Abstract
In the present study, some 9-aminoacridine derivatives have been synthesized by condensation of 9-aminoacridine with substituted phenacyl, benzoyl, and benzyl halides (RM1-RM6). Compounds were investigated for acetylcholinesterase and butyrylcholinesterase inhibition potential, considering these enzymes playing a key role in Alzheimer’s disease. All derivatives showed better inhibition of enzymes than the standard galantamine, whereas except RM4, all exhibit better results than tacrine, a well-known acridine derivative used for the treatment of Alzheimer’s disease.
Keywords: 9-aminoacridine (9AA), Alzheimer, cholinesterases (ChE), acetylcholinesterase (AChE), butyrylcholinesterase (BuChE), tacrine
Introduction
Acridine-based pharmacophore 1,2 has been found to be associated with a wide spectrum of biological activities, especially for the treatment of Alzheimer’s disease (AD) by inhibiting cholinesterases (ChE). 3 In symptomatic improvement of AD, acetylcholinesterase (AChE) has proven to be the most viable therapeutic goal because cholinergic deficit is a constant and early finding in this AD. There are 2 types of ChEs, AChE and butyrylcholinesterase (BuChE). Several available drugs target both AChE and BuChE in AD, but some are more selective. 4 9-Aminoacridine (9AA) and its derivatives have long been known to be reversible inhibitors of acetyl cholinesterase, the most familiar of which is 9-amino-1,2,3,4-tetrahydroacridine, tacrine (Cognex, Pfizer Pharmaceuticals). 5 This was the first nonclassical inhibitor of AChE approved by the Food and Drug Administration (FDA) in 1993 6 that binds to both AChE and BuChE. 7 However, widespread use of tacrine was limited as it caused a number of side effects including nausea, vomiting, dizziness, diarrhea, seizures, and liver toxicity. Short half-life of tacrine was another major problem. 8 Eventually, tacrine was discontinued due to hepatotoxicity. 9
Physostigmine, first-tested AChE inhibitor (AChEI), originally extracted from calabar beans. Physostigmine could improve memory in people with or without dementia, but this property has been limited by the very short half-life. 10 A number of physostigmine derivatives were synthesized, and some are failing in clinical trials due to severe side effects. 4 Rivastigmine (Exelon, Novartis Pharmaceuticals), a physostigmine derivative, is a centrally selective ChEI that was approved in 2000. Rivastigmine exhibits a low level of hepatotoxicity 11,12 but frequently associated with side effects such as nausea, vomiting, anorexia, diarrhea, headache, fatigue, malaise, sweating, somnolence, dyspepsia, and sinusitis. 13,14
Donepezil (Aricept, Pfizer Pharmaceuticals) is another nonclassic, centrally acting, reversible, noncompetitive AChEI that was approved in 1997 for mild to moderate AD and dementia and has little potential for hepatotoxicity, 11,15 but on high dose, transient nausea, diarrhea, and insomnia were reported. 16
Galantamine (Razadyne, Global Pharmaceuticals), approved in 2000, an alkaloid found in plants of the family Amaryllidaceae, is a reversible inhibitor of AChE but does not inhibit BuChE and used for mild to moderate AD and dementia, and no hepatotoxicity is reported 11,12 but associated with adverse events such as nausea, vomiting, diarrhea, anorexia, muscle cramps, headaches, dizziness, fatigue, and insomnia as a result of cholinergic stimulation. 17,18
Naturally derived drugs such as huperzine A and huperzine B are potent ChEIs. The huperzine A had the least amount of activity against BuChE. 19 Huperzine A is considered to be the drug of choice in China for the treatment of memory disorders. Side effects would be expected to be similar in nature to other ChEIs. 4
Although available cholinesterase inhibitors (AChEIs) provide benefit in AD, all these drugs exhibit varied side effects and complications such as low bioavailability, shorter half-life, and hepatotoxicity. 20- 22
The present study is an attempt to find better ChEI, a crucial target for the treatment of AD. All synthesized acridine derivatives showed promising AChE and BuChE inhibition. Pharmacophoric regions are also determined, highlighting the important structural features of ligands, which may be a great help and guidance for the designing of effective anti-Alzheimer drug. 23
Chemicals and Instruments
All reagents were reagent grade purchased from Merck (Germany) and Sigma-Aldrich Company (Germany) and distilled twice. The monitoring of the reaction and purity of the final product were determined by thin-layer chromatography on silica gel 60 GF254 (Merck). Thin-layer chromatography spot was visualized in UV light at 254 and 365 nm on HPUVIS Desaga (Heidelberg, Germany). Melting points were recorded on STUART (Bibby Sterlin Ltd, UK), SMP3 melting point apparatus, and were uncorrected. Spectroscopic data were recorded on ultraviolet spectrophotometer (CECIL 7200, USA), IR spectrophotometer (FTIR-8900; Shimadzu, Japan) using KBr disc, mass spectrometer (JEOL JMS-HX110, Japan), and 1 H-NMR (Bruker Advance 300, France) in Dimethyl sulfoxide deprotonated (DMSO) at 300 MHz using tetramethylsilane as an internal standard.
