Abstract
Research has identified unique cultural factors contributing to dementia caregiving in Latin America but very few caregiver interventions have been systematically piloted and evaluated in this region. The purpose of this study was to examine the effectiveness of a group cognitive–behavioral intervention in improving the mental health of dementia caregivers from Cali, Colombia. Sixty-nine caregivers of individuals with dementia were randomly assigned to the cognitive–behavioral intervention or an educational control condition, both spanning 8 weeks. Compared to controls, the treatment group showed higher satisfaction with life and lower depression and burden over the posttest and 3-month follow-ups although there was no effect of the condition on participants’ stress levels.
Keywords: caregivers, intervention, mental health, dementia, Latin America
Introduction
Alzheimer’s disease, the most common form of dementia, is a progressive disorder, which causes changes in the brain, resulting in the loss of neurons and altering the ability to think clearly, plan and solve problems, remember, and perform other mental functions. 1 Currently, there are an estimated 35.6 million people with dementia worldwide; this figure is likely to double every 20 years and reach 65.7 million by 2030 and 115.4 million by 2050. 2 Today more than 60% of people with dementia live in developing countries. 3 In Colombia, the focus of the current study, the estimated age-adjusted dementia prevalence ranges from 13.1 cases/1000 in persons older than 50 years of age to 30.4 cases/1000 in those older than 70 years of age. 4 The total estimated worldwide costs of dementia were US$604 billion in 2010; it is projected to become an epidemic among older adults and a major public health problem in the coming decades. 5 –7
Dementia affects not only the individual but also the family. 8 As the disease progresses, individuals with dementia usually need increasing amount of assistance with activities such as dressing, preparing meals, managing finances, and completing other activities of daily living, most of which is provided by family members. 3 Studies have shown that caring for an individual with dementia is extremely stressful and can have a negative effect on family caregivers’ physical health, 9 such as decreased immune system functioning, hypertension, cardiovascular disease, and sleep problems. 10 –16 It can also affect caregivers’ psychological and emotional health 17 via increased burden, anxiety, and depression, 18 –20 reduce their social life 21 by activity restrictions and reduced personal time, 22,23 restrict their finances through lost wages due to time off work, medication, and other care-related expenses, 24 –26 and lead to increased mortality rates. 27
In the past 20 years, many different interventions aiming to alleviate the negative consequences of providing care to a family member with dementia have been designed and implemented. Research has shown that combined interventions targeting multiple sources of caregiving stress produce significant improvements in caregiver burden, depression, and subjective well-being as well as caregiving ability and knowledge about dementia. 28,29 Cognitive–behavioral therapy interventions (with a focus on modifying beliefs and developing a new behavioral repertoire to deal with the demands of caregiving) have been shown to have a significant effect on caregiver burden and depression, and psychoeducational interventions that include active participation of the participants (eg, role playing, applying new knowledge to individual problems) have had the most robust effects across various outcome measures and may reduce the likelihood of institutionalization of care recipients. 29 –31 Additionally, interventions conducted in a group setting are effective in increasing caregivers’ social support. 32 On the other hand, group-based cognitive–behavioral interventions have been shown to have little effect on care recipients’ symptoms or on caregivers’ physical health problems. 32
Although the quantity, as well as quality, of dementia caregiver interventions has improved greatly in the past decade, 32 nearly all of them have taken place in developed countries. 33 In developing countries, such as Colombia in South America, family dementia caregivers have a well-documented lack of access to resources, services, education, support groups, and residential programs and caregiver treatments or interventions have not been available to this population. 34 –36 This is very important, especially when considering the increasing number of individuals with dementia and the caregiving roles that family members have to assume without support or preparation, which can possibly lead to decreased quality of life and increased psychological morbidity. 29 Dementia caregivers in Latin America are a unique population because of cultural, historical, ethnic, and racial factors. They are more likely to be religious, collectivist (ie, placing a significant value on the well-being of the group), and have strong familial ties and a sense of obligation to support family members who are sick or in need (ie, familismo). 37
Because of increased risk of mental health problems 38,39 and lack of services available for caregivers of individuals with dementia, 8 research suggests the need to develop and implement effective intervention strategies for this population. 40 Because no interventions specifically for informal caregivers of individuals with dementia have been systematically piloted and evaluated in Colombia, the goal of this study was to test the effectiveness of a caregiver intervention developed in the United States with caregivers of individuals with dementia from Colombia, Latin America.
Method
Participants
The sample was comprised of informal family caregivers who provided care to an individual diagnosed with dementia, from Cali, Colombia. Caregivers were eligible to participate in this study if they (1) were related to the person with dementia, (2) were the primary caregiver of that person, (3) had been providing care for at least 3 months, (4) were knowledgeable about the patient’s family and medical history, and (5) had no self-reported history of neurological and psychiatric disorders or learning disabilities. Participants were from the Alzheimer’s Foundation in Cali, Colombia, a nonprofit facility that provides medical, psychological, occupational, speech therapy, and rehabilitation services to individuals with dementia and their caregivers (eg, music, dance, and art therapies).
