Abstract
Lack of engagement in meaningful activities is associated with poor quality of life in dementia; thus, the development of these activities has been recommended. This pilot study aimed to develop a multisensory and motor-based group activity program for residents with dementia and assess its impact on residents’ behavior. The program was designed using a multisensory and motor-based approach in sixteen 45-minute weekly sessions tailored to residents’ characteristics. Four residents with advanced dementia participated in the program. The frequency and duration of the residents’ behavior were assessed using video recordings. All residents participated in the proposed activities, although they were more participative and communicative in some sessions than in others. Group activity programs based on multisensory and motor stimulation can be a promising approach for people with advanced dementia; however, further research is needed. This study may serve as reference to the implementation of future programs aiming to increase person-centeredness of the care provided.
Keywords: multisensory stimulation, motor stimulation, engagement, activity program
Introduction
Older people with dementia living in residential care homes may spend the majority of their time engaged in no activity, apart from the usual personal care activities.1–3 This is more evident in moderate to severe stages of dementia.4,5 The lack of meaningful activities has been associated with a decrease in residents’ functional status, increased behavior problems, social isolation and poor quality of life.3,5 Activity has therefore been recommended in people with dementia as a means of retaining human abilities and function, 6 by maintaining their connection with the environment and encouraging social interaction.7,8 Previous research suggests that the involvement in meaningful activities can have a positive effect on the quality of life of residents with dementia and is related to increased levels of relaxation and enjoyment,2,8 a lower frequency of behavior problems,8,9 increased alertness and improved functional ability. 3 It has been recommended that, in the middle to late stages of the disease, the activities should focus on fine- and gross-motor and sensory activities.2,7,10 Consistent with this recommendation, there are currently 2 main approaches with promising results in people with moderate to severe dementia: multisensory stimulation (MSS) and motor stimulation (MS).11–14
The purpose of MSS is to provide appropriate and pleasurable experiences through the stimulation of the senses (olfaction, tact, vision, hearing and taste),11,15 without the need for complex intellectual reasoning.16,17 By stimulating the senses in a format that can be understood by the individual, it is expected that people with dementia will respond appropriately to their surroundings and communicate with others, 18 for example, by giving a smile or thanking to the caregiver. 15 The MSS has been found to reduce the frequency of behavior problems and apathy,14,16 improve communication7,19 and functional performance, 20 and increase residents’ attentiveness.12,21 Motor stimulation aims to maintain or improve, as long as possible, the remaining physical abilities of people with dementia.12,22 It is related to movement and exercise and, when tailored to each individual’s abilities, MS can provide people with dementia with an activity in which they can succeed. 15 This approach has been found to improve mobility, balance and cognition, reduce falls and delay the decline of performance in daily activities in residents with dementia.22,23 By combining these 2 approaches, it is likely that residents will show more awareness to the environment through the stimulation of senses and more active involvement in the activities planned through the stimulation of mobility and participation.
There are few studies which have combined MSS and MS approaches in structured group activity programs for people with dementia.7,13,24 Those have reported improvements in strength and flexibility, 24 physical activity, mood, 13 and a reduction of agitation 24 after program implementation. However, these studies fell short in offering guidance regarding engagement strategies during the activity programs, 25 hindering their replication. Moreover, to our knowledge, only 1 study 7 reported the level of residents’ engagement during the activity sessions and, therefore, the question of whether people with advanced dementia can actively participate in these programs remains unanswered, as attendance at programs does not guarantee residents’ engagement. 5 Hence, it is essential to include direct observation of residents’ behavior during activity programs.
This pilot study aimed to (i) develop a multisensory and motor-based activity program designed for institutionalized older people with moderate to severe dementia consisting of structured group-session activities and (ii) assess residents’ behaviors during the program sessions, focused on the aspects of engagement. The term engagement was previously defined by Cohen-Mansfield and colleagues as “the act of being occupied or involved with an external stimulus.” 25 It includes the level of attention to the stimulus and the attitude/action toward it. In this study, it was expected that providing residents with activities appropriate to their cognitive and functional levels and tailored to their interests would result in their active involvement in the proposed activities and facilitate their social engagement.
Methods
Setting
The study was conducted in a traditional care home for older people, in the central region of Portugal. Care homes are defined as a social response developed to provide temporary or permanent accommodation for older people at increased risk of loss of independence and/or autonomy, 26 including people with dementia. The manager of the care home was first contacted to assess the willingness of the institution to collaborate in the study, after the description of its purpose and methods. No simultaneous participation in similar studies during the program implementation was ensured. The care home had 53 licensed beds for older people and 21 were occupied by people with a clinical diagnosis of dementia.