Synthesis of Compounds
To a solution of 0.0025 mol/L 9AA in Acetone (15-20 mL), the substituted phenacyl, benzoyl, and benzyl halides (0.0025 mol/L) were added. Reaction mixture was stirred for 5 to 12 hours at room temperature and then refluxed for 15 to 78 hours at 50°C. After cooling, the solid precipitates were separated through vacuum filtration and washed with acetone. Purification was done through recrystallization using double solvent system (ethanol and ether). Pure compounds were dried in vacuum desiccators over silica beads. Melting point was recorded, and spectral studies were done for the confirmation of the resultant product (Figure 1).
Figure 1.
The synthetic scheme of compounds RM1, RM2, RM3, RM4, RM5, and RM6.
N-(3′-Bromophenyl-2″-oxoethyl)acridine-9-ammonium bromide (RM1)
Light yellow powder, yield: 71.25%. melting point decomposed (m.p. d) 215°C, UV(MeOH) ∊: 1817200. IR (KBr) νmax (cm−1): 3107.1, 2993.3, 1595.0, 1479.3, EIMS (−HBr) [M]+: m/z 390. 1H-NMR (DMSO, 300 MHz): δ 3.498 (s, 2H, H-11), 7.548-7.596 (t, J = 6.3 Hz, 3H, H-2, H-7, H-13), 7.949-8.301 (m, 6H, H-1, H-3, H-6, H-8, H-12, H-14), 8.723-8.752 (d, J = 8.7 Hz, 3H, H-4, H-5, H-15).
N-(Acridine-9-yl)-3′, 5′dinitrobenzamide (RM2)
Dark yellow powder, yield: 99.6%. m.p. d 277°C. UV (MeOH) ∊: 1115120. IR (KBr) νmax (cm−1): 3342.4, 3139.9, 1658.7, 1502.4, EIMS (−HCl) [M]+: m/z 388. 1 H-NMR (DMSO, 300 MHz): δ 7.503-7.552 (t, J = 7.2 Hz. 2H, H-2, H-7), 7.854-7.963 (m, 6H, H-1, H-3, H-4, H-5, H-6, H-8), 8.61-8.630 (d, J = 8.7 Hz, 3H, H-11, H-12, H-13).
N-(4′-Nitrophenyl-2″-oxoethyl)acridine-9-ammonium bromide (RM3)
Mustard yellow powder, yield: 99%. m.p. d 243°C. UV (MeOH) ∊: 1046820. IR (KBr) νmax (cm−1): 3190.0, 3109.0, 1649.0, 1595.0, 1479.3, EIMS (−HBr) [M]+: m/z 357. 1H-NMR (DMSO, 300 MHz): δ 3.352 (s, 2 H, H-11), 7.511-7.560 (d, J = 7.2 Hz, 4H, H-2, H-3, H-6, H-7), 7.847-7.966 (m, 6H, H-1, H-4, H-5, H-8, H-12, H-15), 8.587-8.615(d, J = 8.4 Hz, 2H, H-13, H-14).