Sixty-nine caregivers of individuals with dementia met inclusion criteria and were randomly assigned using the flip of a coin to either the experimental (caregiver intervention) or the control (educational program) condition. There were no statistically significant sociodemographic differences between the groups.
Demographic information for the 2 groups is provided in Table 1. The total sample consisted of 13 (18.8%) men and 56 (81.2%) women, with an average age of 57.5 years (standard deviation [SD] = 11.09) and an average educational level of 12.9 years (SD = 4.93). Caregivers had been providing care to the patient for an average of 28.6 months (SD = 20.6) at the time of entry into the study, for an average of 89.3 hours/week (SD = 51.23). Thirty-six caregivers were children/offspring of the care recipient (52.2%), 13 (18.8%) were married to the care recipient, and the remaining 28.9% had another relationship with the care recipient (ie, sibling, uncle/aunt, etc).
Table 1.
Caregiver Sociodemographic Characteristics by Group.
| Variables | Total Sample (n = 69) | ||
|---|---|---|---|
| Experimental Group (n = 39) | Control Group (n = 30) | P Value | |
| Age | 59.4 ± 10.8 | 55.1 ± 11.2 | .118 |
| Education | 13.26 ± 4.89 | 12.53 ± 5.04 | .55 |
| Months providing care | 27.85 ± 19.07 | 29.67 ± 22.78 | .961 |
| Hours/week providing care | 91.90 ± 52.00 | 85.93 ± 50.89 | .641 |
| Gender | |||
| Female | 34 (87.2%) | 22 (73.3%) | .126 |
| Male | 5 (12.8%) | 8 (26.7%) | |
| Monthly income | |||
| <1 | 2 (5.1%) | 4 (13.3%) | .632 |
| 1-2 | 7 (17.9%) | 4 (13.3%) | |
| 2-3 | 8 (20.5%) | 8 (26.7%) | |
| 4-5 | 7 (17.9%) | 6 (20.0%) | |
| >5 | 15 (38.5%) | 8 (26.7%) | |
| Marital status | |||
| Single | 6 (15.4%) | 2 (6.7%) | .518 |
| Divorced/separated | 10 (25.6%) | 5 (16.7%) | |
| Widowed | 3 (7.7%) | 3 (10.0%) | |
| Married | 15 (38.4%) | 17 (56.7%) | |
| Common law | 5 (12.8%) | 3 (10.0%) | |
| Relationship with patient | |||
| Spouse | 7 (17.9%) | 6 (20%) | .818 |
| Offspring | 22 (56.4%) | 14 (46.7%) | |
| Sibling | 5 (12.8%) | 3 (10%) | |
| Niece/nephew | 2 (5.1%) | 3 (10%) | |
| Other | 3 (7.7%) | 4 (13.3%) | |
Instruments
To measure the effectiveness of the intervention, both the experimental and the control group were administered the following measures at 3 time points: pre-, and post-intervention as well as at 3 months after the conclusion of the intervention.
Patient Health Questionnaire 9
A Spanish version of the Patient Health Questionnaire 9 (PHQ-9), a modified module of the PHQ, was used to measure caregiver depression. 41 The evaluation consists of 9 items that reflect typical symptoms of depression. Respondents are asked to indicate how often they have been troubled by each item, endorsing a 4-point Likert-type scale (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day). Total score ranges from 0 to 27, with higher scores indicating higher levels of depression. Previous research has found the Spanish version of the PHQ-9 42 to be both reliable and valid in assessing depression in Spanish speakers. 43,44
Zarit Burden Interview
The Zarit Burden Interview (ZBI) was used to assess caregiver burden. This self-report questionnaire consists of 22 items that evaluate a caregiver’s health condition, psychological well-being, financial situation, and social life in the context of the caregiver–patient relationship. 45 Participants endorse responses ranging from “never” to “nearly always,” and item scores are added to obtain a total score, which ranges from 0 to 88, with a higher total score indicating higher levels of caregiver distress. The Spanish version of the ZBI utilized in this study has demonstrated good construct validity and internal reliability in previous research. 46
Satisfaction with Life Scale
The satisfaction with life scale (SWLS), created by Pavot and Diener, 47 is 5-item questionnaire used to assess overall life satisfaction. Participants rate each item on a Likert-type scale ranging from “strongly disagree” to “strongly agree” (1-7). In previous research employing the SWLS, good internal consistency has been demonstrated among individuals with neurological conditions. 48 The SWLS was translated into Spanish for this study and internal consistency for this sample indicated a Cronbach’s α of .75.
