epidemiological transition in Brazil, this is a cause for particular concern. 3
Several dysfunctions are observed in patients with AD, particularly those related to β-amyloid and tau protein abnormalities, such as synaptic failure, neuronal loss, vascular damage, oxidative stress, and parenchymal inflammation. However, approximately 95% of cases are sporadic, and the key event that leads to these features has not yet been established, although many hypotheses have been put forward. 2,4
Clinically, AD consists of progressive cognitive decline, frequently presenting initially as short-term memory impairment and affecting judgment, decision making, and orientation skills. Later stages of the disease also present with behavioral disturbances and language abnormalities. 4 According to recent recommendations for diagnostic criteria, clinical investigation can determine whether the disease is probable, possible, or unlikely. 5 Clinical staging scales can be used to classify the dementia as mild, moderate, or severe. 6
Pharmacological intervention is the main form of treatment for AD, although no drug has yet been able to reverse progression of the disorder. 7 Acetylcholinesterase inhibitors (AChEIs) are recommended for the treatment of all stages of dementia in AD. 7 Most recent reviews show that, among nonpharmacological approaches, cognitive enhancement activities are useful strategies for improving cognitive function in patients with dementia. 8,9 Aging causes a self-reinforcing downward spiral of degraded brain function, and behavioral training interventions of this kind may promote recovery or improvement in cognitive skills by modulating brain plasticity. 10
First described in 1966 as a therapy for the rehabilitation of confused elderly patients, 11 reality orientation (RO) is an important cognitive stimulation technique. 9 It involves presenting the patient with continuous memory and orientation information related to personal issues and the patient’s environment. Numerous ways of implementing RO have been described. 8,12 During sessions, the patient is encouraged to discuss various subjects related to recent events and his daily routine. Encouraging the patient to engage socially, especially when this is based on his or her personal interests, is also a very important part of the therapy. 12,13 After the first review of RO was published, 13 interest in the subject increased dramatically and most subsequent articles reported substantial benefits following the use of these strategies. 12,14
Although RO focuses on current social, spatial, and temporal orientation skills, other cognitive stimulation strategies that have emerged in recent decades use broader-based approaches. One example is cognitive stimulation therapy (CST), which was proposed several decades after RO. 15 The CST is based not only on some features assessed in RO but also on a number of additional features, such as reminiscence and multisensory stimulation. 14,16 However, the potential applications of CST are not as extensively documented in the literature as those of RO, which served as the methodological basis for many subsequent interventions. 8
The most recent reviews on RO corroborated earlier findings of substantial benefits and also identified existing and new areas where further work is required. 8,9 Establishing a uniform methodology, identifying long-term benefits and determining to what extent RO is viable in practice are among the greatest challenges still to be faced. A further issue is the need for decisive studies on the subject in Brazil. The findings of the few articles on cognitive stimulation therapies that have been published seem to lack consistency, 17,18 highlighting the need for further studies to provide a body of consistent national evidence supporting RO and other related methods.
In light of this, the present work aims to assess the effectiveness of RO when combined with AChEI in the treatment of mild and moderate AD dementia.
Materials and Methods
Standard Protocol Approval and Patient Consent
This research was approved by the Research Ethics Committee of the State University of Ponta Grossa, Paraná, Brazil. For patients to participate, they and their respective caregivers had to sign an informed consent form.
Selection and Clinical Assessment of Patients
The study sample was selected at the Neurology Outpatient Unit, Hospital Universitário Regional dos Campos Gerais (HURCG), Brazil. All patients were clinically assessed to confirm the diagnosis of AD according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition 19 and given their first cognitive ability tests, which were based on the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery and the Clock Drawing Test (CDT). The score for the grading tests ranged from 1 to 10 points. 20 The CERAD battery is divided into 8 items: verbal fluency, naming test, Mini-Mental State Examination (MMSE), 21 word list learning, constructional praxis, word list recall, word list recognition, and constructional praxis recall. 22 Fourteen patients with a diagnosis of AD dementia confirmed by the proposed criteria who had been receiving standard treatment with donepezil for at least 3 months were selected. To be included in the study, patients had to have an MMSE score between 14 and 27. Exclusion criteria included major aphasia or any disability that could impair therapy and prevent the patient being assessed.
