Abstract
Introduction:
Given the high prevalence of dementia and its devastating consequences, identifying risk factors for dementia is a public health priority. The present study aims to assess whether gastritis increases the odds of dementia.
Methodology:
The data for this study, consisting of 2926 community-dwelling older adults, were obtained from the National survey entitled “Mental Health and Quality of Life of Older Malaysians.” Dementia was diagnosed using the Geriatric Mental State-Automated Geriatric Examination for Computer-Assisted Taxonomy.
Results:
Prevalence of dementia was considerably higher among older adults with gastritis (29.5%) compared to those without gastritis (13.2%). After adjusting for age, gender, marital status, educational attainment, hypertension, stroke, and diabetes, gastritis was significantly associated with more than twice odds of dementia (adjusted odds ratio = 2.42, P < .001, 95% confidence interval = 1.68-3.49).
Conclusions:
The findings from this population-based observational study showing evidence that gastritis may increase the risk of dementia provide avenue for further inquiries into dementia.
Keywords: aged, dementia, gastritis, risk factor
Introduction
As the population is rapidly aging, the numbers of people living with dementia worldwide is increasing. It is estimated by 2050, 71% of 115 million people with dementia will live in the developing countries. 1,2 Given the high prevalence of dementia and its devastating consequences, identifying the factors and understanding the pathways that lead to dementia has been considered as a public health priority. In light of this consideration, numerous studies have been conducted and found that age, 3 ethnicity, 4 gender, 5 genetic factors, 6 physical activity, 7 smoking, 8 drug use, 9 level of education, 10 alcohol consumption, 11 body mass index, 12 hypertension, 13 diabetes, 14 stroke, 15 depression, 16,17 and loneliness 18 are associated with dementia. Although previous studies have found that sociodemographic factors, lifestyle, several chronic medical conditions, and age-related changes are associated with dementia, gastritis as a possible risk factor has not been studied. There is a growing body of evidence to suggest that deficiency of vitamin B12 may contribute to cognitive impairment and dementia. 19 –21 The additional evidence also shows that gastritis leads to vitamin B12 deficiency. 22 However, the association between gastritis and dementia has not been studied among community-dwelling older adults. The present study aims to examine the association between gastritis and dementia in older adults, after controlling for potential confounders.
Methodology
Data for this study, consisting of 2926 community-dwelling older adults, were obtained from a nationally representative survey entitled “Mental Health and Quality of Life of Older Malaysians (MHQoLOM).” The full details of research methodology have been previously reported elsewhere. 3,4 Briefly, the MHQoLOM was conducted in 2003 to 2005 in all 13 Malaysian states and the Federal Territory of Kuala Lumpur. The survey investigated the prevalence and determinants of mental health and quality of life among older Malaysians. The authors selected June 1, 2006, as prevalence day. The MHQoLOM used the sampling frame of the National Household Sampling Frame, in which the country is divided into contiguous quotas called Enumeration Blocks (EBs). The required sample size for the MHQoLOM was calculated as 2935, considering confidence level = 97%, α = .05, design effect = 2, prevalence of 50%, an expected response rate of 80%, and adding a 10% for incomplete answers. A 2-stage proportional stratified random sampling technique was used to obtain the required sample. At the first stage, the EBs were selected, and at the second stage, the Living Quarters (LQ) were selected. Finally, 1 older person from each LQ was interviewed. In total, 2980 Malaysians aged 60 years and older were interviewed in their home by trained interviewers. Among this sample of community-dwelling older adults, 2926 respondents had full information for current analysis.
Assessment of Gastritis and Chronic Medical Conditions
Respondents were asked to self-report whether they have ever been told by the doctor that they have, or have had, any of these chronic medical conditions, gastritis, hypertension, heart disease, and arthritis? If the respondents indicated one of these conditions, they were then asked whether they have been treated by a physician; only cases reported having been treated by a physician were considered positive.