Perceived Stress Scale
The Perceived Stress Scale (PSS) is 14-item scale that measures the degree to which situations in one’s life are perceived as stressful. 49 Items were designed to assess how unpredictable, uncontrollable, and overloaded respondents find their lives over the past month. The scale also includes a number of direct queries about current levels of experienced stress. Moreover, the questions are of a general nature and hence are relatively free of content specific to any subpopulation. The PSS has demonstrated good reliability, with αs ranging from .84 to .86. 49
Procedure
Participants were recruited via written invitations (eg, postcards and letters describing the study) and phone calls by the Alzheimer’s Foundation of Cali, Colombia. Caregivers who expressed initial interest in participation were invited to attend an informational group meeting where the practical components of the program (ie, duration, time commitment per week, etc) as well as potential benefits of participation were presented. Each interested caregiver was scheduled for a follow-up interview, during which informed consent was obtained and pretreatment measures (see subsequently) completed. Subsequently, the participants were assigned to either the experimental or the control treatment condition. After concluding the final session (week 8), and again 3 months later, the participants were administered postintervention and follow-up measures, respectively.
Intervention: Experimental Group
The experimental group participated in the “Coping with Frustration” class. This course is a cognitive–behavioral intervention program developed by Gallagher-Thompson 50 and is based on a cognitive–behavioral model for the management of frustration and anger. 51 –53 The goal of this 8-week intervention is to introduce family caregivers to a variety of cognitive–behavioral strategies that they can use to manage negative feelings (eg, anger and frustration) that arise within the context of caregiving. 50 These cognitive–behavioral strategies and skills include relaxation, identification and challenging of dysfunctional thoughts, the use of positive self-statements, and assertiveness, which are taught within a structured classroom format in small groups ranging in size from 6 to 10 participants. Each session is planned to last 2 hours and is designed to introduce a particular coping strategy, followed by practice of the newly learned skill. Although a similar intervention was originally developed for Anglo/caucasian caregivers, 54 the course and content workbooks were substantially revised and adapted for use with the Hispanic/Latino population in the United States via input from the community through the use of several focus groups with Latino caregivers. The original intervention was modified to include translation of materials by a panel of academics and community leaders, increased use of visual aids throughout the course, and oral presentation of the lessons with the written materials as backup. 50 Local outreach workers, interviewers, and class leaders were employed throughout the intervention.
The first 2 sessions present the treatment rationale and the cognitive–behavioral model. Participants are invited to discuss their sources of frustration and anger regarding their caregiving situation, typical ways that people respond to them, and the relationship between situations, behavior, and emotions. Further, participants are presented with relaxation skills that are useful in anger management, such as deep breathing, guided imagery, and a relaxation log.
Sessions 3 through 5 introduce cognitive techniques (eg, self-talk, identifying, and challenging dysfunctional thoughts) and guide each participant in developing self-statements that are effective for him or her in coping with frustration. The participants are also encouraged to create a log of dysfunctional thoughts (ie, a list of anger-provoking situations, and corresponding thoughts and feelings provoked by such events). The final 3 sessions teach the difference between assertive, passive, and aggressive behavior, teach specific assertiveness skills, continue relaxation exercises and monitoring of dysfunctional cognitions, and finally provide an overall review of the program and its termination. 50
Intervention: Control Group
The control group participated in an educational program of equal duration (8 weeks) and time commitment (2 hours/week) as the experimental group. This educational program was designed by the authors to include an attentional and educational component but not the experimental intervention’s practical application of cognitive–behavioral stress management skills. The educational program presented information related to the dementia, its history, course, and sequelae and included 2 sessions of viewing motion pictures addressing dementia and its effects. 71,72
Statistical Analysis
Analyses were conducted using SPSS 20.0 (IBM Corp., Armonk, NY). Relationships between demographic variables and experimental condition were explored using t-tests and chi-square (χ2) analysis, as appropriate. Multivariate analyses of variance were run to examine the possible differences in treatment conditions on dimensions of mental health at baseline, and a series of longitudinal multi-level analyses for mental health variables were performed to calculate the outcome trajectories by experimental group across the 3-month follow-up, controlling for any baseline differences. A significance level of 5% (α < .05) was used for the analyses.
Results
Chi-square and t-tests were performed using SPSS version 20 to examine the differences between participants in the control and the intervention groups for gender, age, and education. Across conditions, participants showed no differences in age, t(67) = 1.60, P = .12, gender, χ2(1, n = 69) = 2.13, P = .22, or education, χ2(13, n = 69) = 8.33, P = .82 so these variables were not included as covariates in the follow-up analyses.
A multivariate analysis of variance (MANOVA) examined differences in participants’ baseline scores on the mental health variables by condition. Participant group (control vs intervention) was the independent variable and participants’ total scores on each of the 4 mental health variables (satisfaction with life, depression, stress, and burden) were the dependent variables. The MANOVA revealed a statistically significant effect for participant group, Pillai’s trace = .116, F 4,131 = 4.30, P = .002, and η2 = .116, suggesting that participants in the control group generally had better mental health than the intervention group at baseline. As a result, participants’ baseline scores for a mental health variable were included as a covariate in the follow-up analyses for that variable.
