Abstract
Antioxidant agents and cholinesterase inhibitors are the foremost drugs for the treatment of Alzheimer’s disease (AD). In this study, a new peptide from Ziziphus jujuba fruits was investigated for its inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes as well as antioxidant activity. This peptide was introduced as a new peptide and named Snakin-Z. The Snakin-Z displayed considerable cholinesterase inhibition against AChE and BChE. The half maximal inhibitory concentration (IC50) values of Snakin-Z against AChE and BChE are 0.58 ± 0.08 and 0.72 ± 0.085 mg/mL, respectively. This peptide has 80% enzyme inhibitory activity on AChE and BChE at 1.5 mg/mL. The Snakin-Z also had the high antioxidant activity (IC50 = 0.75 ± 0.09 mg/mL). Thus, it is suggested that Snakin-Z may be beneficial in the treatment of AD. However, more detailed researches are still required as in vivo testing its anticholinesterase and antioxidant activities.
Keywords: Alzheimer’s disease, antioxidant, acetylcholinesterase, butyrylcholinesterase, Ziziphus jujuba fruits
Introduction
Peptides are the important compounds of innate immune systems in all creatures. 1,2 These agents have broad biological activity such as antibacterial, 3–5 antifungal, 6–8 antiviral, 9–11 anticancer, antioxidant, spermicidal, and so on. Recently, researchers focused on potential neurobiological activities of peptide vaccine. 12 Dementia is a clinical syndrome caused by disorders that affect the brain. 13,14 Alzheimer’s disease (AD) is the most common type of dementia. 15,16 Evidence show that the inhibition of 2 common enzymes in brain, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), is suitable for the treatment of AD. 17 But present cholinesterase inhibitors have injurious side effects. On the other hand, oxidative stresses lead to neurodegenerative diseases such as AD. 18,19 Hence, discovery of new drugs for the treatment of AD is necessary. On the Earth, there are 250 000 to 500 000 species of plants that some of them are the part of both animal and human food chain, while many others are used for medical purposes. 20,21 Medicinal plants have been always appealing targets for discovery of new bioactive molecules. 22 Different parts of medicinal plant such as fruits, leaves, and seed are used as potent drugs for the treatment of different diseases such as infections, pain, hemorrhage, and so on. Many researches were done on plants in order to discover new drug candidates with neuroprotective activity. Zizyphus jujuba is a small tree with eatable fruit. 23,24 Its dried fruits are used as sedatives, anticancer, antipyretic, analgesic, appetizer, antihemorrhage agents, and as the tonic as well. 25–27 In Chinese medicine, Zizyphus fruit is used for strengthening liver function. Similarly, in Iranian traditional medicine, its fruits are widely used in treating infectious diseases as well as cold. 28
On the basis of necessity of novel candidate for AD therapy as well as regarding the different biological activities of immune system peptides, the purpose of the present study is purification of peptides, from Zizyphus jujube fruits, with appropriate neurobiological effects especially antioxidant and anticholinesterase activity.
Materials and Methods
Preparation of the Protein Extract
In order to obtain the extract, jujube fruit was first soaked in water and then crushed in a porcelain mortar, and a homogeneous solution was finally achieved. The excess salts were removed by centrifugation later, and the extract was filtered by filter paper. Jujube fruit extract was brought from 0% to 85% concentration according to the standard ammonium sulfate precipitation and was saturated with salt via gentle agitation at 4°C. Under this condition, proteins of the extract were suspended in solution as colloidal particles. The deposits containing small and large proteins in the solution were then collected with the help of recentrifugation (12 000 rpm for 20 minutes) and were dissolved in 5 mmol/L phosphate-buffered saline buffer at pH 6.8. To isolate low-molecular-weight compounds, the protein extract was passed through an ultramembrane with a 10-kDa cutoff. The filtrated solution was concentrated using a 1-kDa ultramembrane and lyophilized.
Peptide Isolation and Purification
For the purification purpose, the peptides were lyophilized, and the extract was dissolved in 10 mL phosphate buffer (0.1 mol/L, pH 6.0 + 5 mmol/L EDTA). The sample was applied to a Sephadex G-50 gel filtration column. Elution was performed with the same buffer. The spectrophotometric absorbance of the elute was monitored at 280 nm. According to the absorbance at 280 nm, the fractions were collected and lyophilized. For further purification, the peak-containing active peptide was dissolved in the smallest possible volume of distilled water and purified using a C18 semipreparative reverse phase high-performance liquid chromatography (RP-HPLC) column. Elution was performed using solution A (0.1% trifluoroacetic acid [TFA] in water), combined with a 5% to 65% gradient of solution B (0.098% TFA in acetonitrile) over a period of 70 minutes, at a flow rate of 1 mL/min. According to the absorbance at 220 nm, the fractions were collected and lyophilized. To evaluate the purity of experimental product, a small amount of each peak was checked on the analytical C18 column using RP-HPLC. Each fraction was tested for its antimicrobial activities.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
The electrophoretic pattern of the crude extract and purified peptide was evaluated on Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described in the previous articles. 29 Briefly, a 6.5% (w/v) stacking gel and a 2 parts of 10% and 15% (w/v) separating gel were employed, and then silver staining was used for staining of the gel.
