Abstract
The etiology of Alzheimer’s disease (AD) is multifactorial involving both genetic and environmental factors. Apolipoprotein E (ApoE) gene plays a pivotal role in risk and age of onset of AD. Although it is broadly accepted that ApoE genotype is linked to the pathogenesis of AD, there are still controversial results regarding the association of ApoE levels in cerebrospinal fluid (CSF) with the occurrence of AD. Some studies have shown a positive correlation between CSF ApoE levels and AD, whereas others showed no link. In this study, we measured ApoE levels to assess the usefulness of CSF ApoE as a diagnostic marker of AD by comparing the levels in 3 patient groups and in control participants. No significant difference was observed in CSF ApoE concentrations between the patients with AD and the controls. So, it appears that CSF ApoE measurement does not offer any diagnostic advantage for AD.
Keywords: Alzheimer’s disease, ApoE, cerebrospinal fluid, Indian population
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, accounting for nearly 60% to 70% of cases worldwide. 1 The cardinal features of AD include extracellular amyloid beta (Aβ) deposition, intracellular neurofibrillary tangle (NFT) formation, and neuronal loss in the brain. The Aβ is derived from cleavage of large transmembrane amyloid precursor protein, and NFTs are products of accumulation of phosphorylated microtubule–associated protein, tau, in the brain. The etiology of AD is multifactorial involving both genetic and environmental factors. The risk factors for AD include advancing age, positive family history, head trauma, genetic factors, female gender, and low educational attainment. On the basis of the age of onset, AD has been classified into 2 types early-onset AD (EOAD) and late-onset AD (LOAD). The EOAD occurs at around 30 to 60 years of age and is seen in 1% to 5% of cases with AD, whereas LOAD is manifested after 60 years and accounts for about 90% of cases with AD. Both EOAD and LOAD have genetic component linked to them. 2
Among many susceptible genes proposed for LOAD, the link has been strongly proven only for apolipoprotein E (ApoE). The ApoE gene plays a pivotal role in determining the risk and age of onset of AD. 3 The protein has 3 isoforms, ApoE2, E3, and E4, encoded by alleles ∊2, ∊3, and ∊4 among which ApoE4 has been linked to the pathogenesis of AD. The ∊4 allele frequency is 15% in general population when compared to 40% in the population with AD. The risk of developing AD at earlier age depends on the ApoE isoform (in decreasing order ∊4 > ∊3 >∊2). 4 The incidence of AD increases to 2- to 3-fold in those having a single ∊4 allele and about 12-fold higher in those homozygous for ∊4. Although ApoE∊4 increases the risk of AD, ApoE∊2 is protective and associated with a decrease in risk. Thus, different ApoE isoforms exhibit differential effect on binding Aβ and hence on AD pathogenesis.
Apart from AD, ApoE4 is also involved in the occurrence and progression of other neurodegenerative disorders, such as Parkinson’s disease, multiple sclerosis, Lewy body dementia, and cerebral amyloid angiopathy.
Clinical studies reemphasize the significance of ApoE in the development of AD. In single-photon emission computed tomography, patients with AD homozygous for ∊4 allele exhibited the most severe cerebral hypoperfusion, 5 and in positron emission tomography (PET) the low baseline metabolism in nondemented ∊4 carriers predicted a cognitive decline. 6 Magnetic resonance imaging (MRI) studies have displayed that patients with AD with at least 1 ∊4 allele have decreased volume in hippocampus 5 and entorhinal cortex. 7 In neuropsychological tests also, the ∊4 allele has been associated with the cognitive decline in patients with cognitive impairment. 8
Although the association between ApoE genotype and AD is well established, the association of ApoE levels in cerebrospinal fluid (CSF) and the occurrence of AD has been controversial. Some studies have shown a positive correlation between CSF ApoE levels and AD, a few showing otherwise. In our previous study, 9 we found a significant elevation of the ApoE∊4 allele frequency in patients with AD. In this present work, we analyzed the CSF ApoE levels in AD and assessed its utility as a diagnostic marker.