N-(Acridine-9-yl)-4′-bromobenzamide (RM4)
Florescent yellow powder, yield: 92.2%. m.p. d 275°C. UV (MeOH) ∊: 792960. IR (KBr) νmax (cm−1): 3340, 3138, 1589.2, 1479.3, EIMS (−HCl) [M]+: m/z 377. 1H-NMR (DMSO, 300 MHz): δ 7.505-7.555 (d, J = 7.5 Hz, 2H, H-2, H-7), 7.852-7.964 (m, 6H, H-1, H-3, H-6, H-8, H-12, H-13), 8.597-8.06 (d, J = 8.7 Hz, 4H, H-4, H-5, H-11, H-14).
N-(4′-Bromobenyl)acridine-9-ammonium bromide (RM5)
Fluorescent yellow powder, yield: 39.9%. m.p. d 280°C. UV (MeOH) ∊: 310800. IR (KBr) νmax (cm−1): 3307.7, 3109.0, 1595, 1477.4, EIMS (−HBr) [M]+: m/z 363. 1H-NMR (DMSO, 300 MHz): δ 3.326 (s, 2H, H-11), 8.698-8.726 (d, J = 8.4 Hz, 2H, H-4, H-5), 7.183 (s, 2H, H-12, H-15), 7.559-7.688 (m, 4H, H-2, H-7, H-13, H-14), 7.835-8.039 (m, 4H, H-1, H-3, H-6, H-8).
N-(4′-Methylbenzyl)Acridine-9-Ammonium Chloride (RM6)
Yellow crystals, yield: 25.68%. m.p. 221°C, UV (MeOH) ∊: 858380. IR (KBr) νmax (cm−1): 3182.3, 3049.2, 1485.1, 1392, EIMS (+HCl) [M]+: m/z 334. 1H-NMR (DMSO, 300 MHz): δ 3.323-3.299 (d, J = 4.2 Hz, 5H, H-11 H-16), 7.272-7.322 (t, J = 7.5 Hz, 2H, H-12, H-15), 7.600-7.649 (t, J = 6.9 Hz, 4H, H-2, H-7, H-13, H-14), 7.73-7.821 (m, 4H, H-1, H-3, H-6, H-8), 8.354-8.382 (d, J = 8.4 Hz, 2H, H-4, H-5).
Pharmacology
All the compounds were screened for AChE and BChE inhibition activity. Electric eel AChE (EC 3.1.1.7), equine serum BuChE (EC 3.1.1.8), acetylthiocholine iodide, butyrylthiocholine chloride, and 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB) were purchased from Sigma (St Louis, Missouri). Buffers and other chemicals were of analytical grades.
Enzyme Inhibition Assay
All the assays were performed under 100 mmol/L sodium phosphate buffer, pH 8.0, using a 96-well microplate on SpectraMax340 (Molecular Device [U.S.A]). One hundred and fifty microliters of 100 mmol/L sodium phosphate buffer (pH = 8), 10 μL DTNB, 10 μL of test compound (9AA derivatives) solution, and 20 μL AChE or BuChE solution were mixed and incubated for 15 minutes at 25°C. The reaction was then initiated with the addition of 10 μL acetylthiocholine iodide or butyrylthiocholine chloride in that order. The activity was determined by measuring the increase in absorbance at 412 nm. The AChE and BuChE inhibiting activities were measured by slightly modifying the spectrophotometric method developed by Ellman. 12
Result and Discussion
All the newly synthesized molecules (RM1-RM6) were tested for their inhibition activities toward AChE and BuChE according to the modified Ellman method with parent molecule (9AA) and standards, galantamine and tacrine (Table 1). The ChE inhibition results were summarized in Table 1. The result showed that the target compounds possessed significantly even better ChE inhibition than galantamine and tacrine. In case of galantamine taken as standard, all derivatives showed better activity against AChE and BuChE. As compared to tacrine, compounds showed inhibitory potential against AChE in the order of RM2 > RM3 > RM6 > RM5 > RM1 > RM4. Only RM4 showed weak AChE inhibition than tacrine, whereas against BuChE, all derivatives showed better results than tacrine. All synthesized compounds presented more potent inhibitory activity against BuChE (IC50 value 0.003-0.0715 nmol/L) as compared to AChE (IC50 value 0.0004-0.006 nmol/L).