Neurobiological Assay
Cholinesterase Inhibitory Activity
The AChE and BChE inhibitory activity of purified peptide was determined. This in vitro determination was done by microtiter plate assays 30 but with slight modifications. In this assay, AChE and BChE inhibitory activity of the peptide was determined. In this method, stock serial dilutions of 0.05 to 1.5 mg/mL (0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.5 mg/mL) of the peptide were prepared, and 20 μL of the peptide stocks were added to 140 μL of sodium phosphate buffer (pH 7.6, 0.1 mmol/L), 20 μL of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and 20 μL of AChE solution, which was then poured into a 96-well microplate. Then, this plate was incubated at 25°C for 20 minutes. After this time, the absorbance of each well was read at 410 nm using an enzyme-linked immunosorbent assay reader. The substrate of AChE, acetylthiocholine iodide, was added to the incubated solution. The enzyme produces thiocholines ions, and then this ion react with DTNB, and yellow 5-thio-2-nitrobenzoate anion was formed. This colored solution has absorption at 410 nm. This absorption is criterion of enzyme activity. Similarly, this method was done for the assessment of inhibition of peptide on butyrylcholinesterase activity. Galanthamine was used as the positive control.
The percentage inhibition was calculated with the following equation:
In this equation, Asample is the absorbance of the treated enzyme with peptide, and Acontrol is the absorbance of the blank (ethanol in phosphate buffer pH = 8). The half maximal inhibitory concentration (IC50) was defined as peptide concentration that provides 50% inhibition.
Antioxidant Activity
For assessment of radical scavenging activity of isolated peptide, synthetic radical di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium (DPPH) was used. Radical DPPH was dissolved in methanol (0.15 mmol/L), and then 180 μL of DPPH solution was added to 20 μL stock serial dilutions of the peptide (0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.5 mg/mL) into microplate and was mixed with vortex. The plate was placed in the dark at room temperature for 30 minutes. Then, the absorbance was determined at 570 nm. The butylated hydroxyanisole was used as a positive control. 24 The ability to inhibit DPPH in percentage was calculated using the following equation:
In this equation, Acontrol is the absorbance of control (180 μL DPPH radical + 20 μL methanol), and Asample is the absorbance of the treated DPPH with peptide. Similarity to enzyme inhibitory activity, the peptide concentration that provides 50% inhibition was defined as IC50.
Sequencing
Peptide sequencing was carried out using mass spectrometry (MS), in positive ionization mode on a matrix-assisted laser desorption/ionization time of flight (MALDI-TOF)/TOF instrument. Purified and lyophilized peptide was reconstituted with 10 µL of 0.1% TFA (v:v). A 1-µL aliquot of each peptide solution was applied directly to a ground steel MALDI target plate, followed immediately by an equal volume of a freshly prepared 5 mg/mL solution of 4-hydroxy-α-cyano-cinnamic acid (Sigma, city of York, England) in 50% aqueous (v: v) acetonitrile containing 0.1% TFA (v:v). Bruker flex Analysis software (version 3.3) was used to perform the spectral processing and peak list generation for both the MS and the MS/MS spectra. De novo sequencing was performed by hand, allowing for a maximum mass error of 0.5 Da for any given fragmentation ion. Deduced b- and y-ion series were overlaid onto their fragmentation spectra using the Bruker flex Analysis software (version 3.3).
Phylogenetic Analysis
Ten amino acid sequences of the experimental peptides from different species were obtained from the antimicrobial peptide database (http://aps.unmc.edu/AP/main.php). These sequences, along with those for new peptide, were aligned using the basic local alignment search tool (BLAST) program; the alignment was then adjusted manually. A phylogenetic tree was obtained from the CLC main workbench Ver.5.5 software using the neighbor-joining method. Bootstrap analysis, with 100 replications, was performed on the phylogenetic tree to estimate the reproducibility of the tree topology.