A series of longitudinal multi-level model (MLM) analyses were then performed using the MIXED command in SPSS version 20 to calculate the outcome trajectories. These analyses examined differences in linear trajectories of mental health between participants in both the control and the intervention groups. In these analyses, the independent variables were participant group (control vs intervention), time, and the group × time interaction while controlling for baseline differences in the dependent variable. In each analysis, the dependent variable was one of the mental health variables at posttest and at the 3-month follow-up.
Mental Health
Four MLM analyses were conducted for the 4 mental health variables (satisfaction with life, depression, stress, and burden). None of the interaction terms in these analyses were statistically significant, indicating that there were no differential trajectories for control and intervention participants in their mental health scores over time. In the first MLM, a significant main effect of group emerged on satisfaction with life scores, b = 2.47, t(73.95) = 2.20, P = .03, but no effect of time, b = −0.23, t(69.50) = −0.18, P = .86, suggesting that the intervention group had higher satisfaction with life scores than the control group and that these gains remained constant through the 3-month follow-up. Figure 1 shows the mean satisfaction with life scores of each group at posttest and the 3-month follow-up adjusted for baseline satisfaction with life scores.
Figure 1.
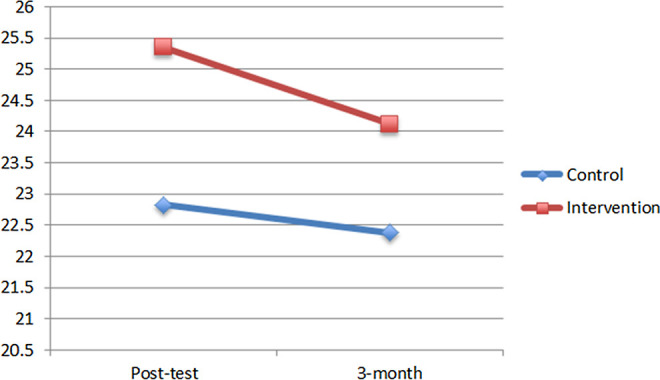
Adjusted satisfaction with life scores by group.
In the second MLM, a significant main effect of group emerged on depression scores, b = −1.82, t(71.97) = −2.41, P = .02, but no effect of time, b = 1.47, t(68.72) = 1.69, P = .10, suggesting that the intervention group had lower depression scores than the control group through the 3-month follow-up. Figure 2 shows the mean depression scores of each group through the 3-month follow-up adjusted for baseline depression.
Figure 2.
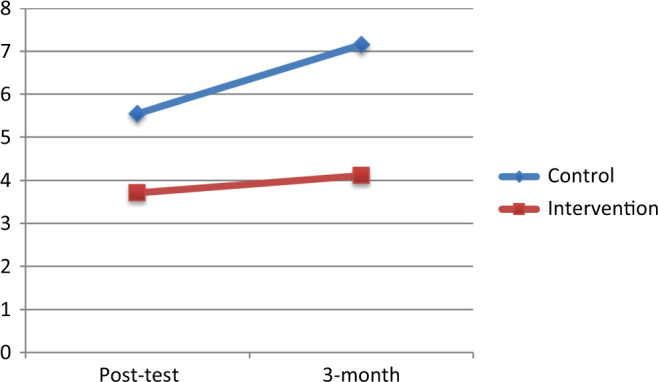
Adjusted depression scores by group.
In the third MLM, a significant main effect of group emerged for burden scores, b = −10.93, t(69.43) = −3.88, P < .001, but no effect of time, b = 2.81, t(64.09) = 0.87, P = .39, suggesting that the intervention group had lower burden than the control group through the 3-month follow-up. Figure 3 shows the mean burden scores of each group adjusted for baseline burden.
Figure 3.
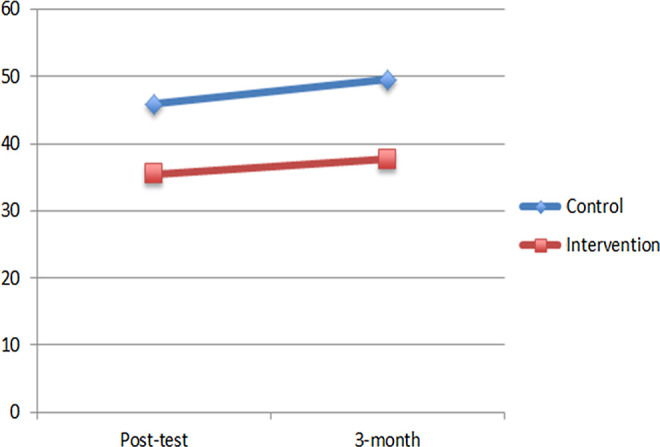
Adjusted burden scores by group.
In the fourth MLM, no significant main effect of group emerged for stress scores, b = −0.85, t(66.42) = −0.83, P = .41, nor an effect of time, b = 0.92, t(61.20) = 0.80, P = .43, suggesting that the intervention did not have an effect on participants’ stress levels.
