Abstract
Sleep disturbances including excessive daytime sleepiness (EDS) are encountered in frontotemporal dementia (FTD). To investigate the relationship between the plasma orexin-A levels and sleep disturbance patterns, we measured the plasma orexin-A levels and performed sleep studies in patients with FTD. The orexin-A levels were measured in 10 consecutive patients with FTD and controls by enzyme-linked immunosorbent assay. Nocturnal polysomnography (PSG) and Multiple Sleep Latency Test (MSLT) were performed in 2 patients with FTD. The orexin-A levels were significantly lower in patients with FTD compared to controls. The PSG revealed increased rapid eye movement (REM) latency in patients, whether or not they reported EDS. Mean sleep latency in MSLT was less than 10 minutes in both the patients, being shorter in patient without EDS, but none of them had REM sleep onset. Some patients with FTD may develop narcolepsy-like involuntary sleep attacks, even without complaining of EDS. Involvement of hypothalamus and a subsequent alteration in the orexin levels might be one of the determining factors in this sleep disturbance.
Keywords: orexin-A, frontotemporal dementia, sleep disturbances, polysomnography, plasma
Introduction
Sleep–wake cycle disturbances are common in elderly patients with dementia. 1 Especially, the presence of excessive daytime sleepiness (EDS) may be an early indicator of cognitive impairment and onset of dementia. 1,2 The neurodegenerative diseases, most frequently represented by Alzheimer’s disease (AD), Parkinson’s disease (PD), and other parkinsonian disorders, are often accompanied by altered sleeping patterns and EDS. 3–7 Frontotemporal dementia (FTD) is characterized by progressive deterioration in cognition, language, behavior, and personality associated with focal atrophy of frontal and temporal regions. Sleep disturbances are also commonly encountered in patients with FTD. 8–11 Although sleep problems of patients with PD have been widely studied, 12–17 there are much less data available about sleep disturbances and sleep recording studies in FTD. In an actigraphy study, patients with FTD showed increased nocturnal activity and decreased morning activity, lower sleep efficiency, and total sleep time (TST) when compared to patients with AD. 8 Other sleep studies have shown rest/activity disturbances and disturbed sleep continuity in patients with both AD and FTD. 9,18
The hypothalamic hypocretin–orexin system plays a critical role in the regulation of sleep and wakefulness. 19 The understanding of the physiological roles of the orexin system in sleep disturbances came forward with the discovery of the hypocretin gene/ligand, and subsequently, decreased plasma and cerebrospinal fluid (CSF) orexin-A levels were found to be predictive of narcolepsy–cataplexy syndrome. 20 By contrast, the CSF orexin-A levels were reported to be within the normal limits in the majority of patients with chronic neurologic diseases. 21 In this study, we investigated the plasma orexin-A levels in patients with FTD in relation to sleep disturbances.
Materials and Methods
Patients
The study was performed in a group of 10 consecutive patients with FTD followed in our outpatient clinic. A control group included 50 patients with PD and 27 patients with AD that statistically matched the FTD group by means of age, gender, and disease duration. Data regarding medical history and demographic characteristics were collected for each patient. All patients were diagnosed according to the revised Lund and Manchester criteria for FTD, 22,23 the international criteria for PD, 24,25 and National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association criteria for AD. 26 Patients or caregivers (in case patients were not able to) gave informed consent for the study, which had been approved by our local ethics committee.
Patients with FTD were described in detail. Comorbid disorders and the present pharmacological therapy were evaluated. Cranial magnetic resonance imaging (MRI) was performed in all patients. The Mini-Mental State Examination (MMSE) 27 and clinical dementia rating (CDR) 28 were used to stage the severity of disease in the FTD group. The MMSE is a 30-point brief cognitive rating screen. The CDR rates performance in 6 areas of function (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) based on a semistructured interview with an informant. A global score ranging from 0 (no dementia) to 3 (severe dementia) was computed. Staging of dementia was assessed by means of the global deterioration scale (GDS). 29 Based on the GDS scores, the patients were categorized into 3 subgroups according to dementia severity: normal (GDS stage 1), age-associated memory impairment (GDS stage 2), minimal cognitive impairment (GDS stage 3), mild (GDS stage 4), moderate (GDS stages 5), and severe or very severe (GDS stages 6 and 7) dementia. 29 Data from the Neuropsychiatric Inventory (NPI) were analyzed. 30 This is a validated, caregiver-based behavioral rating system developed for the assessment of dementia that evaluates the presence or absence of 12 major behavioral disorders, including delusions, hallucinations, aggression/agitation, depression, anxiety, elation/euphoria, apathy, disinhibition, irritability/liability, aberrant motor behavior, sleep disturbances, and eating disorders.