Participants
Eligible participants were identified by the physician of the care home. To participate in the study, residents had to meet the following criteria: presenting a clinical diagnosis of moderate to severe dementia according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria, 27 having no major psychiatric comorbilities and not being bedridden. A total of 13 residents were identified, but 2 died before collecting informed consent. Given the progressive decline that people with dementia experience in their ability to fully understand the context of the research and the implications of their participation, 28 the informed consent was obtained from proxy consent. The researchers contacted legal guardians (all family members), provided them with information about the study and asked to sign the informed consent. Written informed consent was obtained from 7 legal guardians. Four declined due to lack of interest (n = 2) or not wishing the resident to be video recorded (n = 2). One of the recruited residents died before the study began and his or her information had to be removed from the study. In each program session, the residents were asked to participate to obtain their assent, that is, their ongoing willingness to participate in the study. Two residents refused to participate in all sessions and therefore their information was removed from the study.
The sample comprised 4 residents with moderate to severe dementia. Data regarding residents’ characteristics were collected at baseline with the aim of describing the sample and adjusting the program accordingly: residents’ sociodemographic data, lifetime history and stimulus preference list (eg, favorite hobbies, music, dishes, etc) were obtained from family members; residents’ global functional ability was measured using the Barthel Index 29 ; the level of cognitive impairment was assessed using the Portuguese version of the Cognitive Impairment Test of the Elderly Assessment System (EASYcare), 30 which has been proved to be a faster and simpler test of cognition with better sensitivity and specificity than Mini-Mental State Examination. 31
The characteristics of the residents are summarized in Table 1. The assessment of global functional ability revealed that 2 patients were totally dependent on performing activities of daily living, while the other 2 showed moderate levels of dependency. Three residents had a restriction in mobility and were prescribed with walking aids.
Table 1.
Characteristics of the Residents With Dementia.
| Residents | Age | Gender | Clinical Diagnosis | EASYcare Cognitive Impairment Testa | Barthel Indexb | Use of Walking Aid |
|---|---|---|---|---|---|---|
| 1 | 85 | Female | Severe | 25 | 75 | No |
| 2 | 92 | Female | Moderate | 12 | 90 | Walking stick |
| 3 | 74 | Male | Moderate | 25 | 10 | Wheelchair |
| 4 | 75 | Male | Moderate | 16 | 0 | Wheelchair |
a Scores from 0 to 28 points. A score ≥11 indicates moderate to severe cognitive impairment.
b Scores from 0 to 100 points. Cutoff scores of the Barthel Index according to Shah and colleagues 32 were 0 to 20 total dependency; 61 to 90 moderate dependency; 91 to 99 slight dependency.
Multisensory and Motor-Based Group Activity Program
Program design
The program was designed according to an extensive literature review regarding therapeutic interventions for people with moderate to severe dementia, specifically MSS7,12,15,17,24,33 and MS.12,13,15,23 These interventions are based on principles that fit in a person-centered approach, that is, they need to be adapted to residents’ needs, preferences and abilities to enable positive meaningful connections between caregivers and people with advanced dementia.10,34 Therefore, the program was adjusted to participants’ cognitive and functional ability levels and tailored to their interests.5,35
The program was planned to be implemented in a small-group format. The number of participants in these programs should be limited from 4 to 9 people, to avoid the occurrence of challenging behaviors, encourage residents’ involvement in the proposed activities and promote social interaction without compromising the individualized approach.3,15 The program description and implementation are presented in the Results section.
Data Collection
The behaviors of residents with dementia were assessed using video recordings collected during the sessions. As proposed by Martin and colleagues, 34 the observation of residents’ behavior is the best way to recognize when a meaningful connection is occurring and, therefore, the only viable method to assess the well-being of residents with advanced dementia. Video recordings were chosen instead of direct observation because they enable reviewing the events as often as necessary 36 and provide important information that can be lost during direct observation. 37 In each session, researchers fastened the video camera to a top of a tripod and turned on just before the session started. The camera was placed in a specific location in the room where it would not interfere with participants’ movements and enabled video recording of all participants, including their faces. A total of 16 video recordings were collected, 1 per session.
Data Reduction
The selection of video footage for analysis was defined as observation time started when the resident appeared on the screen and it ended when the resident completed the gustatory stimulus task. The length of the smallest video recording was 17 minutes and 53 seconds. Thus, it was preestablished that the other video recordings would be cut from the starting point to standardize observation time, to allow comparisons between participants and sessions. This methodology has been used previously. 12
Outcome Measures
Residents’ behaviors were studied by analyzing the frequency and duration of a list of behaviors (ethogram). It was derived from previous research12,34,38,39 and preliminary observations of the video recordings regarding engagement aspects (ie, level of attention to the stimulus and attitude/action toward it 25 ). The list was developed prior to video recording analyzes and comprised the following categories: engagement in the task, interaction with objects, verbal communication, smiling, laughing, nodding the head and closed eyes. The behavior categories are described in Table 2. According to Cohen-Mansfield, 25 the first 3 categories are related to action toward the stimulus, the next 3 with a positive attitude toward the stimulus, and the last with reduced attention to it.
Table 2.