Participants were divided into 2 groups according to their order of arrival for treatment at the HURCG outpatient unit. In group A, patients were given only conventional therapy (essentially pharmacological) with no other kind of cognitive intervention, while in group B, patients took part in RO sessions and received conventional therapy.
Description of the Therapy and its Application
Patients from group B took part in weekly 30- to 60-minute long individual RO sessions applied by the researchers themselves. Each session consisted of continuous exposure to memory- and orientation-related information using several approaches.
First, to stimulate personal orientation, patients had to fill out a form with their personal information (name, date of birth, place of birth, marital status, occupation, religion, address, number of children, and children’s names). These answers were obtained by means of constructive questioning led by the examiners.
The next step consisted of temporal and spatial orientation. Patients were required to fill out another form about the current weather and time-related information (weather, approximate time, day of the week, and date) using the same process adopted in the first stage. They then had to walk through the room where the sessions took place and attempt to recognize and assimilate its characteristics, including the layout of the furniture, the number of doors and windows and, more importantly, 5 reference objects placed around the room by the examiners.
The following stage was based on intensive dialog. Patient and examiner discussed the patient’s personal interests and a variety of subjects, such as the latest news and important social events (for example, imminent commitments, birthdays, holidays, and other reference events). Patients were always encouraged to express their own opinions and to participate actively in the discussions.
Finally, there was intense stimulation of social engagement to encourage patients to restore social ties and habits that predated disease onset. This was always based on the patient’s situation and interests. Guidance was also given to caregivers as part of the routine so that patients could be constantly stimulated. The therapy was, therefore, tailored to the patients’ personal characteristics and preferences, and the benefits associated with it extended beyond the weekly meetings.
Outcomes Assessment
Cognition was assessed in both groups with the CERAD neuropsychological battery 22 and the CDT 20 during selection and every 2 months for 6 months (25.7 weeks) after the RO sessions had begun.
Statistical Analysis
All the data were tested according to whether they had a normal distribution or not. Statistical differences between the means of the groups were determined using the 2-tailed student t test for normal distributions and the Mann-Whitney test for nonnormal distributions. The Fisher exact test was used for the differences between the expected values and the values actually found. The results are given as mean ± standard deviation. Differences were considered significant if P < .05.
Results
Mean patient age was 80.14 ± 6.04 years, and 9 (64%) patients were men. Alzheimer’s disease was diagnosed on average 5.36 ± 1.78 years before the start of the study. There were no statistically significant differences between the characteristics of the 2 groups (Table 1). Weekly RO activities and follow-up were completed successfully without any complications in either group. Follow-up lasted 6 months and revealed cognitive benefits in the patients who received RO.
Table 1.
Characteristics of Participants at Study Entry.a
| Group A (n = 7) | Group B (n = 7) | P | |
|---|---|---|---|
| Age, years | 79.43 ± 7.11 | 80.86 ± 5.24 | .338 |
| Ratio M:F | 2.5:1 | 1.33:1 | 1 |
| Education, years | 8.71 ± 4.64 | 11.71 ± 3.73 | .103 |
| Duration of disease, years | 4.86 ± 1,68 | 5.86 ± 1.86 | .156 |
| Duration of treatment, years | 4.14 ± 1.68 | 5 ± 2.24 | .216 |
| CERAD neuropsychological battery score | 58.86 ± 15.04 | 57.71 ± 15.78 | .89 |
| MMSE score | 22.71 ± 2.69 | 22.14 ± 3.13 | .72 |
| CDT score | 4.28 ± 2.87 | 3.57 ± 2.64 | .64 |
Abbreviations: CDT, Clock Drawing Test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, Mini–Mental State Examination; SD, standard deviation; M, male; F, female.
aMean values ± SD.