Discussion
The purpose of this study was to examine the efficacy of a group cognitive–behavioral intervention in improving the mental health of dementia caregivers from Cali, Colombia. When controlling for baseline differences between the treatment and control groups, the treatment group showed higher satisfaction with life and lower depression and burden than the control group across the posttest and 3-month follow-ups, although there was no effect of the intervention on participants’ stress level. To the best of our knowledge, the current study is one of the first to examine the effect of a cognitive–behavioral intervention for family dementia caregivers in Spanish in Latin America.
Results were similar to those obtained in cognitive–behavioral interventions for dementia caregivers conducted in other countries, such as the United States, 55 Spain, 56 Switzerland, 57 and Sweden. 58 In addition, other research studies, which obtained similar results in improving mental health in family dementia caregivers, have been carried out using different intervention methods, such as home-based exercise counseling, 59 occupational therapy recommendations, 60 polarity therapy, 61 yoga and meditation, 62 skills training, 63 and multicomponent interventions. 30,64
When considering the lack of an effect for caregiver stress, it is important to note that only 6 caregiver intervention studies identified in the current review have included the PSS as an outcome. The results of these studies were similarly inconclusive: 3 studies reported reductions in caregivers’ stress on the PSS 59,61,63 while the other 2 failed to obtain significant results. 65,66 This could be due to a number of differences among the interventions and methodologies used. Castro and colleagues 59 conducted an exercise-based intervention while Korn et al 61 used an alternative medicine-based polarity therapy (an experiential touch-based therapeutic technique, which optimizes the reduction in sympathetic activity). In contrast, Bourgeois and colleagues 63 employed a skills training protocol in their dementia caregiver intervention. Positive effects of movement and exercise on perceived stress are known 67,68 which may account for significant reductions in caregiver stress observed in the first 2 studies. The current intervention, and others not explicitly focusing on the reduction in stress, may be comparably more effective for improving caregiver satisfaction with life, depression, and burden.
Perhaps the most similar intervention to that in the current study was piloted in Portuguese with a group of dementia caregivers in Brazil. 69 In that study, caregivers participated in an 8-week social skills group intervention involving cognitive–behavioral techniques at the end of which caregivers reported fewer patient neuropsychiatric symptoms and an improvement in patients' quality of life as well as improved caregiver coping strategies and reduced anxiety. As the findings from that study suggest, as well as those from the current study, cognitive–behavioral dementia caregiver interventions in Latin America may be particularly effective.
Clinical Implications
Results of this study have several implications for clinicians and rehabilitation professionals. First, family caregivers play an integral role in the care of individuals with dementia and the current findings suggest that cognitive–behavioral interventions designed to improve caregiver mental health may make it possible to prevent or reduce the negative consequences of long-term caregiving in Latin America. Helping caregivers in this region to identify the sources of negative emotions in their immediate environment and deal with them in a constructive way may enable them to continue to provide care for their family members with dementia for longer than might otherwise have been possible. These interventions may also help reduce the costs of their own health care, potential burden on the public health system, and the likelihood of institutionalization of the family member with dementia. Other potential benefits that warrant investigation are the effects of these interventions on family functioning and the provision of high-quality informal care.
Limitations and Future Directions
Although the current results suggest that cognitive–behavioral interventions for dementia caregivers in Latin America may be effective means of improving mental health, findings should be interpreted in light of several limitations. First, this study operationalized “mental health” using measures of satisfaction with life, depression, burden, and stress. It could be that other aspects of mental health not measured in the present study (eg, anxiety, anger, hostility, and suicidality) were also affected by the intervention. Similarly, results of this study cannot be generalized to other aspects of caregiver functioning (eg, spirituality, fatigue, social interaction, physical functioning, family functioning, communication, and quality of care provision) so future research using these variables as outcomes should be conducted. Second, dementia caregiving is a long-term endeavor during which caregiver and patient circumstances (eg, financial resources, physical health) can change considerably; thus, the current results should not be generalized beyond 3 months postintervention and longer follow-ups in future studies should be used. Third, data on patient characteristics were lacking in the present study, including stage of disease, level of cognitive impairment, functional independence, presence of neuropsychiatric symptoms, type of dementia, and time since diagnosis, all of which are likely to influence caregiver burden and stress and perhaps as a result could influence the benefits of this intervention. Future research would greatly benefit from including these variables as potential moderators of the intervention’s effects. Similarly, because all outcome variables in the current study were caregiver report, interference from caregivers’ emotional distress could have affected the accuracy of information they provided and clinician ratings could be more accurate in future research, as other researchers have argued. 70
Fourth, the current study included a relatively small sample of family caregivers and baseline differences emerged in mental health that were controlled for in the statistical analysis. Although caregivers were similar in terms of sociodemographic characteristics such as age, education, gender, income, and care provision variables (eg, time providing care and hours of care provided per week), it is not known whether the control group commanded any additional resources in providing care to their loved one with dementia, such as in-home (paid) caregivers, outside mental health support, or other respite services that may impact the findings. However, this concern can be somewhat tempered because these baseline differences were controlled for in all analyses and therefore could not have contributed to the significant treatment effects found in the current study. And finally, participants were part of an Alzheimer’s Foundation in Colombia, an organization that provides a variety of services to dementia caregivers. It is possible that the results of this intervention would not have the same effect in dementia caregivers from more remote or rural areas of Latin America, where there are fewer services and resources.