Results
Purification and Neurobiological Assay
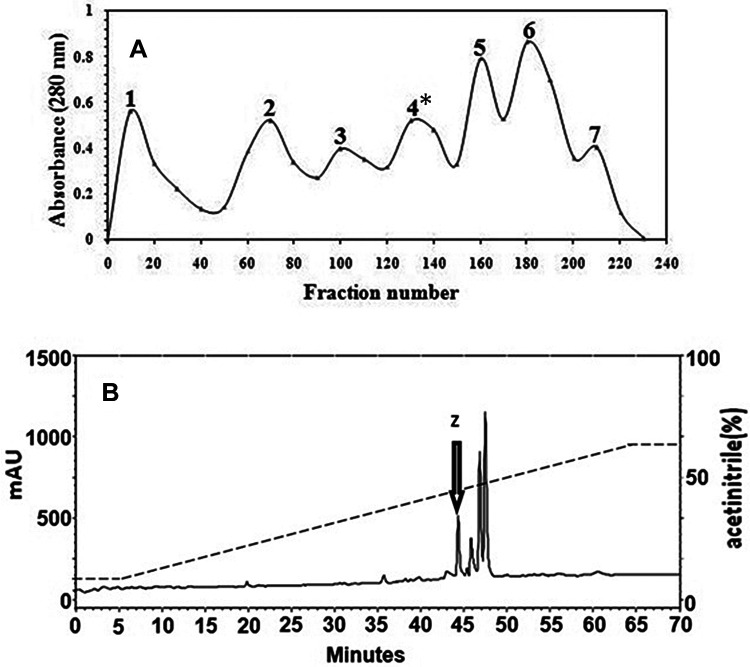
Totally, 7 peaks were collected from a Sephadex G-50 (Figure 1A), and the neurobiological activity showed that the peak number 4 has inhibitory activities against 2 tested enzyme, AChE and BChE, as well as antioxidant activity. Thus, for further purification, this peak was loaded in RP-HPLC column, and 5 common peaks were obtained from this separation that was shown in Figure 1B. The peak 1(z) has antioxidant and anticholinesterase activity. These activities have been illustrated in Figures 2 and 3, respectively. So, this peak, eluted from the HPLC, was collected and subjected to MALDI-TOF mass spectrometric analysis.
Figure 1.
Purification of peptides from Zizyphus jujuba fruit. A, Sephadex G-50 gel filtration of plant extract was applied on a Sephadex G-50 column equilibrated with phosphate buffer (0.1 mol/L, pH 6.0). B, The peak 4 (indicated with asterisk), with anticholinesterase activity from Sephadex G-50, was further purified on a C18 reverse phase-high-performance liquid chromatography (RP-HPLC) column. A 400-μL aliquot of filtrated extract from gel filtration was loaded onto a semipreparative C18 reverse phase column. Elution was performed using solution A (0.1% TFA in water), combined with a 5% to 65% gradient of solution B (0.098% TFA in acetonitrile) over a period of 70 minutes, at a flow rate of 1 mL/min. The absorbance was monitored at 220 nm, and the fractions were tested for anticholinesterase activity. The active peak has been indicated by an arrow. This peak was named Z (Zizyphus jujuba). TFA indicates trifluoroacetic acid.
Figure 2.
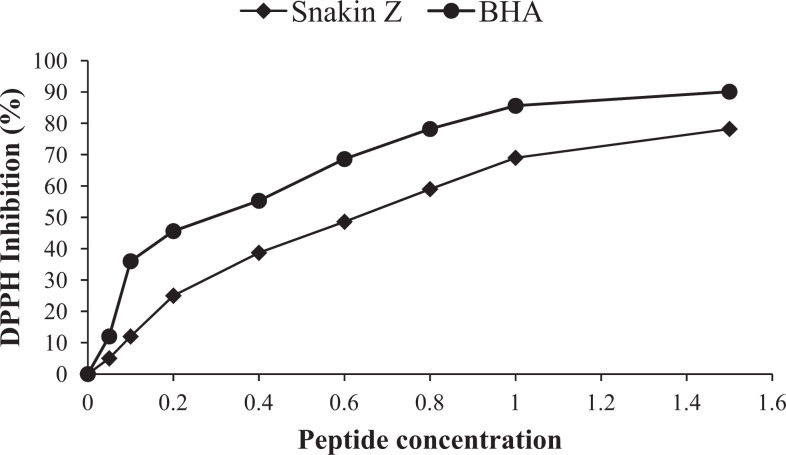
DPPH radical scavenging activity (%) of Snakin-Z from Ziziphus jujuba fruits. butylated hydroxyanisole (BHA) is positive control.