Behavior Categories of the Ethogram.
| Category | Description of the Category |
|---|---|
| Engagement in the task | The resident moves the body or a body part in order to perform a task, or a part of it, related to a specific task of the session (eg, tossing the ball to a facilitator or putting land into the jar). The task has a specific and predefined purpose and its completion can be accomplished with the assistance of a facilitator, for example through verbal commands or physical guidance. |
| Interaction with objects | The resident moves the body or a body part in the direction of an object, reaching it. He or she can explore the object or not. This action is voluntary, that is, the facilitator do not give any instructions to the resident to reach the object (for example, pick up the sunglasses and try them on). |
| Verbal communication | The resident articulates words or sentences with meaning, voluntarily and purposely, in order to communicate with other person (a facilitator, a staff element or another resident). Verbal aggression is excluded. |
| Smiling | The resident produces a facial expression characterized by an upward curving of the corners of the mouth indicating pleasure or amusement, which is directed to a person or an object. |
| Laughing | The resident smiles and produces a sound commonly associated with the act of laughing. |
| Nodding the head | The resident nods his or her head in an affirmative response to an auditory and/or visual stimulus, directed by another person (a facilitator, a staff element or another resident). |
| Closed eyes | The resident closes his or her eyes and keeps them closed for more than 1 second. |
Data Analysis
Analysis of the video recordings
Two observers analyzed the video recordings and independently rated residents’ behaviors according to the ethogram using specialized software, Noldus The Observer XT 10.0 (Noldus International Technology, Wageningen, the Netherlands). Observers were trained to use the software prior to video recording analyzes. This methodology was used in previous studies in people with dementia to reduce observation bias with good reliability results.12,14 Frequency and duration of the categories were measured for each resident in all sessions by both observers. Interobserver reliability analysis was then performed for each behavior category using the recommended methods for conducting reliability studies with continuous data, 40 intraclass correlation coefficient (ICC) 41 and Bland and Altman method. 42 The ICC equation (2, 1 – two-way random effects model) 43 was used.
The ICC values showed excellent to moderate interobserver reliability for both frequency and duration of the categories engagement in the task, interaction with objects, verbal communication, and laughing. 41 Lower ICC values were obtained for duration of smiling, nodding the head, and for frequency and duration of closed eyes. These low ICC values may be attributed to the influence of between-subjects variance on the ICC value; 40 in these categories, the between-subjects variance may not be large enough to obtain a high value in ICC. This is one of the main reasons why the interobserver reliability is recommended to be performed using both methods (ICC and Bland and Altman plots). There was a reasonable agreement between observers according to Bland and Altman method with no evidence of systematic bias. Table 3 provides a detailed description of ICC and Bland and Altman values.
Table 3.
Results of the Interobserver Reliability Analysis.
| ICC | ICC 95% CI | đ | SDdifferences | SE of đ | 95% Limits of Agreement | ||
|---|---|---|---|---|---|---|---|
| Engagement in the task | F | 0.84 | 0.75; 0.90 | 0.38 | 5.87 | 0.75 | −11.36; 12.11 |
| D | 0.90 | 0.82; 0.95 | −44.47 | 110.98 | 15.10 | −266.44; 177.49 | |
| Interaction with objects | F | 0.73 | 0.58; 0.83 | 0.07 | 2.98 | 0.38 | −5.89; 6.02 |
| D | 0.48 | 0.25; 0.66 | −0.40 | 71.12 | 9.68 | −142.64; 141.85 | |
| Verbal communication | F | 0.91 | 0.84; 0.95 | −4.70 | 13.19 | 1.80 | −31.08; 21.68 |
| D | 0.45 | 0.21; 0.64 | −10.99 | 98.04 | 13.34 | −207.07; 185.07 | |
| Closed eyes | F | 0.38 | 0.14; 0.57 | 0.24 | 3.82 | 0.52 | −7.39; 7.87 |
| D | 0.40 | 0.15; 0.60 | −12.23 | 98.88 | 13.46 | −209.99; 185.53 | |
| Smiling | F | 0.51 | 0.30; 0.68 | −0.11 | 1.46 | 0.20 | −3.04; 2.81 |
| D | 0.37 | 0.11; 0.58 | −0.38 | 4.26 | 0.58 | −8.90; 8.15 | |
| Laughing | F | 0.86 | 0.78; 0.91 | 0.32 | 1.32 | 0.18 | −2.32; 2.96 |
| D | 0.62 | 0.42; 0.76 | 0.93 | 3.07 | 0.42 | −5.20; 7.06 | |
| Nodding the head | F | 0.70 | 0.54; 0.81 | −0.72 | 2.12 | 0.29 | −4.97; 3.52 |
| D | 0.33 | 0.07; 0.55 | 0.42 | 5.95 | 0.81 | −11.48; 12.33 |
Abbreviations: F, Frequency (number of times the behavior was present); D, duration (total time the behavior was present, in seconds); ICC, intraclass correlation coefficient; ICC 95% CI, ICC 95% confidence intervals; đ, mean of the differences between results obtained from the 2 observers; SDdifferences, standard deviation of the differences; SE of đ, standard error of the mean difference (SE = SDdifferences/√n); 95% limits of agreement using the Bland and Altman method (đ ± 1.96 × SDdifferences).