Measurement of the Orexin-A Levels
Blood samples of all patients and controls were collected at 8 am before breakfast. The plasma orexin-A levels were measured with a commercially available EIA kit (Peninsula Laboratories, San Carlos, California) as per manufacturer's instructions.
Sleep History and Recordings
Two patients with FTD (case 1 and 2) gave consent for sleep studies. They were evaluated with Epworth Sleepiness Score (ESS) 31 and Pittsburgh Sleep Quality Index (PSQI), 32 and a medical history regarding other sleep disorders was taken in detail. But the ESS and PSQI could not be performed in other patients either due to lack of consent or severe cognitive disturbance. Higher ESS scores indicated increased likelihood of falling asleep during passive activities. In a range of scores between 0 and 24, the score of 11 to 18 indicated “sleepy” and >18 indicated “very sleepy.” In the evaluation of PSQI, lower scores (<5) indicate good sleep quality, whereas higher scores (>5) indicate poor sleep quality. Full night polysomnography (PSG) recordings (Grass software) were performed by 2 sleep specialists who were blinded for the clinical characteristics of the patients. The PSG included 16-channels electroencephalography (placed according to the 10 to 20 international electrode placement system), right and left electrooculography, chin electromyography, and electrocardiography. Leg movements were recorded by left and right tibial electromyography. 33 The following PSG parameters were evaluated: time in bed, TST, sleep efficiency index (TST per time in bed), sleep continuity index (TST per total sleep period), sleep latency, rapid eye movement (REM) latency (time from sleep onset to the first REM sleep epoch), percentages of each sleep stage, wake time after sleep onset, and periodic leg movements in sleep (PLMS) index. Following PSG, multiple sleep latency test (MSLT) was performed, which consists of 4 daytime naps with 2-hour intervals. In MSLT, mean sleep latency (MSL) and the presence of REM sleep onset were examined.
Statistical Analysis
Comparison of the plasma orexin-A levels among 3 groups was established by both analysis of variance (ANOVA, multiple group comparison) and Student’s t test for pairwise comparisons (FTD vs AD; FTD vs PD, AD vs PD). Data were presented as mean ± standard deviation or as percentages. A P value of <.05 was regarded as statistically significant.
Results
Demographic Characteristics
In all, 10 patients (4 women, 6 men) with FTD were studied. The mean age of the FTD group was 59 ± 14 years (range: 38-83 years), and the mean disease duration of the FTD group was 10 ± 4 years (range: 5-16 years). The patients with FTD had a mean disease duration of 5 years (range: 0.5-8 years) during the time of initial diagnosis. All patients were only followed in our outpatient clinic and were diagnosed during the early stages of disease (mean CDR = 1 ± 0.3; mean GDS = 4 ± 0.5).
In all, 27 patients (11 women, 16 men) with AD and 50 patients (20 women, 30 men) with PD were included as control groups. The mean age of the patients with AD and PD was, respectively, 54 ± 10 years (range: 35-91 years) and 55 ± 15 years (range: 38-86 years). The mean disease durations were 12 ± 3 years (range 6-17 years) and 11 ± 4 years (range: 5-15 years) for AD and PD groups, respectively.
Of patients with FTD, 5 had comorbid disorders including parkinsonism, hypertension, diabetes, hyperlipidemia, or anemia. Patients with parkinsonism were receiving moderate doses of antiparkinsonian drugs (levodopa or levodopa + carbidopa + entacapone). Sleep studies were not performed in these patients. Relevant medications were used for other medical problems. Of patients with FTD, 9 were taking standard medications for dementia (galantamine, memantine, rivastigmine, or donepezil), and 6 patients with FTD were on psychiatric drugs (2 with only antidepressants, 2 with only antipsychotics, and 2 with antidepressants and antipsychotics).