Figure 3.
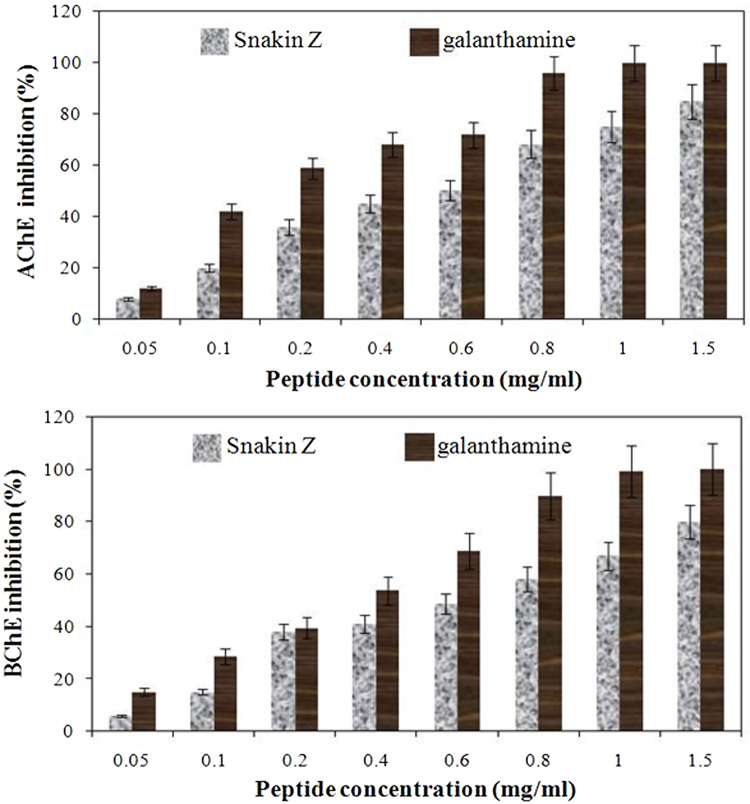
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities (%) of Snakin-Z from Ziziphus jujuba fruits.
Tricine-SDS-PAGE
Figure 4 shows the electrophoretic pattern of the crude extract and purified peptide on SDS-PAGE. According to this figure, a sharp band was obtained with a molecular weight less than that of insulin (5.8 kDa). This result is well overlapping with those of acquired molecular weight from peptide sequencing.
Figure 4.
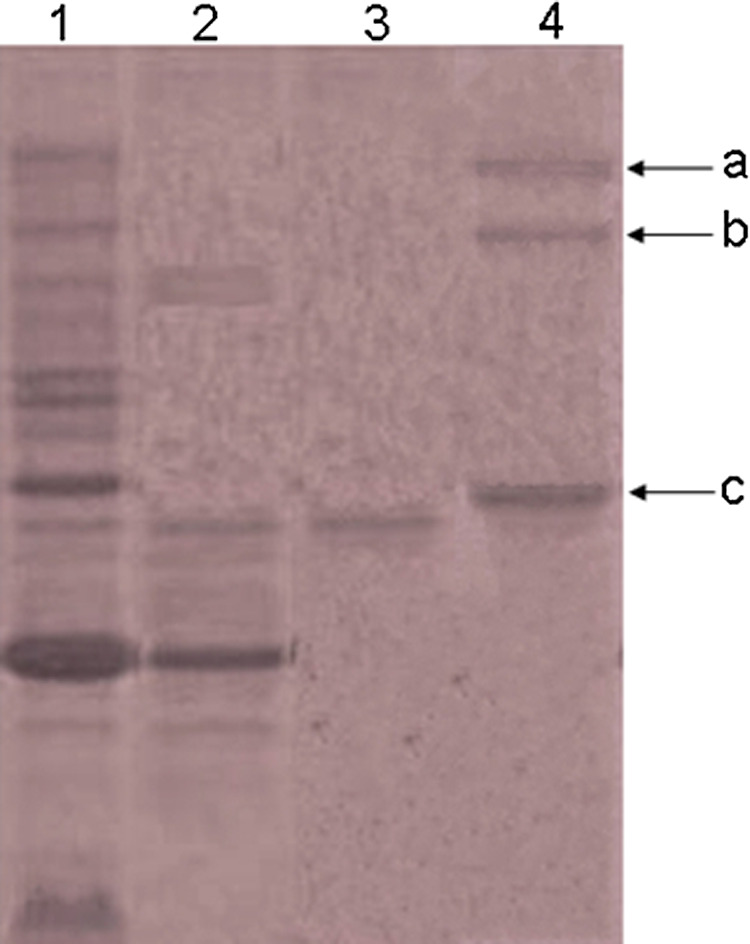
Tricine-SDS-PAGE of raw extract (1), active fraction (peak 4) from gel filtration column (2), purified peptide from C18 reverse phase-high-performance liquid chromatography (RP-HPLC) column (3), and size marker (4). A, Trypsinogen (23 kDa); B, lysozyme (14 kDa); and C, insulin (5.8 kDa).