Residents’ Attendance and Behavior in Program Sessions
At the beginning of each session, residents were asked to participate; therefore, residents’ attendances as well as reasons for nonattendance were assessed. Descriptive statistics of the behavior categories were calculated for all residents and for each resident independently using PASW Statistics (Predictive Analytics Software) version 18.0 for Windows (SPSS Inc, Chicago, Illinois). Nonparametric multiple comparison Friedman test was carried out for each behavior category to assess the significant differences between sessions. Pearson correlation coefficients (r) were also performed to assess the relationships between the behavior categories. The level of significance considered was .05.
Results
Multisensory and Motor-Based Activity Program
Program Description
The program consisted of 16 sessions using a multisensory and motor-based approach and it was developed by a multidisciplinary team including 2 physical therapists, 1 gerontologist and 1 educational scientist. The sessions followed a well-defined structure and were organized by a hierarchy of presentation of different stimuli: olfaction, movement (motor activities), touch, vision, hearing and taste. This concept of “hierarchy” was previously used by Bowlby 15 and Trudeau 7 and is based on the introduction of stimuli in a sequential manner, from the simplest to the most complex. According to this model of stimulation, olfaction should be the first sense to be stimulated because it is the most primitive sense and the olfactory nerve has projections to the limbic system, the area of the brain responsible for the emotions. 44 Therefore, the stimulation of olfaction can have an arousing and pleasant effect in people with dementia, even in those with some deterioration of the olfactory capacity. Movement should be the second stimulus to be performed because it helps to improve arousal and alertness and is less complex than the following stimuli. Motor function should be stimulated in a simple format without requiring complex motor planning and having a concrete and logical reason for movement (eg, throwing a ball into a basket). The senses of touch, sight and hearing should be the next to be explored because they are more complex and usually require more time for interpretation. Taste should be stimulated at the end of the session as it is perceived as rewarding and reinforces socialization.
Each session was designed based on a specific theme consistent with residents’ preferences and lifetime history. The themes were also chosen in accordance with the season or date in which they would be implemented (eg, Christmas or Valentine Day), to offer temporal orientation to participants. The list of themes is presented in Table 4.
Table 4.
Themes of the Sessions.
| Themes | |
|---|---|
| 1. Grape harvest | 9. New Year |
| 2. Celebration—roasted chestnutsa | 10. Relaxation |
| 3. Gardening | 11. Remembering Aveirob |
| 4. Coffee and table games | 12. Old traditional festivities |
| 5. Music | 13. Sports |
| 6. Arts | 14. School time |
| 7. Beach | 15. Valentine day |
| 8. Christmas | 16. Self care |
a This is a traditional festivity of Portugal named “Magusto” which occurs on November 11th. People celebrate it eating roasted Portuguese chestnuts.
b Aveiro is a city in the central region of Portugal, in which the program took place.
For each stimulus, a structured task or sensory cue regarding the theme of the session was planned to encourage residents’ involvement, following the hierarchy of stimulation mentioned above. Although a single object may provide different types of stimulation (eg, a pine can provide olfactory, visual and tactile stimulation), emphasis was given to only 1 sense at a time. The materials selected for providing stimulation were simple, inexpensive and available in most care homes. An example of a session is described in Table 5.
Table 5.
Description of 1 Session With the Theme Beach.
| Stimulation Cue/Task | Description |
|---|---|
| Olfaction | Olfactory stimulation is introduced through a sea-scented air freshener. |
| Movement | A beach ball game is planned to stimulate movement. The game consists of throwing the ball and receiving it (similar to the usual ball games). The ball can be thrown to the facilitator or other participants. Facilitators can give simple step-by-step instructions, either verbal (“Throw the ball forward!” “Send the ball to me!”) or nonverbal (making gestures to explain what is intended with the game or exemplifying the activity). People who are able to walk independently can be asked to walk barefoot on sand previously placed on a floor area of the room (in a protective plastic to prevent contamination). |
| Touch | People are encouraged to touch the sand (dry and wet), water, or other beach-related objects (eg, sea shells, beach towels and sunglasses). |
| Vision | The vision is stimulated by presenting images related to the beach, such as the beach the participants used to go, images of fishes, shells and colored sunshades. Images must be clear and unambiguous, with bright colors and high contrast, and can be presented either on paper or through an image projector. |
| Hearing | The hearing can be stimulated simultaneously with the vision by playing the sound of ocean waves or seagulls. |
| Taste | The taste can be stimulated by offering ice cream. |
The sessions were planned to last 45 minutes each, which is consistent with previous recommendations. 15 Estimated time frames of approximately 10 minutes for each stimulation cue were set to guide the facilitators (ie, the professionals who implemented the program sessions). However, these time frames could be extended or shortened depending on the participants’ responses. Additionally, if a person had a limitation in one sense, the other senses were stimulated to compensate for that one.