Patients and Methods
This study was performed in 223 patients divided into 4 groups. These included 44 patients with AD, 63 patients with dementias other than AD (NAD), 70 patients with neurological illnesses other than dementia (NC), and 46 age-matched healthy controls (HC). Among the patients with AD, 11 belonged to preclinical or very mild stage (VMAD), 13 were of moderate stage (MAD), and 20 were of advanced stage (SAD). The demographic profile of patients is shown in Table 1. The diagnosis of AD was made according to the criteria of the National Institute of Neurological and Communicative Disorders and stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) and International Statistical Classification of Disease and Related Health Problems (ICD-10). The diagnosis of other dementias or NAD was based on the guidelines of Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition; American Psychiatric Association, 1994). Global cognitive functions were assessed using the Mini-Mental State Examination (MMSE). The patients with AD, NAD, and NC were recruited from the Department of Neurology, Nehru Hospital, Postgraduate Institute of Medical Education and Research, Chandigarh. The HCs were recruited from the Departments of Nephrology and Orthopedics. The study was approved by the institute’s human ethics committee, and informed consent was obtained from all the patients or their caregivers.
Table 1.
Demographic Profile of AD, NAD, NC, and HC Patients.
| Group I (AD) | Group II (NAD) | Group III (NC) | Group IV (HC) | |
|---|---|---|---|---|
| N | 44 | 63 | 70 | 46 |
| Age | 61.84 ± 8.97 | 57.15 ± 10.47 | 55.04 ± 7.38 | 60.84 ± 12.29 |
| Sex (M:F) | 26:18 | 57:06 | 51:19 | 32:14 |
| CSFT ApoE μg/mL | 22.36 ± 2.35 | 22.10 ± 3.49 | 22.55 ± 2.93 | 23.22 ± 2.21 |
| MMSE | 10.45 ± 4.03 | 19.11 ± 2.36 | 23.81 ± 2.24 | 28.43 ± 1.00 |
Abbreviations: AD, Alzheimer’s disease; ApoE, apolipoprotein; CSF, cerebrospinal fluid; F, female; HC, age-matched healthy controls; M, male; MMSE, Mini-Mental State Examination; NAD, patients with dementias other than AD; NC, patients with neurological illnesses other than dementia. (Kandimalla et. al, Current Alzheimer Research, 2011).
The CSF Collection
Cerebrospinal fluid samples were obtained from lumbar punctures at L3/L4 or L4/L5 interspace using routine protocol. A total of 2 mL of CSF was collected in polypropylene tubes and subjected to centrifugation at 4000g for 10 minutes to remove cell debris and insoluble material. Aliquots of 100 μL were prepared and immediately stored at −70°C until biochemical assays were carried out. Total ApoE levels were measured in all the patients; ApoE4 was estimated only in AD and NAD groups.
Estimation of CSF Total ApoE and ApoE4 Proteins
Assays for both total ApoE and ApoE4 were performed using commercially available enzyme-linked immunosorbent assay kit as per the manufacturer’s recommendations (MBL Nagoya, Japan). The assay uses affinity-purified polyclonal antibody against ApoE and monoclonal antibody against ApoE4. The samples were incubated in antibody-coated wells. After washing, a peroxidase-conjugated anti-antibody was added into the microwell and incubated. After washing, the peroxidase substrate was added; an acid solution was then added to terminate the enzyme reaction and to stabilize the developed color. The optical density was measured at 450 nm using a microplate reader. The concentration of the proteins was derived from reference standards.
Statistical Analysis
The values were expressed as mean ± standard deviation for all the samples. The statistical analyses were performed using SPSS for windows V.13 software. The 1-way analysis of variance with Bonferroni post hoc analysis was used to compare differences in means between the diagnostic groups. The statistical significance was set at P < .005.