Dementia
Dementia was diagnosed using the Geriatric Mental State-Automated Geriatric Examination for Computer-Assisted Taxonomy (GMS-AGECAT), which has been widely used and applied with good levels of agreement in a variety of settings. 23,24 The score of 3 or higher on the GMS-AGECAT is considered as dementia. 25 The GMS-AGECAT has been compared with psychiatrists’ diagnoses and Diagnostic and Statistical Manual of Mental Disorders (Third Edition) showed good overall agreement. 26 In this study, the GMS-AGECAT was administered by trained enumerators. Training of enumerators was conducted by an experienced investigator from India who was a member of the 10/66 research group.
Control Variables
To control for the possibility that the effect of gastritis on dementia is due to some factors that may cause both gastritis and dementia, a variety of sociodemographic and health factors were measured. The controlling variables included age, gender, marital status, educational attainment, hypertension, stroke, and diabetes.
Statistical Analysis
Data were analyzed using SPSS version 21 for Windows (IBM, Chicago, Illinois). Univariate analysis was used to describe the frequencies and proportions of the variables. A multiple binary logistic regression was performed to determine the unique role of gastritis on dementia while controlling for potential confounders. A 2-tailed P value of ≤.05 was used to determine statistical significant results. The Hosmer and Lemeshow goodness-of-fit test was used to verify the model’s fit.
Ethics and Approvals
The study was approved by the Ministry of Health, Malaysia, and it was in compliance with the Helsinki Declaration, World Medical Association.
Results
The sample consisted of 2926 community-dwelling Malaysians aged 60 years and older, including 1469 women and 1457 men, with an average age of 70.47(standard deviation = 7.21) years. Table 1 presents the distributions of some of the sociodemographic characteristics and physical health status of the respondents. The prevalence of dementia was 14.3% in the whole study population; 8.8% for men; and 19.7% for women.
Table 1.
Descriptive Statistics of Study Variables.
| Variable | Category | n | % |
|---|---|---|---|
| Age | Young-old (60-74) | 2125 | 72.6 |
| Old-old (75-84) | 665 | 22.7 | |
| Oldest-old (85+) | 136 | 4.6 | |
| Gender | Male | 1457 | 49.8 |
| Female | 1469 | 50.2 | |
| Marital status | Married | 1633 | 55.8 |
| Unmarried | 1293 | 44.2 | |
| Education | No formal education | 1309 | 45.4 |
| Primary | 1292 | 44.8 | |
| Secondary and tertiary | 281 | 9.8 | |
| Hypertension | Yes | 892 | 30.5 |
| No | 2034 | 69.5 | |
| Diabetes | Yes | 414 | 14.1 |
| No | 2512 | 85.9 | |
| Stroke | Yes | 49 | 1.7 |
| No | 2877 | 98.3 | |
| Gastritis | Yes | 190 | 6.5 |
| No | 2736 | 93.5 |
The crude prevalence rate was 12 333 per 100 000. Table 2 shows age- and sex-specific prevalence of dementia per 100 000 in Malaysia as of June 1, 2006. As it can be seen from Table 2, women are more likely than men to have dementia at the same age groups.
Table 2.
Age- and Sex-Specific Prevalence of Dementia Per 100 000 in Malaysia.
| Age Group | Population | Estimated Cases | Prevalence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| 60-64 | 637 300 | 326 000 | 963 300 | 40 174 | 30 497 | 70 671 | 6304 | 9355 | 7336 |
| 65-69 | 472 900 | 230 400 | 703 300 | 30 043 | 35 487 | 65 530 | 6353 | 15 402 | 9318 |
| 70-74 | 317 300 | 146 600 | 463 900 | 33 686 | 26 313 | 59 999 | 10 616 | 17 949 | 12 934 |
| 75+ | 359 300 | 157 900 | 517 200 | 44 335 | 52 763 | 97 098 | 12 339 | 33 415 | 18 774 |
| Total | 1 786 800 | 860 900 | 264 7700 | 157 189 | 16 9357 | 32 6546 | 8797 | 19 672 | 12 333 |
The prevalence rate of dementia among older adults with and without gastritis was 29.5% and 13.2%, respectively. Of the respondents, 6.5% said that they have been told by a doctor that they have been receiving treatment for gastritis. The results from chi-square (χ2) test revealed that prevalence of dementia was significantly higher among elderly people with gastritis (29.5%) compared to those without gastritis (13.2%; χ2 (1, n = 2926) = 38.28, P < .001, odds ratio [OR] = 2.74, 95% confidence interval [CI] = 1.97-3.82).