During the presentation of stimuli, facilitators provided participants with simple verbal prompts to help stimulating their communication, such as “What is your favorite color?” “Do you like this fragrance?,” or “Do you like touching the sand?” Because communication is defined as the core of all effective interventions in dementia, 45 a number of recommendations were followed by the facilitators to effectively communicate with the participants during the sessions:
the facilitator was located close to the resident and called the person by his or her name;
the facilitator spoke slowly and clearly, using simple and short sentences. When repeating a statement, the same words were used;
verbal prompts were asked 1 at a time, giving time for the resident to respond;
the facilitator made eye contact when talking to the resident and reinforced verbal cues with visual ones whenever possible. In addition, touch was used to communicate with the residents, although with caution to avoid the occurrence of challenging behaviors;
gestures and facial expressions were also used with appropriate verbal cues.
The provision of MS followed specific strategies and recommendations about the varying levels of assistance offered to participants. This ensured that all residents could participate actively regardless of his or her physical status, giving him or her the best change to be successful. Facilitators planned in advance what tasks each person could perform and adapted them according to the following recommendations:
the person was properly positioned to facilitate the participation in the task;
tasks were broken in small steps and simple instructions were given, step-by-step;
the facilitator demonstrated how to perform the task and then asked the person to do it, using gestures to assist its completion;
if the person needed assistance, the facilitator helped the starting of the movement or gave physical guidance;
care was taken to avoid rushing the person during the task;
periods of rest during the task were given if the person felt tired;
the person was encouraged and praised after task completion.
Program Implementation
The implementation period lasted for 4 months. The sessions were carried out on a weekly basis on the same day of the week, in a room of the care home. The room was quiet and comfortable with proper lightening to ensure residents' participation in activities, but without extraneous stimuli (eg, television, radio) and distant from the passing zones to prevent distractions or interruptions. All sessions were facilitated by a physical therapist and a gerontologist, with the support of the activity organizer of the care home who was aware of program specificities. Sessions were carried out between 2:30 and 3:30 pm, as these were the times when residents were frequently unoccupied.
Residents’ Attendance and Behavior in Program Sessions
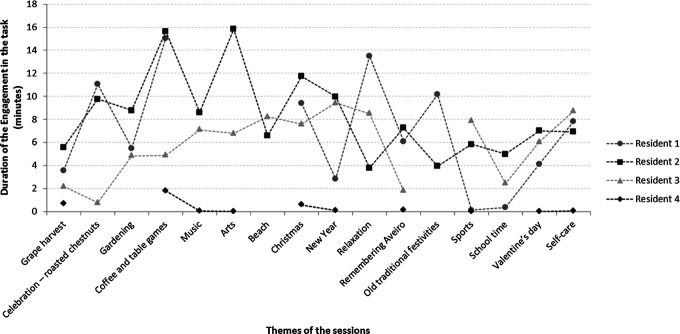
Only 1 resident (resident 2) attended all sessions (Figure 1). Resident 1 did not attend 3 sessions, resident 3 missed 1 session, and resident 4 missed 6 sessions. Reasons given included health-related reasons and hospitalization, family-related reasons, lack of interest to participate and no reason given.
Figure 1.
Duration of the category engagement in the task for each resident in each session.
Table 6 shows the mean frequency and duration of the categories for all residents and for each resident individually. There were no statistically significant differences between the thematic sessions for both indicators of the behavior categories (P > .05) when considering the whole sample. Overall, residents engaged in the proposed tasks for more than 5 minutes (95% confidence interval [CI], 4.61-6.97), communicated verbally about 34 times (95% CI, 27.21-42.39), and interacted with objects with a mean frequency of 2.15 times during the sessions (95% CI, 1.20-3.10), except for resident 4 who did not present this behavior.
Residents 1 and 2 were the most participative and responsive in the program sessions, presenting higher values in the mean frequency and duration of engagement in the task, interaction with objects, verbal communication and laughing, when compared to the other residents (Table 6). In 5 of the attended sessions, these residents spent half or more of the observed time (17 minutes and 53 seconds) engaged in the planned tasks (Figure 1). Furthermore, they were engaged in the tasks for more than 84% of the time in 1 session (coffee and table games); resident 2 was also engaged approximately 88% of the time in other session (arts).
Table 6.