Results
The values for total ApoE are shown in Table 2. The total ApoE levels in AD were 22.36 ± 2.35 μg/mL, and no statistical difference was observed when compared to controls. The mean ApoE4 levels (μg/mL) were 15.01 ± 1.5 in the AD group and 15.4 ± 1.8 in the NAD group. No statistical difference was observed in ApoE4 levels in AD and NAD groups as well as between the different stages of AD. We also estimated the E4/E ratio among the different groups. In patients with AD, E4/E ratio was 67.13 ± 0.81. In the AD subgroups, VMAD, MAD, and SAD, the E4/E ratio was 68.5 ± 0.80, 65.21 ± 1.00, and 67.18 ± 0.812, respectively. In NAD group, of the 63 patients only 3 patients showed ApoE4 protein levels. The E4/E ratio in these 3 patients was 69.7 ± 0.79. It was also observed that only ApoE∊4 allele carriers showed ApoE4 protein in CSF, and the levels were higher among the homozygous individuals. The concentration of CSF ApoE is plotted in Figure 1, according to the presence or absence of E4 allele.
Table 2.
The CSF Total ApoE and ApoE4 Levels in AD, AD Subgroups, NAD, NC, HC.a
| Group | Number | ApoE μg/mL (Mean ± SD) | ApoE4 μg/mL (Mean ± SD) |
|---|---|---|---|
| HC | 46 | 23.22 ± 2.21 | |
| NC | 70 | 22.55 ± 2.93 | |
| NAD | 63 | 22.10 ± 3.49 | 15.41 ± 7.69 |
| AD | 44 | 22.36 ± 2.35 | 15.01 ± 1.5 |
| PAD or VMAD | 11 | 21.76 ± 1.80 | 14.90 ± 1.27 |
| MAD | 13 | 22.02 ± 2.68 | 14.36 ± 1.13 |
| SAD | 20 | 22.70 ± 2.25 | 15.25 ± 1.81 |
Abbreviations: AD, Alzheimer’s disease; ApoE, apolipoprotein; CSF, cerebrospinal fluid; HC, age-matched healthy controls; MAD, moderate-stage Alzheimer’s disease; NAD, patients with dementias other than AD; NC, patients with neurological illnesses other than dementia; SAD, advanced-stage Alzheimer’s disease; VMAD, very mild-stage Alzheimer’s disease.
a Patients with AD: ApoE ∊4/∊4: 14.82 ± 1.71 μg/mL and ApoE ∊3/∊4: 12.85 ± 0.83 μg/mL.
Figure 1.
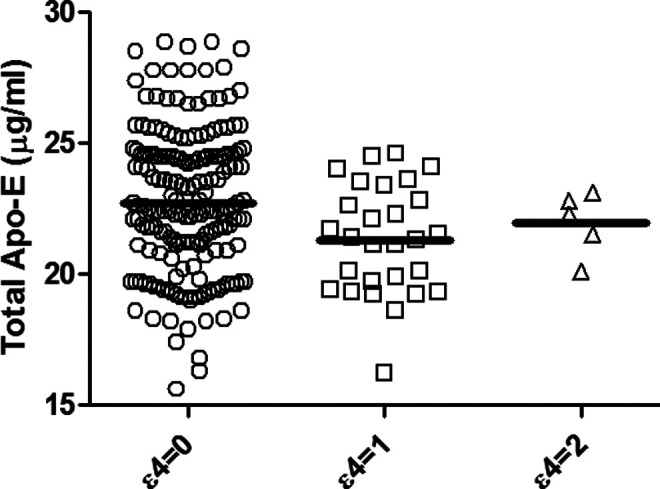
In NAD, NC, HC as well as in the AD subpopulation, the CSF ApoE concentration was not significantly modified by the phenotype, even if a minor increase in ApoE level for individuals having 2 copies of the ∊4 allele was observed. The mean values are no copy of ∊4 allele, 22.7 ± 3.41 µg/mL; 1 copy of ∊ allele, 21.30 ± 2.08 µg/mL; 2 copies of ∊4 allele, 21.96 ± 1.20µg/mL. NAD indicates patients with dementias other than AD; NC, patients with neurological illnesses other than dementia; HC, age-matched healthy controls; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; ApoE, apolipoprotein E.
Figure 2.