Conclusions
Despite these limitations, this study was the first to find support for the effectiveness of a cognitive–behavioral intervention for dementia caregivers in Spanish from Latin America. Compared to controls, caregivers in the treatment group showed improved satisfaction with life, burden, and depression, and these effects persisted over the 3-month follow-up. In showing that a brief, group cognitive–behavioral intervention is feasible, acceptable, and effective to family dementia caregivers in Latin America, this study adds to the growing body of scientific knowledge about the efficacy of this type of intervention among culturally and socioeconomically diverse populations. Given that the burden of family caregivers may be greatest among those with the fewest resources, future culturally appropriate interventions and services for dementia caregivers in diverse global regions should be developed and implemented.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Thies W, Bleiler L, Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. [DOI] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2. [DOI] [PubMed] [Google Scholar]
- 3. Prince M, Acosta D. Ageing and dementia in developing countries-the work of the 10/66 dementia research group. Int Psychiatry. 2006;3(4):3–6. [PMC free article] [PubMed] [Google Scholar]
- 4. Takeuchi Tan Y, Guevara JG. Prevalencia de las enfermedades neurológicas en el valle del Cauca. Estudio neuroepidemiológico nacional (EPINEURO). Colombia Médica. 1999;30(2):74–81. [Google Scholar]
- 5. Das SK, Pal S, Ghosal MK. Dementia: Indian scenario. Neurol India. 2012;60(6):618. [DOI] [PubMed] [Google Scholar]
- 6. Wimo A, Jönsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 7. Trojanowski JQ, Arnold SE, Karlawish JH, Naylor M, Brunden KR, Lee VM. A model for improving the treatment and care of Alzheimer's disease patients through interdisciplinary research. Alzheimers Dement. 2012;8(6):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11(2):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golodetz A, Evans R, Heinritz G, Gibson CD, Jr. The care of chronic illness: the “responsor” role. Med Care. 1969;7(5):385–394. [DOI] [PubMed] [Google Scholar]
- 10. Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53(4):345–362. [DOI] [PubMed] [Google Scholar]
- 11. Kiecolt Glaser JK, Marucha PT, Mercado A, Malarkey W, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–1196. [DOI] [PubMed] [Google Scholar]
- 12. Shaw WS, Patterson TL, Ziegler MG, Dimsdale JE, Semple SJ, Grant I. Accelerated risk of hypertensive blood pressure recordings among Alzheimer caregivers. J Psychosom Res. 1999;46(3):215–227. [DOI] [PubMed] [Google Scholar]
- 13. Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in US women: a prospective study. Am J Prev Med. 2003;24(2):113–119. [DOI] [PubMed] [Google Scholar]
- 14. Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64(3):418–435. [DOI] [PubMed] [Google Scholar]
- 15. Mausbach BT, Patterson TL, Rabinowitz YG, Grant I, Schulz R. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychol. 2007;26(5):539–544. [DOI] [PubMed] [Google Scholar]
- 16. Fetveit A, Bjorvatn B. Sleep duration during the 24-hour day is associated with the severity of dementia in nursing home patients. Int J Geriatr Psychiatry. 2006;21(10):945–950. [DOI] [PubMed] [Google Scholar]
- 17. George LK, Gwyther LP. Caregiver well-being: a multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26(3):253–259. [DOI] [PubMed] [Google Scholar]
- 18. Mahoney R, Regan C, Katona C, Livingston G. Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. Am J Geriatr Psychiatry. 2005;13(9):795–801. [DOI] [PubMed] [Google Scholar]
- 19. Black W, Almeida OP. A systematic review of the association between the behavioral and psychological symptoms of dementia and burden of care. Int Psychogeriatr. 2004;16(3):295–315. [DOI] [PubMed] [Google Scholar]
- 20. Papastavrou E, Kalokerinou A, Papacostas SS, Tsangari H, Sourtzi P. Caring for a relative with dementia: family caregiver burden. J Adv Nurs. 2007;58(5):446–457. [DOI] [PubMed] [Google Scholar]
- 21. Stoltz P, Udén G, Willman A. Support for family carers who care for an elderly person at home—a systematic literature review. Scand J Caring Sci. 2004;18(2):111–119. [DOI] [PubMed] [Google Scholar]
- 22. Morris LW, Morris RG, Britton PG. The relationship between marital intimacy, perceived strain and depression in spouse caregivers of dementia sufferers. Br J Med Psychol. 1988;61(3):231–236. [DOI] [PubMed] [Google Scholar]