Residents’ Behavior During Participation in the Multisensory and Motor-Based Activity Program.
| Resident 1 | Resident 2 | Resident 3 | Resident 4 | All Residents | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | 95% CI | ||
| Engagement in the task | F | 16.38 ± 12.32 | 16.78 ± 6.24 | 14.87 ± 6.11 | 1.10 ± 1.41 | 11.73 ± 9.90 | 9.20-14.26 |
| D | 6.90 ± 4.75 | 8.28 ± 3.65 | 5.82 ± 2.83 | 0.34 ± 0.57 | 5.79 ± 4.34 | 4.61-6.97 | |
| Interaction with objects | F | 5.12 ± 6.36 | 2.72 ± 2.71 | 1.40 ± 1.29 | 0 | 2.15 ± 3.71 | 1.20-3.10 |
| D | 1.25 ± 1.54 | 0.76 ± 0.88 | 0.38 ± 0.40 | 0 | 0.63 ± 1.00 | 0.36-0.90 | |
| Verbal communication | F | 56.08 ± 27.11 | 63.00 ± 17.73 | 17.60 ± 10.29 | 12.20 ± 10.74 | 34.08 ± 29.63 | 27.21-42.39 |
| D | 1.99 ± 1.38 | 2.54 ± 0.92 | 0.76 ± 0.60 | 1.21 ± 1.66 | 1.67 ± 1.33 | 1.30-2.03 | |
| Closed eyes | F | 0.23 ± 0.56 | 0 | 0 | 4.15 ± 5.57 | 0.73 ± 2.66 | 0.05-1.41 |
| D | 0.01 ± 0.03 | 0 | 0 | 1.73 ± 2.57 | 0.10 ± 0.07 | 0.08-0.12 | |
| Smiling | F | 1.54 ± 2.11 | 0.47 ± 0.85 | 0.17 ± 0.52 | 0 | 0.49 ± 1.21 | 0.18-0.80 |
| D | 0.05 ± 0.09 | 0.01 ± 0.03 | 0.01 ± 0.04 | 0 | 0.02 ± 0.05 | 0.01-0.03 | |
| Laughing | F | 1.62 ± 2.39 | 2.87 ± 3.29 | 0.10 ± 0.28 | 0 | 1.12 ± 2.31 | 0.53-1.71 |
| D | 0.03 ± 0.06 | 0.06 ± 0.07 | 0.01 ± 0.02 | 0 | 0.03 ± 0.05 | 0.01-0.04 | |
| Nodding the head | F | 1.38 ± 1.26 | 3.50 ± 3.39 | 1.77 ± 2.29 | 0 | 1.65 ± 2.47 | 1.01-2.28 |
| D | 0.03 ± 0.03 | 0.05 ± 0.07 | 0.05 ± 0.11 | 0 | 0.04 ± 0.07 | 0.02-0.05 | |
Abbreviations: F, frequency (number of times the behavior was present); D, duration (total time the behavior was present, in minutes); SD, standard deviation; 95% CIs, 95% confidence intervals for the mean of the behaviors presented by all residents.
Resident 3 showed high levels of engagement in the proposed tasks, being engaged in more than one-third of the total amount of time in 8 of the 15 attended sessions. Lower levels of participation in the sessions were observed in resident 4. In fact, he did not present some of the positive behaviors observed in other residents (interaction with objects, smiling, laughing and nodding the head), although he did engage and verbally communicate in some sessions.
When assessing correlations between behavior categories, statistically significant results were found. It was possible to observe an inverse correlation between the duration (D) and frequency (F) of engagement in the task and closed eyes (r = −.34, P = .012 [D]; r = −.35, P = .008 [F]). The frequency of engagement in the task was positively correlated to the frequency of smiling (r = .44, P = .001 [F]) and laughing (r = .39, P = .004 [F]). A relationship was also found between verbal communication and the categories engagement in the task (r = .33, P = .014 [D]; r = .52, P = .001 [F]), interaction with the objects (r = .37, P = .005 [F]), smiling (r = .49, P = .001 [D]; r = .51, P = .001 [F]), laughing (r = .32, P = .020 [D]; r = .60, P = .001 [F]), and nodding the head (r = .39, P = .003 [F]), suggesting that residents who talked more also presented more positive nonverbal communication behaviors and were more participative in the sessions. The duration of laughing was positively related to the same indicator of interaction with the objects (r = .29; P = .036 [D]).
Discussion
This pilot study developed a multisensory and motor-based group activity program tailored to cognitive and functional abilities, preferences and lifetime history of residents with advanced dementia in care homes. This was needed as the literature has emphasized the importance of developing meaningful and suitable activities to increase engagement in this population,8,13,17 especially in advanced stages, for which the activities are either not available or fail to match their skill levels.46,47 There is relatively little research exploring the impact of the implementation of structured activity programs in residents’ behavior and the few published studies failed to fully characterize the intervention, 48 making it difficult to replicate and/or compare different studies. This study tried to overcome this gap by developing and presenting a detailed group activity program for people with advanced dementia, including the rationale that formed the basis for the program design and information about the strategies used to interact with the residents (communication strategies and assistance).