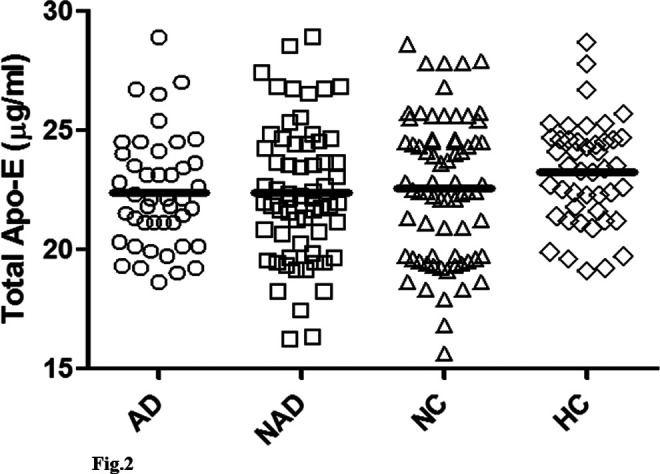
The CSF ApoE values are plotted according to the pathology. The statistical analysis with the Mann-Whitney U and Kruskal-Wallis tests did not show any significant differences between the different subpopulations. The mean values are AD group, 22.36 ± 2.35 µg/mL; NAD, 22.10 ± 3.49 µg/mL; NC, 22.55 ± 2.93 µg/mL; HC, 23.22 ± 2.21µg/mL. CSF indicates cerebrospinal fluid; ApoE, apolipoprotein E; AD, Alzheimer’s disease; NAD, patients with dementias other than AD; NC, patients with neurological illnesses other than dementia; HC, age-matched healthy controls.
Discussion
Apolipoprotein E, the 299-amino acid protein, is associated with lipoproteins in the plasma and the CSF. The 3 major genetic variants of ApoE in the general population differ by a single amino acid. Among the different susceptibility genes associated with the risk of LOAD, ApoE has been identified as a strong genetic determinant. The amino acid substitutions alter the charge and structure of ApoE,10 affecting its binding to lipoprotein receptors and thus impairs lipoprotein transport. The role of ApoE4 in AD neuropathology is not yet clear. However, various potential mechanisms have been shown to play a part in associating ApoE with AD. 11 Various studies have linked ApoE to AD via its ability to modulate Aβ metabolism. Although the exact mechanism of ApoE-Aβ interaction is not clear, ApoE is proposed to influence Aβ conformation and hence clearance. 11 It has been suggested that ApoE binds to Aβ peptide and targets it for protease digestion. However, arginine in ApoE4 acts as protease inhibitor and thus results in Aβ accumulation.
The ApoE4 is also known to influence neurofibrillary pathology. 12 Previous studies have demonstrated that young individuals depicting NFTs have ApoE∊4 allele when compared to controls, and the presence of ∊4 allele correlates with neurofibrillary pathology in both elderly individuals and patients with AD. The role of ApoE in NFTs is also attributed to the differential ability of ApoE isoforms to bind to tau protein, with ApoE∊2 as guardian and ∊4 as risk factor. 12 The ApoE4 has also been demonstrated to take part in producing inflammation and oxidative stress.
In view of this, knowledge studies have attempted to bring out any correlation between CSF levels and the disease. In this present study, we analyzed the total ApoE and ApoE4 protein concentration in CSF of patients with AD, and we observed that the levels of both the proteins in CSF were not significantly different from those of the controls. The levels of both the proteins were also similar across the different AD subgroups. In our previous study, we found a significantly high frequency of the ApoE∊4 allele in the AD group (P < .001). 9 The mean ± SD values of CSF total ApoE and ApoE4 proteins in different groups are shown in Table 2. We analyzed the impact of the ApoE genotype on CSF protein levels and observed that it had no effect on the CSF ApoE level in Indian population. Both these findings indicate the fact that estimation of ApoE levels may not hold a diagnostic importance.