- 23. Sainsbury P, de Alarcon JG. The psychiatrist and the geriatric patient. The effects of community care on the family of the geriatric patient. J Geriatr Psychiatry. 1970;4(1):23–52. [PubMed] [Google Scholar]
- 24. Rose Rego SK, Strauss ME, Smyth KA. Differences in the perceived well-being of wives and husbands caring for persons with Alzheimer's disease. Gerontologist. 1998;38(2):224–230. [DOI] [PubMed] [Google Scholar]
- 25. Bell CM, Araki SS, Neumann PJ. The association between caregiver burden and caregiver health-related quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(3):129–136. [DOI] [PubMed] [Google Scholar]
- 26. Clyburn LD, Stones MJ, Hadjistavropoulos T, Tuokko H. Predicting caregiver burden and depression in Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 2000;55(1):S2–S13. [DOI] [PubMed] [Google Scholar]
- 27. Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. Gerontologist. 1995;35(6):771–791. [DOI] [PubMed] [Google Scholar]
- 28. Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51(5):657–664. [DOI] [PubMed] [Google Scholar]
- 29. Schulz R, O'Brien A, Czaja S, et al. Dementia caregiver intervention research in search of clinical significance. Gerontologist. 2002;42(5):589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belle SH, Burgio L, Burns R, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial Groups A randomized, controlled trial. Ann Intern Med. 2006;145(10):727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. [DOI] [PubMed] [Google Scholar]
- 32. Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577–596. [DOI] [PubMed] [Google Scholar]
- 33. Llanque SM, Enriquez M. Interventions for Hispanic caregivers of patients with dementia a review of the literature. Am J Alzheimers Dis Other Demen. 2012;27(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lehan T, Arango-Lasprilla JC, Macias MÁ, Aguayo A, Villaseñor T. Distress associated with patients' symptoms and depression in a sample of Mexican caregivers of individuals with MS. Rehabil Psychol. 2012;57(4):301–307. [DOI] [PubMed] [Google Scholar]
- 35. Arango Lasprilla J, Moreno A, Rogers H, Francis K. The effect of dementia patients’ physical, cognitive, and emotional/ behavioral problems on caregiver well-being: Findings from a Spanish-speaking simple from Colombia, South America. Am J Alzheimers Dis Other Dement. 2009;24(5):384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuentes MG, Baker JG, Markello SJ, Wood KD. Discharge to home among Hispanic and non-Hispanic stroke survivors: does family make a difference? Int J Rehabil Res. 1999;22(4):317–320. [DOI] [PubMed] [Google Scholar]
- 37. Arciniega GM, Anderson TC, Tovar-Blank ZG, Tracey TJ. Toward a fuller conception of machismo: development of a traditional machismo and Caballerismo scale. J Counsel Psychol. 2008;55(1):19. [Google Scholar]
- 38. Cox C, Monk A. Strain among caregivers: comparing the experiences of African American and Hispanic caregivers of Alzheimer's relatives. Int J Aging Hum Dev. 1996;43(2):93–105. [DOI] [PubMed] [Google Scholar]
- 39. Harwood DG, Barker WW, Cantillon M, Loewenstein DA, Ownby R, Duara R. Depressive symptomatology in first-degree family caregivers of Alzheimer disease patients: a cross-ethnic comparison. Alzheimer Dis Assoc Disord. 1998;12(4):340–346. [DOI] [PubMed] [Google Scholar]
- 40. Pinquart M, Sörensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: a meta-analysis. Gerontologist. 2005;45(1):90–106. [DOI] [PubMed] [Google Scholar]
- 41. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wulsin L, Somoza E, Heck J. The feasibility of using the Spanish PHQ-9 to screen for depression in primary care in Honduras. Prim Care Companion J Clin Psychiatry. 2002;4(5):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679–686. [DOI] [PubMed] [Google Scholar]
- 44. Donlan W, Lee J. Screening for depression among indigenous Mexican migrant farmworkers using the patient health questionnaire-9. Psychol Rep. 2010;106(2):419–432. [DOI] [PubMed] [Google Scholar]
- 45. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. [DOI] [PubMed] [Google Scholar]
- 46. Marín M. Adaptación para nuestro medio de la escala de sobrecarga del cuidador de zarit. Revista Multidisciplinar de Gerontología. 1996;6(4):338. [Google Scholar]
- 47. Pavot W, Diener E. Review of the satisfaction with life scale. Psychol Assess. 1993;5(2):164. [Google Scholar]
- 48. Corrigan JD, Bogner JA, Mysiw WJ, Clinchot D, Fugate L. Life satisfaction after traumatic brain injury. J Head Trauma Rehabil. 2001;16(6):543–555. [DOI] [PubMed] [Google Scholar]