In the FTD group, MRI showed prominent bilateral symmetrical or unilateral atrophy in the frontotemporoparietal regions. Detailed demographic and neuropsychological data (mean MMSE, CDR, and GDS) of the FTD group are shown in Table 1. The proportion of patients with evidence for each behavioral disorder included in the NPI is summarized in Table 2.
Table 1.
Detailed Demographic and Neuropsychological Data for the FTD Group.a
| Patients | Age, year | Disease duration, year | Diagnosis time,b year | MMSE | CDR | GDS |
|---|---|---|---|---|---|---|
| FTD (n = 10) | 59 ± 14 (38-83) | 10 ± 4.1 (5-16) | 5 ± 3.1 (0.5-8) | 13 ± 12 (1-22) | 2 ± 1.2 (1-3) | 5 ± 1.3 (4-6) |
Abbreviations: FTD, frontotemporal dementia; MMSE, Mini-Mental State Examination; CDR, clinical dementia rating; GDS, Global deterioration scale; SD, standard deviation; min, minimum; max, maximum.
a Values are mean ± SD (min–max).
b Disease durations during the initial diagnosis.
Table 2.
Number of Patients (%) in Each Diagnostic Group With Behavioral Disorders on the NPI.
| Feature | FTD |
|---|---|
| Delusions | 2 (20) |
| Hallucinations | 2 (20) |
| Aggression/agitation | 3 (30) |
| Depression | 1 (10) |
| Anxiety | 0 (0) |
| Elation/euphoria | 1 (10) |
| Apathy | 4 (40) |
| Disinhibition | 1 (10) |
| Irritability/lability | 2 (20) |
| Aberrant motor behavior | 0 (0) |
| Sleep disturbances | 1 (10) EDS |
| 1 (10) insomnia | |
| 1 (10) irregular sleep | |
| 1 (10) frequent awakening | |
| Eating disorders | 3 (30) |
Abbreviations: NPI, neuropsychiatric inventory; FTD, frontotemporal dementia; EDS, excessive daytime sleepiness.
Orexin-A Levels
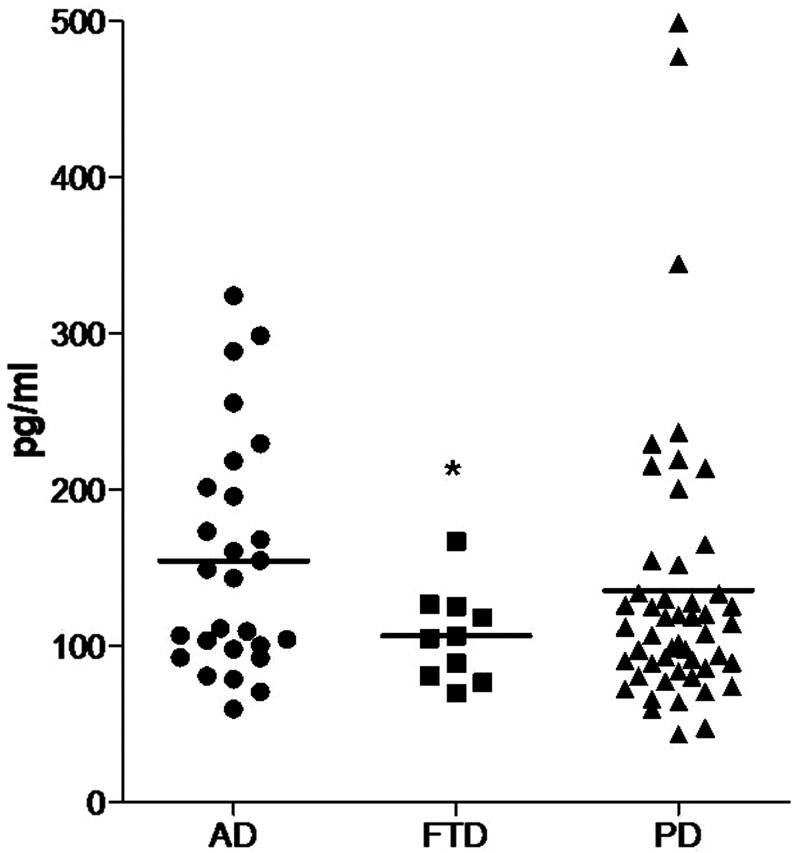
The mean levels of plasma orexin-A were 106 ± 29 pg/mL (70-167 pg/mL) in FTD, 154 ± 74 pg/mL (60-324 pg/mL) in AD, and 135 ± 92 pg/mL (43-499 pg/mL) in PD. The orexin-A levels in the patients with FTD were statistically lower than both the patients with AD and patients with PD (FTD vs AD, P = .003; FTD vs PD, P = .030 by Student’s t test). There was no statistically significant difference in the orexin-A levels between the patients with AD and patients with PD (P = .100 by Student’s t test). Multiple group comparison also showed a statistical difference in the orexin-A levels among 3 groups (P = .042 by ANOVA; Figure 1). In the FTD group, the plasma orexin-A level of case 1 was 88 pg/mL and that of case 2 was 167 pg/mL.