Results of Multiple Binary Logistic Regression
The likelihood ratio test (χ2 (8) =296.68, P < .001) indicated that the logistic model was more effective than an intercept-only model. Table 3 summarizes the results of logistic regression. The Hosmer–Lemeshow test showed that the model fits the data well (χ2(8) = 8.18, P = .416). The findings of logistic regression revealed that gastritis was significantly associated with more than twice the odds of dementia (adjusted OR = 2.42, P < .001, 95% CI = 1.68-3.49) while controlling for potential confounders including age, gender, educational attainment, marital status, hypertension, stroke, and diabetes.
Table 3.
Summary of Multiple Binary Logistic Regression Analysis.
| Variable | B | SE | OR | 95% CI for OR | |
|---|---|---|---|---|---|
| Age (ref: young-old, 60-74) | |||||
| Old-old (75-84) | 0.61 | 0.13 | 1.84a | 1.42 | 2.36 |
| Oldest-old (85+) | 1.03 | 0.22 | 2.80a | 1.84 | 4.27 |
| Gender | −0.44 | 0.14 | 0.65b | 0.49 | 0.85 |
| Marriage | −0.12 | 0.13 | 0.88 | 0.69 | 1.14 |
| Education (ref: no formal education) | |||||
| Primary | −1.24 | 0.14 | 0.29a | 0.22 | 0.38 |
| Secondary and tertiary | −2.15 | 0.39 | 0.12a | 0.05 | 0.25 |
| Hypertension | −0.17 | 0.13 | 0.85 | 0.66 | 1.09 |
| Diabetes | −0.09 | 0.17 | 0.92 | 0.65 | 1.29 |
| Stroke | 0.72 | 0.35 | 2.05c | 1.02 | 4.10 |
| Gastritis | 0.89 | 0.19 | 2.42a | 1.68 | 3.49 |
Abbreviations: CI, confidence interval; OR, odds ratio; SE, standard error.
a P < .001.
b P < .01.
c P ≤ .05.
Discussion
The prevalence of dementia in this community-dwelling elderly population of Malaysia was 14.3. Overall prevalence of self-reported gastritis was 6.5%, which is similar to the German study of 6% in a sample of 9444 women and men aged 50 to 74 years. 27 Our findings, in accordance with previous studies concerning gender differences in dementia, 28 –31 indicated that women were significantly at higher risk of dementia than men, where older men appeared to be as much as 35% less likely to have dementia compared to women, after adjusting for age, marital status, educational attainment, and chronic medical conditions. The higher risk of dementia for women can be explained by statement that risk factors for dementia are more common in women.
The main purpose of the present study was to examine whether gastritis is associated with increased dementia after adjusting for potential confounders. The findings from adjusted multiple binary logistic regression revealed that gastritis was significantly associated with increased odds of dementia. Figure 1 shows underlying mechanism by which gastritis may increase risk of dementia. The gastritis may lead to vitamin B12 deficiency 32 –34 through 2 ways. First, gastritis results in a low acid–pepsin secretion by the gastric mucosa, which in turn results in a reduced release of free vitamin B12 from food proteins. 34,35 Second, prolonged therapy can be responsible for a vitamin B12 deficiency due to protein-bound dietary vitamin B12 malabsorption. 36,37 As it can be seen from Figure 1, there are several possible pathways that could explain how vitamin B12 deficiency might be related to dementia. One of the possible mechanisms by which vitamin B12 deficiency could lead to dementia is homocysteine. 38 It seems that low level of vitamin B12 results in the accumulation of homocysteine. 39 Finally, the elevated plasma homocysteine level may be associated with dementia through several biologically plausible pathways. 40 –43 Another possible mechanism is that vitamin B12 deficiency leads to deficiency of S-adenosylmethionine (SAM) 44 thereby may cause impaired methylation reactions in the central nervous system. 45 The SAM levels have been reported to be decreased in the brains 46 and cerebrospinal fluid of Alzheimer’s disease. 47 It has also found that vitamin B12 deficiency is associated with white matter damage and thereby to cerebral disconnection and may lead to cognitive impairment and dementia. 48,49 The last possible mechanism that may link vitamin B12 deficiency to dementia is atrophy of the brain. The finding from a study among community-dwelling elderly patients showed that low-normal vitamin B12 status predicts whole-brain atrophy in community-dwelling elderly patient. 50 In addition, it is well documented that loss of brain tissue causes cognitive decline and dementia. 51
Figure 1.