Table 1.
Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE) Inhibiting Activity.
| Compounds Codes | AChE Inhibition, Inhibitory Concentration 50% (IC50) ± SEM (nmol/L) | BuChE Inhibition, Inhibitory Concentration 50% (IC50) ± SEM (nmol/L) |
|---|---|---|
| RM1 | 0.022 ± 0.00046 | 0.0011 ± 0.00 |
| RM2 | 0.0033 ± 0.0011 | 0.0011 ± 0.00 |
| RM3 | 0.010 ± 0.00 | 0.00046 ± 0.00 |
| RM4 | 0.0715 ± 0.00195 | 0.0062 ± 0.000208 |
| RM5 | 0.015 ± 0.001 | 0.0004 ± 0.00 |
| RM6 | 0.0127 ± 0.00 | 0.0004 ± 0.00 |
| 9AA (lead) | 0.046 ± 0.00 | 0.0014 ± 0.00 |
| Galantamine (standard) | 1.6 ± 0.000167 | 6.6 ± 0.00038 |
| Tacrine (standard) | 0.021 ± 0.002 | 0.051 ± 0.005 |
Abbreviation: SEM, standard error of the mean.
Ligand-Based Pharmacophoric Study of Synthesized Compounds
Pharmacophoric study was carried out using the software LigandScout 3.02 [i1_10] with ChemDraw Ultra 8.0 for structure drawing. Important features were identified after analyzing the structures of the synthesized compounds along with the standards generated by LigandScout. The obtained main features of all the synthesized compounds were then superimposed into different standard ligands to get the shared features indicating the similar important binding regions, which may be involved in binding with the target.
Acetylcholinesterase and BuChE Inhibition
Pharmacophoric regions of the synthesized molecules determined with the help of the software “LigandScout” revealing the important structural features (Table 2). Donepezil, rivastigmine, galantamine, and tacrine approved by the United States Food and Drug Administration (FDA) and marketed for the treatment of AD. 24 -27
Table 2.
Pharmacophoric Features of Parent, Synthesized Derivatives, and Standards.
| Compounds | Aromatic Region(s) | Hydrophobic Region(s) | Hydrogen Bond Acceptor(s) | Hydrogen Bond Donor(s) | Ionic Interaction Positive/Negative |
|---|---|---|---|---|---|
| 9AA | 3 | 2 | 1 | 1 | 0 |
| RM1 | 4 | 4 | 2 | 1 | 1/0 |
| RM2 | 4 | 3 | 6 | 1 | 0 |
| RM3 | 4 | 2 | 4 | 1 | 1/0 |
| RM4 | 4 | 5 | 2 | 1 | 0 |
| RM5 | 5 | 4 | 1 | 1 | 1/0 |
| RM6 | 4 | 4 | 1 | 1 | 1/0 |
| Galantamine | 1 | 2 | 3 | 1 | 1/0 |
| Physostigmine | 1 | 2 | 2 | 1 | 1/0 |
| Rivastigmine | 1 | 4 | 2 | 0 | 1/0 |
| Tacrine | 2 | 1 | 1 | 1 | 1/0 |
Tacrine, physostigmine, rivastigmine, and galantamine taken as standard in this study are extensively investigated for the ChE inhibition as a major targets in AD. 6 In pharmacophoric generation, these standards displayed 5 to 9 pharmacophoric features (Figures 2 –5). 9-Aminoacridine has 7 pharmacophoric regions, whereas synthesized molecules (RM 1-6) comprised around 10 to 14 pharmacophoric regions (Figure 6) including hydrogen bond donor(s), hydrogen bond acceptor(s), ionic interaction, hydrophobic (HPB), and aromatic region(s).