Structural Properties
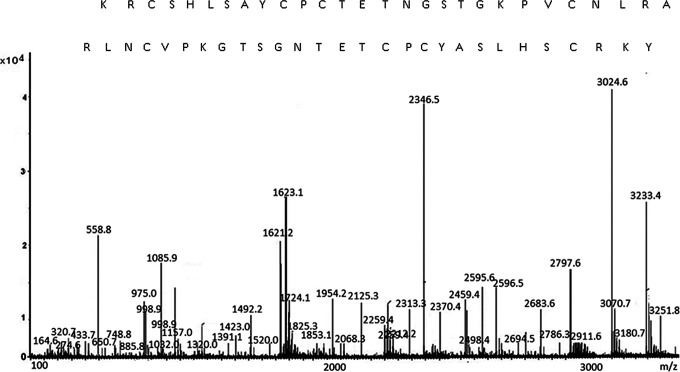
The peak 1(z), marked by an arrow in Figure 1B, was subjected to amino acid sequencing analysis by MS. The MS/MS spectrometric analysis of the peptide was shown in Figure 5. This peptide consists of 31 amino acids and its sequence is CARLNCVPKGTSGNTETCPCYASLHSCRKYG. Our obtained peptide was compared with other reported peptides. This peptide shows no 100% sequence homology to any reported peptide in the database, suggesting that it is novel.
Figure 5.
Identification of the molecular mass and amino acid sequence of the new peptide from the fruit of Ziziphus jujube using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) spectrometer.
Phylogenetic Analysis
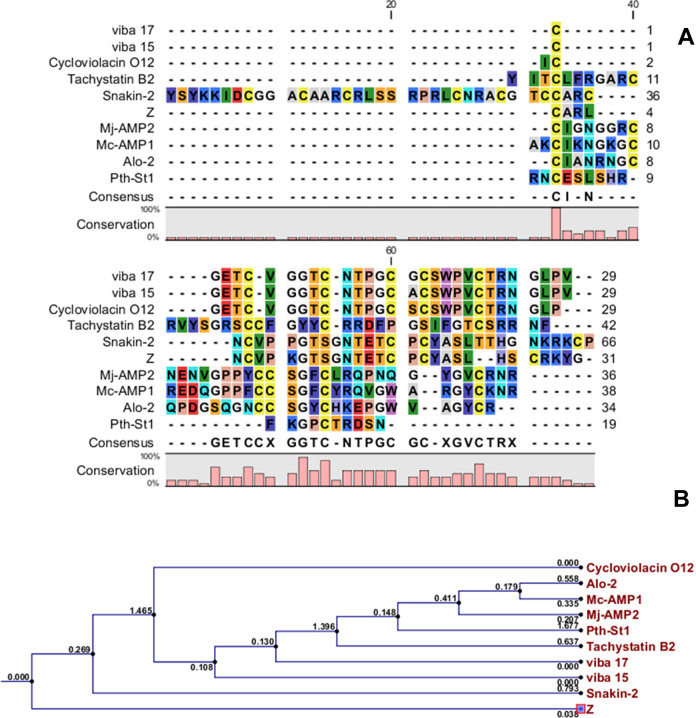
Multiple sequence alignment of new peptide was done along with 10 antimicrobial peptides from different species. The results are illustrated in Figure 6A. The BLAST search shows that this peptide has similarity to antimicrobial peptide Snakin-2 from Solanum tuberosum to some extent. 8 Phylogenetic analysis of the amino acid sequences was done by constructing a phylogenetic tree. This peptide is most similar (66% identity) to Snakin-2 from S tuberosum (Figure 6B). According to these data, this peptide is a new peptide, identified for the first time by our research group. Thus, the new peptide was named Snakin-Z, based on a systematic nomenclature for bioactive peptides. 31
Figure 6.
The alignment and phylogenetic tree of Snakin-Z. A, The alignment of amino acid sequences of Snakin-Z with the sequences of other antimicrobial peptides. The alignment was carried out with CLC Main Work Bench Ver.5.5 software. B, Phylogenetic tree of Snakin-Z. Amino acid sequences of the 10 reference peptides obtained from the APD database were incorporated into the tree using the neighbor-joining method. The name of each sequence is typed at the end of the corresponding branch. Reliability of the tree was assessed by bootstrap analysis with 100 replications. The substitutions per amino acid position are typed above each branch.