In group A, mean CERAD neuropsychological battery score fell by 4 ± 9.56 points during follow-up, while in group B, it increased by on average 5 ± 6.11 points. Although not statistically significant, there were important differences between the groups at the end of the follow-up period (P = .169). In addition, when scores at the end of the 6-month follow-up were compared with initial scores, a significant improvement was observed in the treatment group (P = .037). Mean scores for each group from the beginning of the study to the end of follow-up are given in Figure 1.
Figure 1.
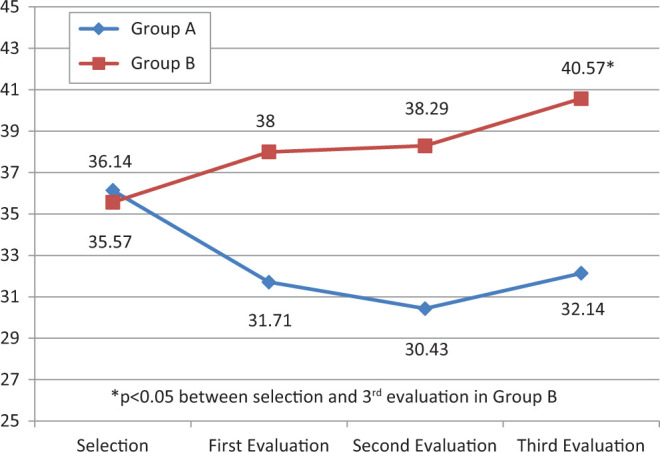
Mean Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery scores for the control group (A) and the treatment group (B) during the 6-month follow-up.
Analysis of each item in the CERAD neuropsychological battery separately failed to reveal any statistically significant differences between mean scores for the 2 groups during selection. However, at the third follow-up evaluation, there were statistically significant differences between treatment and control groups in 2 items (naming test and constructional praxis), as shown in Table 2. There was also an important improvement in word list learning in group B, which increased by on average 2.14 ± 2.61 (P = .03) between selection and the third follow-up evaluation.
Table 2.
Mean Scores for Individual Tests in the CERAD Neuropsychological Battery for the Control Group (A) and the Treatment Group (B) at Study Entry and at the Third Follow-Up Evaluation.
| Study Entry | Third Evaluation | |||||
|---|---|---|---|---|---|---|
| Group A (n = 7) | Group B (n = 7) | P | Group A (n = 7) | Group B (n = 7) | P | |
| Verbal fluency | 9.28 ± 4.07 | 11.28 ± 7.89 | .28 | 7.57 ± 3.55 | 10.29 ± 3.64 | .09 |
| Naming test | 8 ± 1.63 | 9.57 ± 13.08 | .15 | 7.57 ± 2.15 | 10.43 ± 3.36 | .04 |
| MMSE | 22.71 ± 2.69 | 22.14 ± 3.13 | .36 | 21.57 ± 3.99 | 23.57 ± 2.70 | .14 |
| Word list learning | 8 ± 2.51 | 8.29 ± 3.63 | .43 | 8.71 ± 5.22 | 10.43 ± 7.29 | .27 |
| Constructional praxis | 5.71 ± 2.14 | 6.57 ± 1.13 | .18 | 5 ± 1.83 | 7.29 ± 1.25 | .009 |
| Word list recall | 2.57 ± 3.1 | 1.57 ± 3.44 | .23 | 1.43 ± 1.81 | 1.29 ± 1.38 | .44 |
| Word list recognition | 2.14 ± 3.08 | 3.14 ± 2.61 | .35 | 3.43 ± 3.31 | 4 ± 3 | .37 |
| Constructional praxis recall | 1.43 ± 2.99 | 0.14 ± 0.38 | .14 | 1.43 ± 2.57 | 1.43 ± 2.7 | .5 |
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, Mini-Mental State Examination.
Mean score in the MMSE, which was performed as part of the CERAD neuropsychological battery, decreased slightly in group A (1.14 ± 3.76 points) and improved by 1.43 ± 2.67 points in group B. Although not statistically significant (P = .146), there was a marked difference between the control and treatment groups. At the second follow-up evaluation, however, there was a statistically significant difference between the groups (P = .039). At this evaluation, there was also a statistically significant change compared with initial scores in the treatment group (P = .0018). Mean MMSE scores in both groups at selection and during follow-up are shown in Figure 2.