Figure 1.
Comparison of plasma orexin-A levels among the disease groups. AD indicates Alzheimer’s disease; FTD, frontotemporal dementia; PD, Parkinson’s disease. * indicates P < .05 and vertical lines indicate mean values.
Sleep History and Recordings
Detailed sleep history was obtained from 2 patients with FTD. One of them was a 62-year-old woman (case 1), who had EDS. Other patient was a 58-year-old man (case 2), who was bedridden for 10 years, but had no complaints of EDS. He had forgetfulness and apathy (first MMSE 20, CDR 1, GDS 4) and then slowly progressed and became bedridden (last MMSE 1, CDR 3, GDS 6).
The ESS of both the patients was within the normal limits (6 and 8), while PSQI indicated slightly poor sleep quality in both the patients (6 for each).
The PSG and MSLT data are given in Table 3. None of the patients had obstructive or central sleep apnea syndrome or PLMS. Case 1 had longer sleep latency than case 2. Sleep efficiency index was slightly lower in case 1 although sleep continuity index was higher. In MSLT, MSL of 4 daytime naps was short in both the patients; case 2 showed a very short latency of 2.8 minutes. Sleep onset REM period (SOREMP) was not observed in any of the patients.
Table 3.
Sleep Parameters of Patients With FTD.
| PSG data | Case 1 | Case 2 |
|---|---|---|
| Time in bed, min | 487.2 | 558.6 |
| Total sleep time, min | 387 | 458 |
| Sleep efficiency index, % | 79.4 | 82 |
| Sleep continuity index, % | 92.9 | 82.6 |
| Sleep latency, min | 65 | 1 |
| REM sleep latency, min | 124 | 132.5 |
| Percentages of sleep stages, % | ||
| N1 | 7.7 | 21.6 |
| N2 | 54.7 | 41.6 |
| N3 | 12.7 | 0.2 |
| REM | 17.8 | 19.2 |
| Wake time after sleep onset, min | 35.2 | 99.6 |
| PLMS index, hours | 11.8 | 0 |
| MSLT data | ||
| Mean sleep latency, min | 9.6 | 2.8 |
| Number of SOREMP (n) | 0 | 0 |
Abbreviations: FTD, frontotemporal dementia; PSG, polysomnography; REM, rapid eye movement; N, nonREM; PLMS, periodic leg movements during sleep; MSLT, multiple sleep latency test; SOREMP, sleep onset REM period.
In addition to case 1, we learned from the caregivers that additionally 3 patients had sleep disorders including insomnia, irregular sleep, and frequent awakening. However, ESS and PSQI could not be performed in these patients due to severe dementia. When the orexin levels of patients with or without reported sleep problems were compared, no significant difference could be found (P = .318 by Student’s t test).
Discussion
Our results showed significantly reduced orexin-A levels in patients with FTD when compared to patients with AD and PD. Moreover, PSG studies revealed increased REM latency, and MSLT showed reduced MSL in 2 patients with FTD whether or not they reported EDS. Although we could obtain very limited data in 2 of our patients with FTD, the PSG findings of these patients were more or less compatible with those obtained in the previous studies. 18 Alternatively, sleep efficiency indices were higher in our patients and REM sleep latency was prolonged with high REM period percentages in comparison with previously reported patients with FTD. The remarkable variability of polysomnographic data between the studies indicates the heterogeneity of sleep status in patients with FTD.