Antioxidant and Cholinesterase Inhibitory Activity
The antioxidant activity of peptide was examined by free radical (DPPH) scavenging test in the well. The result indicated that the new peptide presented a certain degree of antioxidant activity.
Figure 2 shows the DPPH radical scavenging activity of the new peptide. As shown in this figure, DPPH radical scavenging of Snakin-Z was 78.2% ± 2.95% at concentration of 1.5 mg/mL.
The IC50 values of this peptide (concentration of the peptide that scavenges half of the DPPH radical) are shown in Table 1. As shown in this table, the IC50 value of DPPH radical-scavenging activity was found to be 0.75 ± 0.09 mg/mL. The result showed that Snakin-Z had low IC50 values in antioxidant assay which indicate its good antioxidant potential. The obtained peptide could be able to quench DPPH radicals and exhibited an antioxidant activity.
Table 1.
The IC50 Values of Antioxidant and Cholinesterase Inhibitory Activity of Snakin-Z.
| Activity | IC50, mg/mL | ||
|---|---|---|---|
| Snakin-Z | Galanthaminea | BHAa | |
| Anti-AChE | 0.58 ± 0.08 | 0.17 ± 0.065 | – |
| Anti-BChE | 0.72 ± 0.085 | 0.35 ± 0.09 | – |
| Antioxidant | 0.75 ± 0.09 | – | 0.3 ± 0.05 |
Abbreviations: AChE, acetylcholinesterase; BChE, butyrylcholinesterase; BHA, butylated hydroxyanisole; IC50, half maximal inhibitory concentration.
aGalanthamine and BHA were used as the positive control for anticholinesterase and antioxidant activities, respectively.
Moreover, the new peptide Snakin-Z showed potent cholinesterase inhibitory activity. The results of the AChE and BChE inhibitory activities of the tested new peptide are shown in Figure 4. As shown in this figure, this new peptide has inhibitory activity against these enzymes. The IC50 values of the peptide indicating AChE and BChE inhibitory activity are presented in Table 1.
Snakin-Z exhibited the inhibition against AChE with IC50 of 0.58 ± 0.08 mg/mL. The IC50 value of this peptide was also found to be 0.72 ± 0.085 mg/mL against BChE. Low IC50 values are indicative of good inhibition of the enzymes.
Discussion
Zizyphus jujuba is a medicinal plant. The fruits of this plant are used as sedatives, anticancer, antipyretic, analgesic, appetizer, antihemorrhage agents, and so on. 25–27 Polysaccharide and protein content of these fruits also have biological activity. 24,32–35 There are a small number of studies on neurobiological activity of Z jujuba fruits. In this study, protein and peptide content of Z jujuba fruits was isolated and purified. Then, their neurobiological activity (antioxidant and cholinesterase inhibition) was assessed. Oxidative stress occurring by different mechanisms and cholinesterase activity is connected with AD. 36–38 So, antioxidant and cholinesterase inhibitory compound are suitable for the treatment of this disease. In the current study, the new bioactive peptide was identified and purified from Z jujuba fruits. Large numbers of peptides with different structures and functions such as cyclotides, glycine-rich proteins, snakins, albumins, and purothionin from plants have been reported. 39–42 These peptides have different biological activities. In this study, 1 novel peptide was discovered. Primary structural characterization of the new peptide revealed that it was the member of defensin-like Snakin-2. Thus, based on a recent nomenclature recommendation by Conlon, the present peptide was named as Snakin-Z. 31 This peptide has the highest potent activity in inhibiting the activity of AChE and BChE enzymes. This peptide also showed an effect on ions’ DPPH radical quenching. The sequence of peptide and protein affects the antioxidant activity. 43–45 Existence of hydrophobic amino acids in sequence have prominent role in quenching DPPH radicals. 46,47 The new peptide is a hydrophobic moiety, and this property might be effective on antioxidant activity of this new peptide. The results of the cholinesterase inhibitory activities of Snakin-Z showed that this peptide is a good inhibitor. In many articles, it is shown that different parts of medicinal plant such as flavonoids, essential oils, and so on possess anticholinesterase activity. 48–50 In this study, it is shown that peptides also have anticholinesterase activity. The IC50 values of Snakin-Z indicating AChE inhibitory activity are presented in Table 1. A low IC50 value is indicative of good inhibition of the enzymes. The IC50 value of this new peptide is much lower than different agents with anticholinesterase activity. Considering the unique features of Snakin-Z, this peptide may find a potential application in the treatment of AD. However, further work is recommended to be done in investigating the neurobiological activities of this peptide in vivo using appropriate animal models.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was funded by Young Researchers and Elite Club from Islamic Azad University, Yazd, Iran.