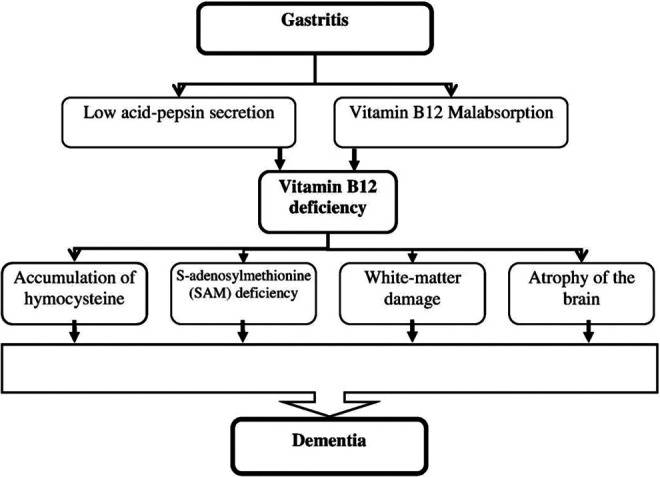
Underlying mechanism by which gastritis may increase risk of dementia.
Although the present study used a large sample size and revealed significant results, it has a few limitations that should be addressed. The most important limitation of the present study is the cross-sectional design, which might limit its ability to capture the causal relationship between gastritis and dementia. Therefore, prospective follow-up studies are needed to further evaluate this issue. Several studies have shown acceptable agreement between self-report and medical records of chronic medical conditions among older adults. 52 –55 Next limitation that should be addressed is the use of self-report technique to assess chronic medical conditions. Another limitation is the fact that the number of individuals who were asymptomatic and taking antacids/proton pump inhibitors. The last limitation that should be mentioned is the fact that the vitamin B12 as well as serum folate and plasma homocysteine levels were not measured in this study.
Conclusions
Despite the above-mentioned limitations, the findings from this population-based observational study, showing evidence that gastritis may be associated with an increased risk of dementia, provide avenue for further inquiries into dementia. In addition, the proposed theoretical model linking gastritis to dementia should be explored through experimental and longitudinal studies.
Footnotes
Authors’ Note: YAM did literature review, data analysis, and writing the article. TAH designed and managed the project. RI shared in writing the first and final version of the article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The authors would like to acknowledge the Ministry of Science, Technology and Innovation for supporting the project (IRPA 06-02-04-0461PR0031).