Figure 2.

Two- and 3-dimensional picture of galantamine. Blue indicates aromatic region; yellow, hydrophobic region; red, hydrogen bond acceptor; green, hydrogen bond donor; blue star, positive ionic interaction. Note: Color version of the figure is available at http://aja.sagepub.com/
Figure 3.

Two- and 3-dimensional picture of physostigmine. Blue indicates aromatic region; yellow, hydrophobic region; red, hydrogen bond acceptor; green, hydrogen bond donor; blue star, positive ionic interaction. Note: Color version of the figure is available at http://aja.sagepub.com/
Figure 4.

Two- and 3-dimensional picture of rivastigmine. Blue indicates aromatic region; yellow, hydrophobic region; red, hydrogen bond acceptor; blue star, positive ionic interaction. Note: Color version of the figure is available at http://aja.sagepub.com/
Figure 5.
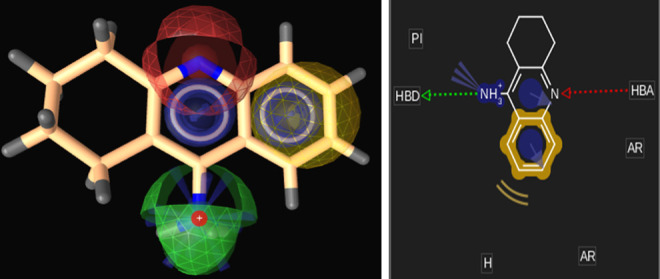
Two- and 3-dimensional picture of tacrine. Blue indicates aromatic region; yellow, hydrophobic region; red, hydrogen bond acceptor; green, hydrogen bond donor; blue star, positive ionic interaction. Note: Color version of the figure is available at http://aja.sagepub.com/
Figure 6.

Three-dimensional picture showing shared features of 9-aminoacridine (9AA), RM1, RM2, RM3, RM4, RM5, and RM6. Yellow, hydrophobic region; red, hydrogen bond acceptor; green, hydrogen bond donor. Note: Color version of the figure is available at http://aja.sagepub.com/
All synthesized molecules showed maximum shared features with tacrine as compared to other standards (Table 3). Tacrine shared 6 pharmacophoric regions with phenacyl, benzoyl, and benzyl derivatives (Figure 7) including 1 hydrogen bond acceptor, 1 hydrogen bond donor, 2 aromatic and 1 HPB region, and 1 ionic interaction. There is a possibility that all of these regions or some of them may be involved to interact with the active binding sites of enzymes.
Table 3.
Shared Pharmacophoric Features of Parent, Synthesized Derivatives, and Standards.
| Compounds | Aromatic Region(s) | Hydrophobic Region(s) | Hydrogen Bond Acceptor(s) | Hydrogen Bond Donor(s) | Ionic Interaction Positive/Negative |
|---|---|---|---|---|---|
| 9AA, RM1, RM2, RM3, RM4, RM5, RM6 | 0 | 2 | 1 | 1 | 0 |
| Galantamine, RM1, RM3 | 1 | 2 | 1 | 0 | 1/0 |
| Galantamine, RM2, RM4 | 0 | 1 | 1 | 0 | 0 |
| Galantamine, RM5, RM6 | 1 | 2 | 1 | 0 | 1/0 |
| Physostigmine, RM1, RM3 | 1 | 0 | 0 | 1 | 1/0 |
| Physostigmine, RM2, RM4 | 0 | 2 | 1 | 0 | 0 |
| Physostigmine, RM5, RM6 | 1 | 1 | 0 | 1 | 1/0 |
| Rivastigmine, RM1, RM3 | 1 | 2 | 1 | 0 | 0 |
| Rivastigmine, RM2, RM4 | 0 | 2 | 0 | 0 | 0 |
| Rivastigmine, RM5, RM6 | 1 | 2 | 1 | 0 | 0 |
| Tacrine RM1, RM3 | 2 | 1 | 1 | 1 | 1/0 |
| Tacrine RM2, RM4 | 2 | 1 | 1 | 1 | 0 |
| Tacrine RM5, RM6 | 2 | 1 | 1 | 1 | 1/0 |
Figure 7.