As attendance at activity programs does not guarantee residents’ involvement, 5 this study also assessed the immediate effects of the program on the behavior of residents with dementia.It was observed that residents were actively involved and engaged in the proposed activities. Such results suggest that even residents with advanced dementia can effectively participate in group activities appropriate to their cognitive and functional levels and considering their past experience and preferences. This is an encouraging finding, given that most individuals with dementia have difficulty with attention and often lack the internal resources needed to initiate, maintain, or complete an activity.7,49 However, further research is needed to explore the extent of these findings. The use of MSS and MS approaches may have facilitated residents’ engagement and enabled them to successfully participate in the activities, as reported by previous studies using these approaches in other contexts.12,14 The communication strategies and assistance provided during the sessions may also have facilitated residents’ involvement. It is not possible to determine which factor most influenced the residents’ behavior; however, these strategies are the key element in all activity approaches and should not be provided separately. 45
Overall, residents seem to enjoy most of the attended sessions, although they evidenced more positive behaviors in some sessions rather than in others. These results may be explained by 2 main factors previously identified in the literature (i) factors related to residents’ characteristics, such as their level of function and cognitive impairment5,8,25 and/or (ii) a combination between the context of past experiences, personal interests and preferences and cultural expectations.2,5,50 A recent guideline developed to support people with dementia and their caregivers 45 highlighted the need of considering the right level of stimulation and challenge for the individual when exploring appropriate activities. The present study attempted to overcome participants’ deficits by designing the program according to their cognitive and functional status and planning the tasks in advance to adapt them accordingly. However, in people with moderate to severe dementia, the function is often highly compromised 15 and may act as a “limiting effect” in their participation. 5 It should not be assumed that the person does not retain abilities to perform an activity and, therefore, creative ways need to be explored to maximize the use of each individual’s remaining abilities. 45
The fact that residents participated more actively in specific sessions and less in others may be attributable to their personal preferences and lifetime history. Previous literature has emphasized that the combination between the context of past experiences, personal interests and cultural expectations may play a role in individuals’ motivation to participate in activities.2,50,51 Therefore, a comprehensive assessment of residents’ physical, mental and social dimensions2,5,6,51 is fundamental in the development of these programs. 52 Although the present program was designed considering these dimensions, sessions were performed in a group format which made the selection of session themes more challenging as residents’ preferences were not always consensual. Nevertheless, group activities are essential to promote an enriched social environment with opportunities for people with dementia to feel valued and included, 10 reducing the risk of social isolation. Therefore, future research should focus on the development of meaningful activities for people with dementia in a small-group format, to improve their active involvement in activities while promoting social engagement.
Recent guidelines recommend that stimulation interventions should be offered to people with dementia on a regular basis (eg, daily or weekly), 48 though in residential care homes, it is well recognized that organizational issues such as lack of staff and the prioritization of physical needs over psychosocial ones may be a barrier to implement these activities as part of fundamental care.2,34 Therefore, important shifts in dementia care need to be conducted in the next decades to achieve a high quality of care. Specifically, the development of meaningful and structured group activities for people with advanced dementia should become a priority within their care practices in order to promote residents’ comfort, quality of life and human dignity.
Limitations and Future Research
This study adds knowledge to the literature on structured group activity programs for people with advanced stages of dementia in the context of residential care by showing that residents can effectively participate in the program sessions. However, the sample was small and, thus, the program should be replicated with a larger sample to investigate whether similar results are obtained. The program was implemented in only 1 care home because the main focus of this pilot study was to assess the adequacy of the intervention to the target population. This is required before the implementation of the program in a larger study. 53 Therefore, as the results look promising, the inclusion of more care homes should be addressed in future studies.
Conclusion
The findings suggest that structured group activity programs based on MSS and MS approaches can be a promising approach for people with advanced dementia. Given the well-documented lack of residents’ engagement observed in most care homes and its potential to increase excess disability and behavior problems, similar interventions are urgently needed to promote residents’ comfort, quality of life and human dignity. The present program may serve as reference to the development of future programs exploring residents’ engagement aiming to increase person-centeredness of the care provided.
Acknowledgments
The authors would like to thank the residents and their families for participating in this study and the managers and staff members of the care home where the study was conducted.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Fundação Calouste Gulbenkian (project reference 100131/FCG/2009).