It is a surprise to know that even after numerous studies, the levels of ApoE in CSF and its relation to AD have been controversy. Studies have found conflicting results and provided conclusions with increased, 13 decreased, 14,15 or normal 16 -18 CSF ApoE levels in AD. Since inflammatory activity is associated with the neuronal degeneration in AD, it is possible that ApoE levels might increase. On the other hand, decreased CSF levels may result since ApoE coaggregates with Aβ to form amyloid, and reutilization of ApoE lipid complexes is increased due to neuronal repair. Thus, it is perhaps not unexpected that the discrepant reports on ApoE levels in CSF of patients with AD appear. Studies have tried quantification of ApoE messenger RNA (mRNA) and protein levels in hippocampus of AD brains, and the results have shown that the levels of mRNA may be unchanged although protein levels were decreased by about 20%. 19
The question often arises as to what is the source of ApoE protein in CSF? The ApoE is expressed highly in the liver and brain tissues, and it is shown that ApoE does not cross the blood–brain barrier. Studies have shown that both the species are independent of each other, and the ApoE in CSF originates from the brain tissue directly. 20 In the periphery, ApoE is predominantly produced by the liver and is preferentially found in VLDL. 21 In the central nervous system (CNS), ApoE is produced by astrocytes and microglia. Peripheral blood ApoE metabolism is partially understood, 22 -24 and studies have shown that turnover of ApoE4 is twice as ApoE3. 24 Not much is currently known about ApoE turnover kinetics in the CNS, and it is unclear whether a similar isoform-specific effect on ApoE turnover exists in the human CNS. 19,25 It is not known whether the isoforms of ApoE are different among different population and ethnic groups, considering the differential origin and kinetics of the protein between periphery and CNS it would not be a surprise. It is also possible that the different isoforms in proteins alter the kinetics and have implications on protein structure, thus altering the epitope presentation. Most of the studies that attempted ApoE measurement, including the present one, used immunoassays; hence, it is not known whether the differences observed in this study are due to differences in populations or based on differences in the analytical procedures used or both. A recent mass spectrometry-based study on a large sample size has been performed trying to absolutely quantify the ApoE levels in patients and controls. 26 The study also observed the fact that ApoE levels showed no difference between cases and controls, reaffirming the fact that ApoE measurement may not provide any diagnostic significance. 26
Furthermore, in this study we found patients with AD who were homozygous for ApoE∊4 allele showed increased ApoE∊4 protein levels in their CSF when compared with the heterozygous ApoE∊4 allele carriers, but there was no change in total ApoE levels in patients with AD compared to control groups even though the mean MMSE score of the AD group was around 10. So, here it is strongly evident that ApoE∊4 allele plays a role in pathogenesis of AD. Previous studies have shown that ApoE∊4 protein is involved in Aβ42 oligomerization resulting in the development of senile plaques extracellularly, thereby leading to lesser release of Aβ42 peptides into the CSF. These results are supported by our previous study, wherein we have shown less Aβ42 in the CSF of patients with AD carrying ApoEe4 allele compared to patients with AD with non-ApoEe4 allele carrier.9 Intriguingly, in our study, ApoE∊4 allele carrier AD patients and NAD patients have shown ApoE∊4 proteins in their CSF, but there was no mean difference in their levels. So based on our study results, we confirm that although ApoE genotyping may be useful to identify the individuals who are at risk of developing AD, the measurement of CSF ApoE level does not hold any diagnostic significance for the detection and/or classification of the severity of AD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Indian Council of Medical Research (ICMR), 52/3/2003, New Delhi, India.