References
- 1. Krantic S. Peptides as regulators of the immune system: emphasis on somatostatin. Peptides. 2000;21(12):1941–1964. [DOI] [PubMed] [Google Scholar]
- 2. Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung WS, Park Y, Choi CH, Hahm KS, Lee DG. Mode of antibacterial action of a signal peptide, Pep27 from Streptococcus pneumoniae . Biochem Biophys Res Commun. 2007;363(3):806–810. [DOI] [PubMed] [Google Scholar]
- 4. Duval E, Zatylny C, Laurencin M, Baudy-Floc'h M, Henry J. KKKKPLFGLFFGLF: a cationic peptide designed to exert antibacterial activity. Peptides. 2009;30(9):1608–1612. [DOI] [PubMed] [Google Scholar]
- 5. Daoud R, Dubois V, Bors-Dodita L, et al. New antibacterial peptide derived from bovine hemoglobin. Peptides. 2005;26(5):713–719. [DOI] [PubMed] [Google Scholar]
- 6. Ng TB. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides. 2004;25(7):1215–1222. [DOI] [PubMed] [Google Scholar]
- 7. Lin P, Ng TB. A novel and exploitable antifungal peptide from kale (Brassica alboglabra) seeds. Peptides. 2008;29(10):1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ngai PH, Ng TB. Coccinin, an antifungal peptide with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from large scarlet runner beans. Peptides. 2004;25(12):2063–2068. [DOI] [PubMed] [Google Scholar]
- 9. Pan Y, Lee A, Wan J, et al. Antiviral properties of milk proteins and peptides. Int Dairy J. 2006;16(11):1252–1261. [Google Scholar]
- 10. Jenssen H, Andersen JH, Mantzilas D, Gutteberg TJ. A wide range of medium-sized, highly cationic, α-helical peptides show antiviral activity against herpes simplex virus. Antiviral Res. 2004;64(2):119–126. [DOI] [PubMed] [Google Scholar]
- 11. Mutchnick MG, Ehrinpreis MN, Kinzie JL, Peleman RR. Prospectives on the treatment of chronic hepatitis B and chronic hepatitis C with thymic peptides and antiviral agents. Antiviral Res. 1994;24(2-3):245–257. [DOI] [PubMed] [Google Scholar]
- 12. Fachim HA, Cunha AO, Pereira AC, et al. Neurobiological activity of Parawixin 10, a novel anticonvulsant compound isolated from Parawixia bistriata spider venom (Araneidae: Araneae). Epilepsy Behav. 2011;22(2):158–164. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Bouchard RW, Camicioli R, Léger G. Toward a revision of criteria for the dementias. Alzheimers Dement. 2007;3(4):428–440. [DOI] [PubMed] [Google Scholar]
- 14. Grinberg LT, Heinsen H. Toward a pathological definition of vascular dementia. J Neurol Sci. 2010;299(1-2):136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caroli A, Frisoni GB. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's disease neuroimaging initiative cohort. Neurobiol Aging. 2010;31(8):1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta. 2011;1812(12):1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costagli C, Galli A. Inhibition of cholinesterase-associated aryl acylamidase activity by anticholinesterase agents: focus on drugs potentially effective in Alzheimer’s disease. Biochem Pharmacol. 1998;55(10):1733–1737. [DOI] [PubMed] [Google Scholar]
- 18. Mamelak M. Alzheimer’ s disease, oxidative stress and gammahydroxybutyrate. Neurobiol Aging. 2007;28(9):1340–1360. [DOI] [PubMed] [Google Scholar]
- 19. Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic Biol Med. 2002;32(12):1264–1275. [DOI] [PubMed] [Google Scholar]
- 20. Borris RP. Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol. 1996;51(1-3):29–38. [DOI] [PubMed] [Google Scholar]
- 21. Moerman DE. An analysis of the food plants and drug plants of native North America. J Ethnopharmacol. 1996;52(1):1–22. [DOI] [PubMed] [Google Scholar]
- 22. Gomes NG, Campos MG, Órfão JM, Ribeiro CA. Plants with neurobiological activity as potential targets for drug discovery. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1372–1389. [DOI] [PubMed] [Google Scholar]
- 23. Yoon JI, Al-Reza SM, Kang SC. Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem Toxicol. 2010;48(5):1350–1354. [DOI] [PubMed] [Google Scholar]
- 24. Memarpoor-Yazdi M, Mahaki H, Zare-Zardini H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J Funct Foods. 2013;5(1):62–70. [Google Scholar]