References
- 1. Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2010;9(1):1–11.e13. [DOI] [PubMed] [Google Scholar]
- 2. Lakey L, Chandaria K, Quince C, Kane M, Saunders T. Dementia 2012: A National Challenge. London: Alzheimer’s Society; 2012. [Google Scholar]
- 3. Hamid TA, Krishnaswamy S, Abdullah SS, Momtaz YA. Sociodemographic risk factors and correlates of dementia in older Malaysians. Dement Geriatr Cogn Disord. 2010;30(6):533–539. [DOI] [PubMed] [Google Scholar]
- 4. Momtaz YA, Hamid TA, Yusoff S, Ibrahim R. Do depression and educational attainment mediate the association between ethnicity and dementia? Gerontology. 2013;59(3):206–212. [DOI] [PubMed] [Google Scholar]
- 5. Zhang M, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–437. [DOI] [PubMed] [Google Scholar]
- 6. Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soci. 2003;51(2):169–177. [DOI] [PubMed] [Google Scholar]
- 7. de Bruijn RF, Schrijvers EM, de Groot KA, et al. The association between physical activity and dementia in an elderly population: the Rotterdam study. Eur J Epidemiol. 2013;28(5):447–448. [DOI] [PubMed] [Google Scholar]
- 8. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Craen AJ, Gussekloo J, Vrijsen B, Westendorp RG. Meta-analysis of nonsteroidal antiinflammatory drug use and risk of dementia. Am J Epidemiol. 2005;161(2):114–120. [DOI] [PubMed] [Google Scholar]
- 10. Basu R. Education and dementia risk: results from the aging demographics and memory study. Res Aging. 2013;35(1):7–31. [Google Scholar]
- 11. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam study. Lancet. 2002;359(9303):281–286. [DOI] [PubMed] [Google Scholar]
- 12. Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–119. [DOI] [PubMed] [Google Scholar]
- 13. Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2009;23(2):116–124. [DOI] [PubMed] [Google Scholar]
- 14. Zilkens RR, Davis WA, Spilsbury K, Semmens JB, Bruce DG. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am J Epidemiol. 2013;177(11):1246–1254. [DOI] [PubMed] [Google Scholar]
- 15. Pinkston JB, Alekseeva N, Gonzalez TE. Stroke and dementia. Neurol Res. 2009;31(8):824–831. [DOI] [PubMed] [Google Scholar]
- 16. Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke. Stroke. 2004;35(6):1264–1268. [DOI] [PubMed] [Google Scholar]
- 18. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234–240. [DOI] [PubMed] [Google Scholar]
- 19. Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86(5):1384–1391. [DOI] [PubMed] [Google Scholar]
- 20. Agarwal R. Vitamin B12 deficiency & cognitive impairment in elderly population. Indian J Med Res. 2011;134(4):410–412. [PMC free article] [PubMed] [Google Scholar]
- 21. Reay JL, Smith MA, Riby LM. B vitamins and cognitive performance in older adults: review. ISRN Nutr. 2013;2013:7. doi:10.5402/2013/650983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sipponen P, Laxen F, Huotari K, Harkonen M. Prevalence of low vitamin B12 and high homocysteine in serum in an elderly male population: association with atrophic gastritis and helicobacter pylori infection. Scand J Gastroenterol. 2003;38(12):1209–1216. [DOI] [PubMed] [Google Scholar]
- 23. Copeland JR, Prince M, Wilson KCM, Dewey ME, Payne J, Gurland B. The geriatric mental state examination in the 21st century. Int J Geriatr Psychiatry. 2002;17(8):729–732. [DOI] [PubMed] [Google Scholar]
- 24. Copeland JR, McCracken CF, Dewey ME, et al. Undifferentiated dementia, Alzheimer’s disease and vascular dementia: age-and gender-related incidence in Liverpool. The MRC-ALPHA Study. Br J Psychiatry. 1999;175(5):433–438. [DOI] [PubMed] [Google Scholar]
- 25. Holwerda TJ, Deeg DJ, Beekman AT, et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam study of the elderly (AMSTEL) [published online December 10, 2012.]. J Neurol Neurosurg Psychiatry. 2012. doi:10.1136/jnnp-2012-302755. [DOI] [PubMed] [Google Scholar]
- 26. Copeland JR, Dewey ME, Saunders P. The epidemiology of dementia: GMS-AGECAT studies of prevalence and incidence, including studies in progress. Eur Arch Psychiatry Clin Neurosci. 1991;240(4):212–217. [DOI] [PubMed] [Google Scholar]