- 49. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 50. Gallagher-Thompson D. Controlling Your Frustration: A Class for Caregivers. Palo Alto, CA: Department of Veterans Affairs Medical Center; 1992. [Google Scholar]
- 51. Novaco RW. Anger Control: The Development and Evaluation of an Experimental Treatment. Lexington, KY: Lexington Books; 1975. [Google Scholar]
- 52. Novaco RW. Anger and its therapeutic regulation. In: Chesney MA, Rosenman RH, eds. Anger and Hostility in Cardiovascular and Behavioral Disorders. Washington, DC: Hemisphere; 1985:203–226. [Google Scholar]
- 53. Feindler EL, Ecton RB. Adolescent Anger Control: Cognitive–Behavioral Techniques. New York: Pergamon Press; 1986. [Google Scholar]
- 54. Gallagher-Thompson D, DeVries HM. “Coping with frustration” classes: development and preliminary outcomes with women who care for relatives with dementia. Gerontologist. 1994;34(4):548–552. [DOI] [PubMed] [Google Scholar]
- 55. Gallagher-Thompson D, Arean P, Rivera P, Thompson LW. A psychoeducational intervention to reduce distress in Hispanic family caregivers: results of a pilot study. Clin Gerontol. 2001;23(1-2):17–32. [Google Scholar]
- 56. Baltar AL, Rojo GP. Estudio e intervención sobre el malestar psicológico de los cuidadores de personas con demencia: El papel de los pensamientos disfuncionales. Madrid, Spain: Instituto de Mayores y Servicios Sociales (IMSERSO); 2006. [Google Scholar]
- 57. Perren S, Schmid R, Wettstein A. Caregivers’ adaptation to change: the impact of increasing impairment of persons suffering from dementia on their caregivers’ subjective well-being. Aging Ment Health. 2006;10(5):539–548. [DOI] [PubMed] [Google Scholar]
- 58. Signe A, Elmståhl S. Psychosocial intervention for family caregivers of people with dementia reduces caregiver's burden: development and effect after 6 and 12 months. Scand J Caring Sci. 2008;22(1):98–109. [DOI] [PubMed] [Google Scholar]
- 59. Castro CM, Wilcox S, O’Sullivan P, Baumann K, King AC. An exercise program for women who are caring for relatives with dementia. Psychosom Med. 2002;64(3):458–468. [DOI] [PubMed] [Google Scholar]
- 60. Dooley NR, Hinojosa J. Improving quality of life for persons with Alzheimer’s disease and their family caregivers: brief occupational therapy intervention. Am J Occup Ther. 2004;58(5):561–569. [DOI] [PubMed] [Google Scholar]
- 61. Korn L, Logsdon RG, Polissar NL, Gomez-Beloz A, Waters T, Rÿser R. A randomized trial of a CAM therapy for stress reduction in American Indian and Alaskan native family caregivers. Gerontologist. 2009;49(3):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677–687. [DOI] [PubMed] [Google Scholar]
- 63. Bourgeois MS, Schulz R, Burgio LD, Beach S. Skills training for spouses of patients with Alzheimer's disease: outcomes of an intervention study. J Clin Geropsychol. 2002;8(1):53–73. [Google Scholar]
- 64. Newcomer R, Yordi C, DuNah R, Fox P, Wilkinson A. Effects of the medicare Alzheimer's disease demonstration on caregiver burden and depression. Health Serv Res. 1999;34(3):669. [PMC free article] [PubMed] [Google Scholar]
- 65. Gallagher-Thompson D, Gray HL, Tang PC, et al. Impact of in-home behavioral management versus telephone support to reduce depressive symptoms and perceived stress in Chinese caregivers: results of a pilot study. Am J Geriatr Psychiatry. 2007;15(5):425–434. [DOI] [PubMed] [Google Scholar]
- 66. Javadpour A, Ahmadzadeh L, Bahredar MJ. An educative support group for female family caregivers: Impact on caregivers psychological distress and patient's neuropsychiatry symptoms. Int J Geriatr Psychiatry. 2009;24(5):469–471. [DOI] [PubMed] [Google Scholar]
- 67. Starkweather AR. The effects of exercise on perceived stress and IL-6 levels among older adults. Biol Res Nurs. 2007;8(3):186–194. [DOI] [PubMed] [Google Scholar]
- 68. Rueggeberg R, Wrosch C, Miller GE. The different roles of perceived stress in the association between older adults' physical activity and physical health. Health Psychol. 2012;31(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fialho PPA, Köenig AM, Santos MDL, Barbosa MT, Caramelli P. Positive effects of a cognitive–behavioral intervention program for family caregivers of demented elderly. Arquivos de Neuro-Psiquiatria. 2012;70(10):786–792. [DOI] [PubMed] [Google Scholar]
- 70. de Medeiros K, Robert P, Gauthier S, et al. The neuropsychiatric inventory-clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cassavetesr N. The Notebook [DVD]. United States: New Line Cinema; 2004. [Google Scholar]
- 72. Jenkins T. The Savages. [DVD]. United States: 20th Century Fox Home Entertainment; 2007. [Google Scholar]