References
- 1. Alzheimer's disease facts and figures. Alzheimers Dement. 2008;4(2):110–133. [DOI] [PubMed] [Google Scholar]
- 2. Ashford JW, Mortimer JA. Non-familial Alzheimer's disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4(3):169–177. [DOI] [PubMed] [Google Scholar]
- 3. Panza F, Solfrizzi V, D'Introno A, et al. Genetics of late-onset Alzheimer's disease: vascular risk and beta-amyloid metabolism [article in Italian]. Recenti Prog Med. 2002;93(9):489–497. [PubMed] [Google Scholar]
- 4. Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25(5):641–650. [DOI] [PubMed] [Google Scholar]
- 5. Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60(6):644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Small SA, Wu EX, Bartsch D, et al. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron. 2000;28(3):653–664. [DOI] [PubMed] [Google Scholar]
- 7. Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Sr, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer's disease carrying the apolipoprotein E epsilon4 allele. J Neurol Neurosurg Psychiatry. 1998;65(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dik MG, Deeg DJ, Bouter LM, Corder EH, Kok A, Jonker C. Stroke and apolipoprotein E epsilon4 are independent risk factors for cognitive decline: a population-based study. Stroke. 2000;31(10):2431–2436. [DOI] [PubMed] [Google Scholar]
- 9. Kandimalla RJ, Prabhakar S, Binukumar BK, et al. Apo-Eepsilon4 allele in conjunction with Abeta42 and tau in CSF: biomarker for Alzheimer's disease. Curr Alzheimer Res. 2011;8(2):187–196. [DOI] [PubMed] [Google Scholar]
- 10. Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31(8):445–454. [DOI] [PubMed] [Google Scholar]
- 11. Carter DB. The interaction of amyloid-beta with ApoE. Subcell Biochem. 2005;38:255–272. [DOI] [PubMed] [Google Scholar]
- 12. Lovestone S, Anderton B, Betts J, et al. Apolipoprotein E gene and Alzheimer's disease: is tau the link? Biochem Soc Symp. 2001;(67):111–120. [DOI] [PubMed] [Google Scholar]
- 13. Taddei K, Clarnette R, Gandy SE, Martins RN. Increased plasma apolipoprotein E (apoE) levels in Alzheimer's disease. Neurosci Lett. 1997;223(1):29–32. [DOI] [PubMed] [Google Scholar]
- 14. Blennow K, Hesse C, Fredman P. Cerebrospinal fluid apolipoprotein E is reduced in Alzheimer's disease. Neuroreport. 1994;5(18):2534–2536. [DOI] [PubMed] [Google Scholar]
- 15. Gupta VB, Laws SM, Villemagne VL, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76(12):1091–1098. [DOI] [PubMed] [Google Scholar]
- 16. Lehtimaki T, Pirttila T, Mehta PD, Wisniewski HM, Frey H, Nikkari T. Apolipoprotein E (apoE) polymorphism and its influence on ApoE concentrations in the cerebrospinal fluid in Finnish patients with Alzheimer's disease. Hum Genet. 1995;95(1):39–42. [DOI] [PubMed] [Google Scholar]
- 17. Slooter AJ, de Knijff P, Hofman A, et al. Serum apolipoprotein E level is not increased in Alzheimer's disease: the Rotterdam study. Neurosci Lett. 1998;248(1):21–24. [DOI] [PubMed] [Google Scholar]
- 18. Panza F, Solfrizzi V, Colacicco AM, et al. Apolipoprotein E (APOE) polymorphism influences serum APOE levels in Alzheimer's disease patients and centenarians. Neuroreport. 2003;14(4):605–608. [DOI] [PubMed] [Google Scholar]
- 19. Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res Mol Brain Res. 1995;33(1):174–178. [DOI] [PubMed] [Google Scholar]
- 20. Linton MF, Gish R, Hubl ST, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88(1):270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990;31(8):1503–1511. [PubMed] [Google Scholar]
- 22. Ikewaki K, Zech LA, Brewer HB, Jr, Rader DJ. Comparative in vivo metabolism of apolipoproteins E2 and E4 in heterozygous apoE2/4 subjects. J Lab Clin Med. 2002;140(5):369–374. [DOI] [PubMed] [Google Scholar]
- 23. Gregg RE, Zech LA, Schaefer EJ, Brewer HB, Jr. Type III hyperlipoproteinemia: defective metabolism of an abnormal apolipoprotein E. Science. 1981;211(4482):584–586. [DOI] [PubMed] [Google Scholar]
- 24. Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB, Jr. Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78(3):815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahrle SE, Shah AR, Fagan AM, et al. Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol Neurodegener. 2007;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon R, Girod M, Fonbonne C, et al. Total ApoE and ApoE4 Isoform Assays in an Alzheimer's Disease Case-control Study by Targeted Mass Spectrometry (n = 669): a Pilot assay for methionine-containing proteotypic peptides. Mol Cell Proteomics. 2012;11(11):1389–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]