The relationship between narcolepsy–cataplexy syndrome and the CSF orexin-A levels is well known. Decreased CSF orexin-A levels are often seen in symptomatic narcolepsy–cataplexy cases and also in other EDS cases with various etiology. 19,20,21,34,35–37 The MSLT has been widely used for the diagnosis of narcolepsy syndrome, often yielding abnormal findings (MSL < 5 minutes and >2 SOREMPs) that were correlated with low orexin-A levels. 20,37 Patients with narcolepsy show reduced sleep and REM sleep latency and increased REM period percentage on nocturnal PSG studies. 38,39 The PSG results of our patients, with or without clinical complaints of EDS, were not strictly compatible with those obtained in patients with narcolepsy. Particularly, REM sleep latencies and REM period percentages were increased in our patients. Nevertheless, in a manner similar to patients with narcolepsy, sleep latencies were reduced in both of our FTD cases. Moreover, narcolepsy-like sleep attacks were observed in MSLT studies of our patients.
Patients with neurodegenerative diseases have usually been reported to have normal CSF orexin-A levels; most of these studies are related to AD and PD. 4,5,7,12–14,16,21,40,41 By contrast, 2 studies have shown low CSF orexin levels in ventricular CSF of patients with PD. 42,43 In a recent study, reduced CSF orexin levels have been reported in dementia with Lewy bodies (DLB). 3 In another study, reduced neocortical orexin immunoreactivity has been shown in patients with DLB correlating with hypersomnolence and α-synuclein levels. 44 Similarly, low CSF orexin-A levels associated with short MSL or increased SOREMPs were also reported in patients with PD. 12,17 In a study by Friedman et al, lower CSF orexin-A levels in 15 patients with AD were significantly correlated with increased fragmentation of sleep. 5 In a recent study, the CSF orexin-A levels were found to be lower in patients with AD compared with controls, and 2 patients with AD with documented EDS showed the lowest CSF orexin-A concentrations. 6 Overall, previous studies, in addition to our data given in Figure 1, demonstrate a significant heterogeneity in the orexin-A levels of patients with AD and PD, which presumably indicate the involvement or preservation of sleep-related structures in different patient subgroups with or without sleep problems. In general, neurodegenerative processes are known to affect hypothalamic orexin neurons in association with disease severity. Although the orexin system is affected by AD, the neurodegeneration seen in this disorder does not specifically target the hypocretin system but damages other neuronal systems involved in sleep–wake regulation, such as the circadian system, to an even greater extent. 45 Thus, it is likely that other factors play a more principal role in the disturbed sleep–wake rhythm of patients with AD. 6 As in AD, the neurodegenerative processes in PD do not selectively target the orexin system. 43 The CSF hypocretin-1 levels only drop when the hypothalamic hypocretin neurons are reduced above 70% even in advanced disease stages. 46 Since a widespread neurodegeneration occurs in advanced PD, impairment of other sleep-related structures other than the orexin system may account for sleep disturbances in PD. 15,43 Similarly, some studies have examined the CSF levels of orexin in DLB; however, the results have been inconclusive, with a study reporting reduction 3 and others finding no alterations 4,40 in DLB. These inconsistencies may be related to disease severity and methodological issues regarding different kits or methods of measurement. 44 To our knowledge, neurodegeneration of orexin-producing neurons in FTD has not been reported. However, since our patients with FTD had long disease durations and mostly moderate to severe dementia, reduced orexin levels might plausibly be the result of widespread loss of neurons including those located in hypothalamus.
The measurement of the plasma orexin-A levels was performed in a number of cases, mostly associated with narcolepsy, showing decreased or normal levels. 47,48 However, to our knowledge, there are no reports regarding the plasma orexin-A levels in neurodegenerative disorders such as FTD. Our study showed for the first time that the remarkable heterogeneity of the plasma orexin-A levels observed in patients with AD and PD was not present in patients with FTD, and the plasma orexin-A levels of all patients with FTD showed trends toward lower values. Furthermore, 2 of these patients with FTD had narcolepsy-like daytime sleep attacks. These results suggest that, in patients with FTD, the plasma orexin-A levels are within a certain range that may cause EDS, and the orexin-A levels might be one of the causal factors behind sleep problems encountered in patients with FTD.