- 27. Weck MN, Stegmaier C, Rothenbacher D, Brenner H. Epidemiology of chronic atrophic gastritis: population-based study among 9444 older adults from Germany. Aliment Pharmacol Ther. 2007;26(6):879–887. [DOI] [PubMed] [Google Scholar]
- 28. Xing Y, Wei C, Chu C, et al. Stage-specific gender differences in cognitive and neuropsychiatric manifestations of vascular dementia. Am J Alzheimers Dis Other Dement. 2012;27(6):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler M. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575–580. [DOI] [PubMed] [Google Scholar]
- 30. Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(suppl 5):v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brieva L, Ara JR, Bertol V, Canellas A, Del Agua C. Polyneuropathy caused by vitamin B12 deficiency secondary to chronic atrophic gastritis and giardiasis. Rev Neurol. 1998;26(154):1019–1020. [PubMed] [Google Scholar]
- 33. Krasinski SD, Russell RM, Samloff IM, et al. Fundic atrophic gastritis in an elderly population. Effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34(11):800–806. [DOI] [PubMed] [Google Scholar]
- 34. Oh RC, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003;67(5):979–986. [PubMed] [Google Scholar]
- 35. Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19(1):357–377. [DOI] [PubMed] [Google Scholar]
- 36. Bellou A, Aimone-Gastin I, De Korwin JD, et al. Cobalamin deficiency with megaloblastic anaemia in one patient under long-term omeprazole therapy. J Intern Med. 1996;240(3):161–164. [DOI] [PubMed] [Google Scholar]
- 37. Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104(5):422–430. [DOI] [PubMed] [Google Scholar]
- 38. Kong HY, Cheng DM, Pang W, et al. Homocysteine levels and cognitive function scores measured with MMSE and BCAT of middle-aged and elderly subjects in Tianjin City. J Nutr Health Aging. 2013;17(16):527–532. [DOI] [PubMed] [Google Scholar]
- 39. Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008;29(2):S143–S172. [DOI] [PubMed] [Google Scholar]
- 40. Oulhaj A, Refsum H, Beaumont H, et al. Homocysteine as a predictor of cognitive decline in Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;25(1):82–90. [DOI] [PubMed] [Google Scholar]
- 41. McCaddon A, Kelly CL. Alzheimer’s disease: a cobalaminergic hypothesis. Med Hypotheses. 1992;37(3):161–165. [DOI] [PubMed] [Google Scholar]
- 42. Regland B, Gottfries CG. Slowed synthesis of DNA and methionine is a pathogenetic mechanism common to dementia in Down’s syndrome, AIDS and Alzheimer’s disease? Med Hypotheses. 1992;38(1):11–19. [DOI] [PubMed] [Google Scholar]
- 43. Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–483. [DOI] [PubMed] [Google Scholar]
- 44. Smith AD, Refsum H. Vitamin B-12 and cognition in the elderly. Am J Clin Nutr. 2009;89(2):707S–711S. [DOI] [PubMed] [Google Scholar]
- 45. Weir DG, Scott JM. Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull. 1999;55(3):669–682. [DOI] [PubMed] [Google Scholar]
- 46. Morrison LD, Smith DD, Kish SJ. Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J Neurochem. 1996;67(3):1328–1331. [DOI] [PubMed] [Google Scholar]
- 47. Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Psychiatry. 1990;53(12):1096–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. [DOI] [PubMed] [Google Scholar]
- 49. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826–832. [DOI] [PubMed] [Google Scholar]
- 51. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31(3):378–386. [DOI] [PubMed] [Google Scholar]
- 52. Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52(1):123–127. [DOI] [PubMed] [Google Scholar]
- 53. Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145(8):762–769. [DOI] [PubMed] [Google Scholar]
- 54. Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician-reported medical history. Am J Epidemiol. 1994;139(8):813–818. [DOI] [PubMed] [Google Scholar]
- 55. Momtaz YA, Hamid TA, Ibrahim R, Yahaya N, Abdullah SS. Moderating effect of Islamic religiosity on the relationship between chronic medical conditions and psychological well-being among elderly Malays. Psychogeriatrics. 2012;12(1):43–53. [DOI] [PubMed] [Google Scholar]