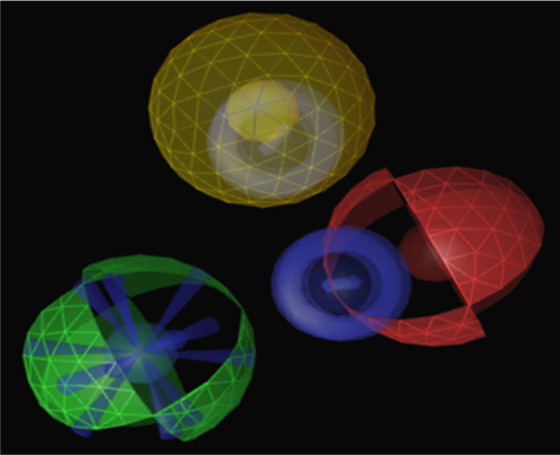
Three-dimensional picture showing shared features of tacrine, RM1, RM2, RM3, RM4, RM5, and RM. Blue indicates aromatic region; yellow, hydrophobic region; red, hydrogen bond acceptor; green, hydrogen bond donor; blue star, positive ionic interaction. Note: Color version of the figure is available at http://aja.sagepub.com/
Overall, this 3-dimensional structural investigation of synthesized compounds with different standards generates pharmacophoric regions showing good sharing of different important regions in structures. Several binding studies of the various cholinesterase inhibitory ligands with the target enzymes indicate the significance of hydrogen bonding (HB) and HPB interactions. 28,29 In the present pharmacophoric investigation, all the synthesized compounds present greater number of hydrogen bondings (HBs 1-6) and HPB interactions (2-5) as compared to all standards (HB 1-3 and HPB 1-4). Presence of more HBs and HPB regions in the synthesized compounds provides possible justification of better enzymes inhibition as compared to standards.
Conclusion
In the given study, synthesized 9AA derivatives displayed highly significant potential for AChE and BuChE inhibition. As these enzymes have been studied and considered as a major target for the treatment of Alzheimer, better result than standards indicating the pronounced potential of these compounds to be used as anti-Alzheimer agents. These compounds have been selected to be investigated further for their toxicity profile as well as studied at molecular level for their binding mode with the target.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work has been done by the support of research grant of University of Karachi, Karachi, Pakistan.
References
- 1. Vilma P, Eduardas T. New ethacridine derivatives as the potential antifungal and antibacterial preparations. Medicina (Kaunas). 2007;43(8):657–663. [PubMed] [Google Scholar]
- 2. Baguey BC, Zhuang L, Marshall EM. Experimental solid tumour activity of N-[2-(dimethylamino)ethyl]-acridine-4-carboxamide. Cancer Chemother Pharmcol. 1995;36(3):244–248. [DOI] [PubMed] [Google Scholar]
- 3. Mehul MP, Mimansha DM, Saurabh KP. Bernthsen synthesis, antimicrobial activities and cytotoxicity of acridine derivatives. Bioorg Med Chem Lett. 2010;20(21):6324–6326. [DOI] [PubMed] [Google Scholar]
- 4. Mehta M, Adem A, Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker TM, Starr B, Dewhurst BB, Atterwill C. Potential neurotoxicity of a novel aminoacridine analogue. Hum Exp Toxicol. 1995;14(6):469–474. [DOI] [PubMed] [Google Scholar]
- 6. Korabecnya J, Musilekb K, Holasa O, et al. Synthesis and in vitro evaluation of N-alkyl-7-methoxytacrine hydrochlorides as potential cholinesterase inhibitors in Alzheimer disease. Bioorg Med Chem Lett. 2010;20(20):6093–6095. [DOI] [PubMed] [Google Scholar]
- 7. Giacobini E. Cholinesterase inhibitors for Alzheimer’s therapy: from tacrine to future applications. Neurochem Int. 1998;32(5-6):413–419. [DOI] [PubMed] [Google Scholar]
- 8. Tacrine. Drugs in Clinical Trials. Alzheimer Research Forum, http://www.alzforum.org/drg/drc/detail.asp?id=90.