An important limitation of our study is the low number of patients and the absence of CSF orexin measurements. The CSF orexin levels are known to be more sensitive and reliable in diagnosis of sleep problems. However, the measurement of the CSF orexin levels is not indicated in diagnosis of FTD or related sleep disorders, especially when patients do not report narcolepsy-like sleep problems. Therefore, we could not obtain the CSF samples with which to measure the CSF orexin-A levels in our groups. Elucidation of the clinical implications of the plasma and CSF orexin-A levels in a greater number of patients with FTD is thus warranted. Also, the analysis of the association between the plasma and the CSF orexin-A levels in future studies might give important clues on the pathogenesis of FTD. Whether the plasma orexin-A levels can be used as a simple method to diagnose sleep problems in patients with FTD and whether orexin agonists might be utilized in future treatment trials targeting sleep problems in neurodegenerative disorders remains to be clarified in future studies.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11(4):372–377. [DOI] [PubMed] [Google Scholar]
- 2. Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12):1628–1632. [DOI] [PubMed] [Google Scholar]
- 3. Wennström M, Londos E, Minthon L, Nielsen HM. Altered CSF orexin and α-synuclein levels in dementia patients. J Alzheimers Dis. 2012;29(1):125–132. [DOI] [PubMed] [Google Scholar]
- 4. Baumann CR, Dauvilliers Y, Mignot E, Bassetti CL. Normal CSF hypocretin-1 (orexin A) levels in dementia with Lewy bodies associated with excessive daytime sleepiness. Eur Neurol. 2004;52(2):73–76. [DOI] [PubMed] [Google Scholar]
- 5. Friedman LF, Zeitzer JM, Lin L, et al. In Alzheimer disease, increased wake fragmentation found in those with lower hypocretin-1. Neurology. 2007;68(10):793–794. [DOI] [PubMed] [Google Scholar]
- 6. Fronczek R, van Geest S, Frölich M, et al. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol Aging. 2012;33(8):1642–1650. [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Rodriguez JE, Seppi K, Cardozo A, et al.; SINBAR (Sleep Innsbruck Barcelona) group. Cerebrospinal fluid hypocretin-1 levels in multiple system atrophy. Mov Disord. 2007;22(12):1822–1824. [DOI] [PubMed] [Google Scholar]
- 8. Anderson KN, Hatfield C, Kipps C, Hastings M, Hodges JR. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol. 2009;16(3):317–323. [DOI] [PubMed] [Google Scholar]
- 9. Harper DG, Stopa EG, McKee AC, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. 2001;58(4):353–360. [DOI] [PubMed] [Google Scholar]
- 10. Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103(6):367–378. [DOI] [PubMed] [Google Scholar]
- 12. Compta Y, Santamaria J, Ratti L, et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson's disease dementia. Brain. 2009;132(pt 12):3308–3317. [DOI] [PubMed] [Google Scholar]
- 13. Poryazova R, Benninger D, Waldvogel D, Bassetti CL. Excessive daytime sleepiness in Parkinson's disease: characteristics and determinants. Eur Neurol. 2010;63(3):129–135. [DOI] [PubMed] [Google Scholar]
- 14. Baumann C, Ferini-Strambi L, Waldvogel D, Werth E, Bassetti CL. Parkinsonism with excessive daytime sleepiness e a narcolepsy-like disorder? J Neurol. 2005;252(2):139–145. [DOI] [PubMed] [Google Scholar]
- 15. Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology. 2002;58(7):1019–1024. [DOI] [PubMed] [Google Scholar]
- 16. Overeem S, van Hilten JJ, Ripley B, Mignot E, Nishino S, Lammers GJ. Normal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepiness. Neurology. 2002;58(3):498–499. [DOI] [PubMed] [Google Scholar]
- 17. Maeda T, Nagata K, Kondo H, Kanbayashi T. Parkinson's disease comorbid with narcolepsy presenting low CSF hypocretin/orexin level. Sleep Med. 2006;7(8):662. [DOI] [PubMed] [Google Scholar]
- 18. Kundermann B, Thum A, Rocamora R, Haag A, Krieg JC, Hemmeter U. Comparison of polysomnographic variables and their relationship to cognitive impairment in patients with Alzheimer's disease and frontotemporal dementia. J Psychiatr Res. 2011;45(12):1585–1592. [DOI] [PubMed] [Google Scholar]