Figure 2.
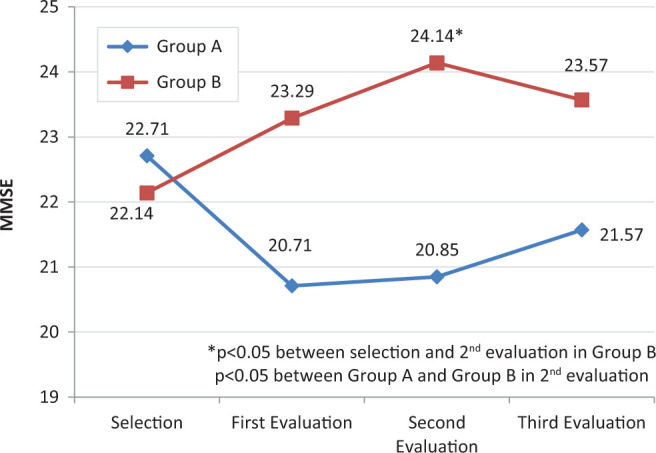
Mean Mini-Mental State Examination (MMSE) scores for the control group (A) and the treatment group (B) during the 6-month follow-up.
Mean CDT score for group A fell by 1.34 ± 2.14 points, while for group B, it increased by 1.43 ± 2.15 points. There was no statistically significant difference between the groups at the end of the follow-up (P = .183). Nevertheless, as shown in Figure 3, there were very clear differences during follow-up.
Figure 3.
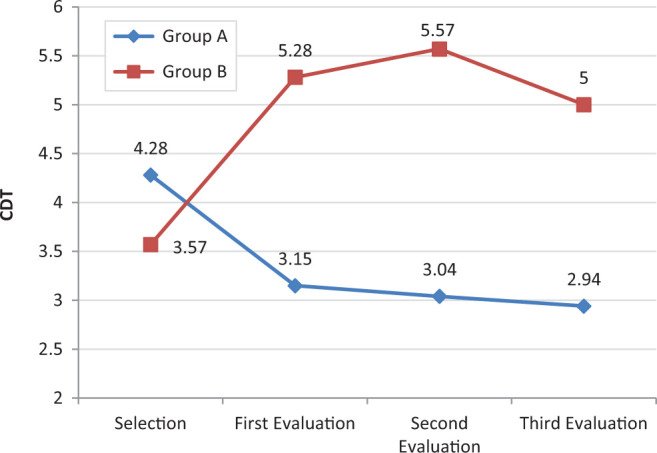
Mean Clock Drawing Test (CDT) scores for the control group (A) and the treatment group (B) during the 6-month follow-up.
Discussion
Our findings show that there was an improvement in cognitive functions in patients with mild to moderate AD dementia who had weekly individual RO sessions for 6 months and that RO enhanced the effectiveness of a conventional pharmacological approach. These findings support recent evidence of the effectiveness of CST 8,9 and are very similar to those of related studies. A multicenter randomized controlled trial conducted by Onder et al 23 also concluded that RO provides cognitive benefits and used MMSE score as one of the parameters for identifying improvements. The authors evaluated the efficacy of RO combined with cholinesterase inhibitors in patients with AD dementia. Their sample was much larger than ours and consisted of 156 patients, of whom 79 had RO sessions. Follow-up lasted on average 25.4 weeks, approximately the same length as in our study. However, RO sessions were performed in the patients’ homes by trained caregivers, a limitation of their work. 23
Of the studies identified when we carried out a literature review, only 1, by Breuil et al, 15 used the CERAD neuropsychological battery to assess the effects of cognitive stimulation in patients with dementia. Furthermore, the assessments were restricted to the MMSE, word list memory, and verbal fluency, and the other assessments in the battery were not included in the results. An improvement was observed in the MMSE and word list memory task for patients who received CST. 15
Although CERAD neuropsychological battery total score is recognized as a valid measure of the progression of AD dementia, 24 to our knowledge no studies have used it to evaluate the effectiveness of cognitive stimulation. In our treatment group, there was a statistically significant improvement in this score, indicating a general cognitive increase in patients who received RO. Three specific items in the battery were observed to have changed significantly, that is, the naming test, word list learning, and constructional praxis. This suggests that RO had most effect on frontal functions.