- 9. Tumiatti V, Minarini A, Bolognesi ML, Milelli A, Rosini M, Melchiorr C. Tacrine derivatives and Alzheimer’s disease. Curr Med Chem. 2010;17(17):1825–1838. [DOI] [PubMed] [Google Scholar]
- 10. Coelho F, Birks J. Physostigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brisks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballard CG, Perry EK. In: Giacobini E, Martin Dunitz, eds. Butyrylcholinesterase its Function and Inhibitors. London, UK: CRC Press; 2003:123. [Google Scholar]
- 13. Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol. 1998;1(2):55–65. [Google Scholar]
- 14. Onor ML, Trevisiol M, Aguglia E. Rivastigmine in the treatment of Alzheimer’s disease: an update. Clin Intervent Aging. 2007;2(1):17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemke T, Williams DA, Roche V, Zito SW. Foye’s Principles of Medicinal Chemsitry, 6th edn. Baltimore, USA: Lippincott, Williams and Wilkins; 2008:361–392. [Google Scholar]
- 16. Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled Study. Arch Intern Med. 1998;158(9):1021–1031. [DOI] [PubMed] [Google Scholar]
- 17. Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer’s disease. Biol Psychiatry. 2001;49(3):289–299. [DOI] [PubMed] [Google Scholar]
- 18. Olin J, Schneider L. Galantamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2000;(3):CD001747. [DOI] [PubMed] [Google Scholar]
- 19. Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer’s disease: an updated meta-analysis. J Neural Transm. 2009;116(4):457–465. [DOI] [PubMed] [Google Scholar]
- 20. Kim FE. Drug affecting cholinergic neurotransmission. In: Thomas LL, David AW, Victoria FR, William ZS, eds. Foye’s Principles of Medicinal Chemistry. New Dehli, India: Wolters Kluwer Pvt Ltd: 361, 363, 374–375, 377–378. [Google Scholar]
- 21. Inestrosa NC, Alvarez A, Pérez CA, et al. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. [DOI] [PubMed] [Google Scholar]
- 22. Kadiyala M, Ponnusankar S, Elango K. Screening of siddha medicinal plants for its in-vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity. Pharmacogn Mag. 2014;10(suppl 2):S294–S298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Jang YJ, Zhov JW, Yu QS, You QD. Identification of ligand features essential for HDACs inhibitors by pharmacophore modeling. J Mol Graph Model. 2008;26(7):1160–1168. [DOI] [PubMed] [Google Scholar]
- 24. Muñoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr Med Chem. 2008;15(24):2433–2455. [DOI] [PubMed] [Google Scholar]
- 25. Relkin NR. Beyond symptomatic therapy: a re-examination of acetylcholinesterase inhibitors in Alzheimer’s disease. Expert Rev Neurother. 2007;7(6):735–748. [DOI] [PubMed] [Google Scholar]
- 26. Wishart DS, Knox C, Guo AC, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34(database issue):668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordberg A. Mechanisms behind the neuroprotective actions of cholinesterase inhibitors in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2006;20(1):S12–S18. [DOI] [PubMed] [Google Scholar]
- 28. Khan I, Nisar M, Khan N, et al. Structural insights to investigate Conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia. 2010;81(8):1020–1025. [DOI] [PubMed] [Google Scholar]
- 29. Gupta S, Mohan CG. Dual binding site and selective acetylcholinesterase inhibitors derived from integrated pharmacophore models and sequential virtual screening. Biomed Res Int. 2014;2014:291214. [DOI] [PMC free article] [PubMed] [Google Scholar]