- 19. Kanbayashi T, Sagawa Y, Takemura F, et al. The pathophysiologic basis of secondary narcolepsy and hypersomnia. Curr Neurol Neurosci Rep. 2011;11(2):235–241. [DOI] [PubMed] [Google Scholar]
- 20. Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59(10):1553–1562. [DOI] [PubMed] [Google Scholar]
- 21. Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005;9(4):269–310. [DOI] [PubMed] [Google Scholar]
- 22. Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57(4):416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 24. Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32(suppl):125–127. [DOI] [PubMed] [Google Scholar]
- 25. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. ‘‘Mini-mental state’’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 28. Morris J. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 29. Reisberg B, Ferris SH, de Leon MJ, Crook T. Global Deterioration Scale (GDS). Psychopharmacol Bull. 1988;24(4):661–663. [PubMed] [Google Scholar]
- 30. Cummings JL. The neuropsychiatric inventory. Assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–S16. [DOI] [PubMed] [Google Scholar]
- 31. Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703–710. [DOI] [PubMed] [Google Scholar]
- 32. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 33. Zucconi M, Ferri R, Allen R, et al.; International Restless Legs Syndrome Study Group (IRLSSG). The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7(2):175–183. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura M, Kanbayashi T, Sugiura T, Inoue Y. Relationship between clinical characteristics of narcolepsy and CSF orexin-A levels. J Sleep Res. 2011;20(1 pt 1):45–49. [DOI] [PubMed] [Google Scholar]
- 35. Ebrahim IO, Sharief MK, de Lacy S, et al. Hypocretin (orexin) deficiency in narcolepsy and primary hypersomnia. J Neurol Neurosurg Psychiatry. 2003;74(1):127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bassetti C, Gugger M, Bischof M, et al. The narcoleptic borderland: a multimodal diagnostic approach including cerebrospinal fluid levels of hypocretin-1 (orexin A). Sleep Med. 2003;4(1):7–12. [DOI] [PubMed] [Google Scholar]
- 37. Baumann CR, Khatami R, Werth E, Bassetti CL. Hypocretin (orexin) deficiency predicts severe objective excessive daytime sleepiness in narcolepsy with cataplexy. J Neurol Neurosurg Psychiatry. 2006;77(3):402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terzano MG, Smerieri A, Del Felice A, et al. Cyclic alternating pattern (CAP) alterations in narcolepsy. Sleep Med. 2006;7(8):619–626. [DOI] [PubMed] [Google Scholar]
- 39. Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20(4):514–521. [DOI] [PubMed] [Google Scholar]
- 40. Yasui K, Inoue Y, Kanbayashi T, Nomura T, Kusumi M, Nakashima K. CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci. 2006;250(1-2):120–123. [DOI] [PubMed] [Google Scholar]
- 41. Abdo WF, Bloem BR, Kremer HP, Lammers GJ, Verbeek MM, Overeem S. CSF hypocretin-1 levels are normal in multiple-system atrophy. Parkinsonism Relat Disord. 2008;14(4):342–344. [DOI] [PubMed] [Google Scholar]
- 42. Drouot X, Moutereau S, Nguyen JP, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61(4):540–543. [DOI] [PubMed] [Google Scholar]
- 43. Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130(pt 6):1577–1585. [DOI] [PubMed] [Google Scholar]
- 44. Lessig S, Ubhi K, Galasko D, et al. Reduced hypocretin (orexin) levels in dementia with Lewy bodies. Neuroreport. 2010;21(11):756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu YH, Fischer DF, Kalsbeek A, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20(11):1874–1876. [DOI] [PubMed] [Google Scholar]
- 46. Gerashchenko D, Murillo-Rodriguez E, Lin L, et al. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Exp Neurol. 2003;184(2):1010–1016. [DOI] [PubMed] [Google Scholar]
- 47. Higuchi S, Usui A, Murasaki M, et al. Plasma orexin-A is lower in patients with narcolepsy. Neurosci Lett. 2002;318(2):61–64. [DOI] [PubMed] [Google Scholar]
- 48. Dalal MA, Schuld A, Haack M, et al. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology. 2001;56(12):1749–1751. [DOI] [PubMed] [Google Scholar]