Although there were few statistically significant outcomes, a marked improvement was observed in patients who received RO, especially when contrasted with the deterioration in the control group. The increase in mean MMSE scores agreed with values reported in similar studies, in which mean MMSE score ranged from 0.2 to 3 points. 23,25,26 Likewise, the mean decline in the control group was similar to the declines observed in other trials, which ranged from −0.3 to −2.8 points. 23,25,26 This outcome is extremely important because even small changes in MMSE score imply significant changes in health care expenses for patients with dementia. 27,28
Cognitive stimulation techniques have proved to be affordable and very well-accepted interventions in other countries and appear to be more cost-effective and practical than isolated standard approaches. 29,30 The benefits of these techniques and the worldwide scarcity of health care resources 29 make RO a potentially valuable resource for mitigating AD morbidity and reducing health care expenses.
The main limitation of the present study is the sample size and consequently lower level of evidence. Nonetheless, clear differences were observed in both groups, including statistically significant differences in CERAD neuropsychological battery total scores. Given the limited number of patients, allocation based on order of arrival could be considered a potential source of bias. Furthermore, the fact that outcomes assessment was performed by the same researchers who conducted the RO sessions could have caused subjective bias in the interpretation of the data even though all steps followed the methodology strictly.
Although MMSE scores showed statistically significant changes when the second battery of tests was carried out, this was not maintained until the third follow-up evaluation. This finding could be interpreted as an isolated result or as an indicator that the effect of RO has a limited duration. It could also be objected that these outcomes are the result of a simple increase in attention and social stimulation in patients who received RO. However, it has been proved that complementary activities (including alternative social therapies) offered to control groups have no impact on cognitive results during RO trials. 13
The most significant features of the present study are the novel use of the CERAD neuropsychological battery to assess the effectiveness of RO; the finding that RO seems to influence mainly frontal cognitive functions; and the evidence it provides that this treatment may be suitable for use in Brazil in view of the clear benefits identified in this study and the similarity between our findings and those of trials published in other countries.
Further data and comprehensive studies are required to confirm these findings and provide more accurate confirmation of the benefits of other similar cognitive stimulation techniques. In conclusion, the outcomes reported here suggest that RO is a valuable, low-cost, long-term, complementary intervention for AD dementia and that it has a more pronounced influence on frontal cognitive functions.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Cohen RM. Epidemiology and clinical diagnosis: Alzheimer disease. PET Clin. 2013;8(4):391–405. [DOI] [PubMed] [Google Scholar]
- 2. Querfurth HW, LaFerla FM. Alzheimer’s disease: mechanisms of disease. N Engl J Med. 2010;362 (4):329–344. [DOI] [PubMed] [Google Scholar]
- 3. Marinho FM, Soliz P, Gawryszewski V, Gerger A. Epidemiological transition in the Americas: changes and inequalities. Lancet. 2013;381(Special issue, S89);381:S89. [Google Scholar]
- 4. Galimberti D, Scarpini E. Progress in Alzheimer’s disease. J Neurol. 2012;259 (2):201–211. [DOI] [PubMed] [Google Scholar]
- 5. Frota NAF, Nitrini R, Damasceno BP, et al. Critérios para o diagnóstico de doença de Alzheimer. Dement Neuropsychol. 2011;5(suppl 1):5–10. [Google Scholar]