- 25. Hung CF, Hsu BY, Chang SC, Chen BH. Antiproliferation of melanoma cells by polysaccharide isolated from Zizyphus jujuba . Nutrition. 2012;28(1):98–105. [DOI] [PubMed] [Google Scholar]
- 26. Al-Reza SM, Yoon JI, Kim HJ, et al. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba . Food Chem Toxicol. 2010;48(2):639–643. [DOI] [PubMed] [Google Scholar]
- 27. Al-Reza SM, Rahman A, Lee J, Kang SC. Potential roles of essential oil and organic extracts of Zizyphus jujuba in inhibiting food-borne pathogens. Food Chem. 2010;119(3):981–986. [Google Scholar]
- 28. Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009;112(4):858–862. [Google Scholar]
- 29. Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1(1):16–22. [DOI] [PubMed] [Google Scholar]
- 30. Adewusi EA, Moodley N, Steenkamp V. Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. S Afr J Bot. 2011;77(3):638–644. [Google Scholar]
- 31. Conlon JM. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides. 2008;29(10):1815–1819. [DOI] [PubMed] [Google Scholar]
- 32. Plastina P, Bonofiglio D, Vizza D, et al. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J Ethnopharmacol. 2012;140(2):325–332. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Fan L, Ding S. Isolation, purification and structure of a new water-soluble polysaccharide from Zizyphus jujuba cv. Jinsixiaozao. Carbohydr Polym. 2011;83(2):477–482. [Google Scholar]
- 34. Li J, Ai L, Yang Q, Liu Y, Shan L. Isolation and structural characterization of a polysaccharide from fruits of Zizyphus jujuba cv. Junzao. Int J Biol Macromol. 2013;55:83–87. [DOI] [PubMed] [Google Scholar]
- 35. Li JW, Ding SD, Ding Xl. Optimization of the ultrasonically assisted extraction of polysaccharides from Zizyphus jujuba cv. jinsixiaozao. J Food Eng. 2007;80:176–183. [Google Scholar]
- 36. Feng Z, Qin C, Chang Y, Zhang J-t. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer's disease. Free Radic Biol Med. 2006;40(1):101–109. [DOI] [PubMed] [Google Scholar]
- 37. Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33(2):182–191. [DOI] [PubMed] [Google Scholar]
- 38. Shen ZX. Brain cholinesterases: II. The molecular and cellular basis of Alzheimer's disease. Med Hypotheses. 2004;63(2):308–321. [DOI] [PubMed] [Google Scholar]
- 39. Selitrennikoff CP. Antifungal proteins. Appl Environ Microbiol. 2001;67(7):2883–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Caleya RF, Gonzalez-Pascual B, García-Olmedo F, Carbonero P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol. 1972;23(5):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pelegrini PB, Franco OL. Plant γ-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins. Int J Biochem Cell Biol. 2005;37(11):2239–2253. [DOI] [PubMed] [Google Scholar]
- 42. Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135(1):1–11. [DOI] [PubMed] [Google Scholar]
- 43. Gimenez B, Aleman A, Montero P, Gomez-Guillen MC. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009;114(3):976–983. [Google Scholar]
- 44. Udenigwe CC, Lu Y-L, Han C-H, Hou W-C, Aluko RE. Flaxseed protein-derived peptide fractions: antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem. 2009;116(1):277–284. [Google Scholar]
- 45. Alemán A, Giménez B, Pérez-Santin E, Gómez-Guillén MC, Montero P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011;125(2):334–341. [Google Scholar]
- 46. Rajapakse N, Mendis E, Jung WK, Je J-Y, Kim S-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38(2):175–182. [Google Scholar]
- 47. Ren J, Zhao M, Shi J, et al. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108(2):727–736. [DOI] [PubMed] [Google Scholar]
- 48. Arruda M, Viana H, Rainha N, et al. Anti-acetylcholinesterase and antioxidant activity of essential oils from hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules. 2012;17(3):3082–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khadri A, Serralheiro ML, Nogueira JM, Neffati M, Smiti S, Araujo ME. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem. 2008;109(3):630–637. [Google Scholar]
- 50. Miura GA, Broomfield CA, Lawson MA, Worthley EG. Widespread occurrence of cholinesterase activity in plant leaves. Physiol Plant. 1982;56(1):28–32. [Google Scholar]