- 6. Rikkerd MGMO, Tona KD, Janssen L, et al. Clinical staging scales in dementia: a systematic review. Am J Alzheimers Dis Other Demen. 2011;26 (5):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. do Vale FAC, Corrêa Neto Y, Bertolucci PHF, et al. Treatment of Alzheimer’s disease in Brazil. Dement Neuropsychol. 2011;5 (3):178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguirre E, Woods RT, Spector A, Orrell M. Cognitive stimulation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Ageing Res Rev. 2013;12 (1):253–262. [DOI] [PubMed] [Google Scholar]
- 9. Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropshycol Rev. 2013;23 (1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. [DOI] [PubMed] [Google Scholar]
- 11. Taulbee LR, Folsom JC. Reality orientation for geriatric patients. Hosp Community Psychiatry. 1966;17(5):133–135. [DOI] [PubMed] [Google Scholar]
- 12. Spector A, Orrell M, Woods B. Cognitive Stimulation Therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry. 2010;25 (12):1253–1258. [DOI] [PubMed] [Google Scholar]
- 13. Spector A, Davies S, Bob Woods, Orrell M. Reality Orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist. 2000;40 (2):206–212. [DOI] [PubMed] [Google Scholar]
- 14. Spector A, Woods B, Orrell M. Cognitive stimulation for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2008;8 (5):751–757. [DOI] [PubMed] [Google Scholar]
- 15. Breuil V, De Rotrou J, Forette F. Cognitive stimulation of patients with dementia: preliminary results. Int J Geriatr Psychiatry. 1994;9:211–217. [Google Scholar]
- 16. Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil. 2004;14(4):385–401. [Google Scholar]
- 17. Bottino CMC, Carvalho IAM, Alvarez AMMA, et al. Reabilitação cognitiva em pacientes com doença de Alzheimer. Arq Neuropsiquiatr. 2002;60 (1):70–79. [DOI] [PubMed] [Google Scholar]
- 18. Camara VD, Gomes SS, Ramos F, et al. Reabilitação cognitiva das demências. Rev Bras Neurol. 2009;45(1):25–33. [Google Scholar]
- 19. American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, D.C: American Psychiatry Association; 1994. [Google Scholar]
- 20. Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease: a novel measure of dementia severity. J Am Geriatr Soc. 1989;37 (8):725–729. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein ST, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psichiat Res. 1975;12 (3):189–198. [DOI] [PubMed] [Google Scholar]
- 22. Fillenbaum GG, van Belle G, Morris JC, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4 (2):96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onder G, Zanetti O, Giacobini E, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised controlled trial. Br J Psychiatr. 2005;187:450–455. [DOI] [PubMed] [Google Scholar]
- 24. Rossetti HC, Cullum CM, Hynan LS, Lacrits L. The CERAD Neuropsychological Battery total score and the progression of Alzheimer's disease. Alzheimer Dis Assoc Disord. 2010;24 (2):138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prolo P, Fanto F, Santoro M, et al. Long-term Reality Orientation Therapy (ROT) in subjects with dementia of the Alzheimer’s type. Neurobiol Aging. 2004;25:190–191. [Google Scholar]
- 26. Mapelli D, Di Rosa E, Nocita R, Sava D. Cognitive stimulation in patients with dementia: randomized controlled trial. Dement Geriatr Cogn Disord Extra. 2013;3 (1):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jönsson L, Lindgren P, Wimo A, Jönsson B, Winblad B. Costs of Mini Mental State Examination-related cognitive impairment. Pharmacoeconomics. 1999;16 (4):409–416. [DOI] [PubMed] [Google Scholar]
- 28. Wolstenholme J, Fenn P, Gray A, Keene J, Jacoby R, Hope T. Estimating the relationship between disease progression and cost of care in dementia. Br J Psychiatr. 2002;181:36–42. [DOI] [PubMed] [Google Scholar]
- 29. Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatr. 2006;188:574–580. [DOI] [PubMed] [Google Scholar]
- 30. Alves J, Alves-Costa F, Magalhães R, Gonçalves OF, Sampaio A. Cognitive Stimulation for Portuguese older adults with cognitive impairment: a randomized controlled trial of efficacy, comparative duration, feasibility and experiential relevance. Am J Alzheimers Dis Other Demen. 2014;29 (6):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]