References
- 1.Kuhn D, Kasayka RE, Lechner C. Behavioral observations and quality of life among persons with dementia in 10 assisted living facilities. Am J Alzheimers Dis Other Demen. 2002;17(5):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harmer BJ, Orrell M. What is meaningful activity for people with dementia living in care homes? A comparison of the views of older people with dementia, staff and family carers. Aging Ment Health. 2008;12(5):548–558. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Mansfield J, Thein K, Dakheel-Ali M, Marx MS. Engaging nursing home residents with dementia in activities: the effects of modeling, presentation order, time of day, and setting characteristics. Aging Ment Health. 2010;14(4):471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clare L, Whitaker R, Quinn C, et al. AwareCare: development and validation of an observational measure of awareness in people with severe dementia. Neuropsychol Rehabil. 2012;22(1):113–133. [DOI] [PubMed] [Google Scholar]
- 5.Kolanowski A, Buettner L, Litaker M, Yu F. Factors that relate to activity engagement in nursing home residents. Am J Alzheimers Dis Other Demen. 2006;21(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thelander VB, Wahlin T-BR, Olofsson L, Heikkilä K, Sonde L. Gardening activities for nursing home residents with dementia. Adv Physiother. 2008;10(1):53–56. [Google Scholar]
- 7.Trudeau SA. Bright eyes: a structured sensory-stimulation intervention. In: Volicer L, Bloom-Charette L, eds. Enhancing the Quality of Life in Advanced Dementia. Philadelphia, PA: Taylor & Francis; 1999:93–105. [Google Scholar]
- 8.Riley P, Alm N, Newell A. An interactive tool to promote musical creativity in people with dementia. Comput Human Behav. 2009;25(3):599–608. [Google Scholar]
- 9.Buettner LL. Simple pleasures: a multilevel sensorimotor intervention for nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 1999;14(1):41–52. [Google Scholar]
- 10.Brooker D. Person-Centred Dementia Care: Making Services Better. London, England: Jessica Kingsley Publishers; 2007. [Google Scholar]
- 11.Baker R, Holloway J, Holtkamp CCM, et al. Effects of multi-sensory stimulation for people with dementia. J Adv Nurs. 2003;43(5):465–477. [DOI] [PubMed] [Google Scholar]
- 12.Cruz J, Marques A, Barbosa AL, Figueiredo D, Sousa L. Effects of a motor and multisensory-based approach on residents with moderate-to-severe dementia. Am J Alzheimers Dis Other Demen. 2011;26(4):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyn P. The effect of a multisensory exercise program on engagement, behavior, and selected physiological indexes in persons with dementia. Am J Alzheimers Dis Other Demen. 2003;18(4):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Weert JCM, van Dulmen AM, Spreeuwenberg PMM, Ribbe MW, Bensing JM. Effects of snoezelen, integrated in 24 h dementia care, on nurse-patient communication during morning care. Patient Educ Couns. 2005;58(3):312–326. [DOI] [PubMed] [Google Scholar]
- 15.Bowlby C. Therapeutic Activities With Persons Disabled by Alzheimer's Disease and Related Disorders. Shoal Creek Boulevard, TX: Pro-Ed Inc; 1992. [Google Scholar]
- 16.Verkaik R, Weert JCMv, Francke AL. The effects of psychosocial methods on depressed, aggressive and apathetic behaviors of people with dementia: a systematic review. Int J Geriatr Psychiatry. 2005;20(4):301–314. [DOI] [PubMed] [Google Scholar]
- 17.Vozzella S. Sensory stimulation in dementia care: why it is important and how to implement it. Top Geriatr Rehabil. 2007;23(2):102–113. [Google Scholar]
- 18.Baillon S, van Diepen E, Prettyman R. Multi-sensory therapy in psychiatric care. Adv Psychiatr Treat. 2002;8(6):444–450. [Google Scholar]
- 19.Hope KW, Easby R, Waterman H. ‘Finding the person the disease has’ - The case for multisensory environments. J Psychiatr Ment Health Nurs. 2004;11(5):554–561. [DOI] [PubMed] [Google Scholar]
- 20.Collier L, McPherson K, Ellis-Hill C, Staal J, Bucks R. Multisensory stimulation to improve functional performance in moderate to severe dementia—interim results. Am J Alzheimers Dis Other Demen. 2010;25(8):698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Weert JCM, Janssen BM, Van Dulmen AM, Spreeuwenberg PMM, Bensing JM, Ribbe MW. Nursing assistants’ behaviour during morning care: effects of the implementation of snoezelen, integrated in 24-hour dementia care. J Adv Nurs. 2006;53(6):656–668. [DOI] [PubMed] [Google Scholar]
- 22.Christofoletti G, Oliani MM, Gobbi S, Stella F. Effects of motor intervention in elderly patients with dementia: an analysis of randomized controlled trials. Top Geriatr Rehabil. 2007;23(2):149–154. [Google Scholar]
- 23.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–165. [DOI] [PubMed] [Google Scholar]
- 24.Buettner LL, Lundegren H, Lago D, Farrell P, Smith R. Therapeutic recreation as an intervention for persons with dementia and agitation: an efficacy study. Am J Alzheimers Dis Other Demen. 1996;11(5):4–12. [Google Scholar]
- 25.Cohen-Mansfield J, Dakheel-Ali M, Marx MS. Engagement in persons with dementia: the concept and its measurement. Am J Geriatr Psychiatry. 2009;17(4):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfim CDJ, Garrido MM, Saraiva ME, Veiga SM. Lar para Idosos - Condições de Implantação, Localização, Instalação e Funcionamento. Lisboa, Portugal: Direcção-Geral da Acção Social; 1996. [Google Scholar]
- 27.American Psychiatric Association. DSM-IV-TR - Manual de Diagnóstico e Estatística das Perturbações Mentais. 4th ed. Lisboa: Climepsi Editores; 2000. [Google Scholar]
- 28.Slaughter S, Cole D, Jennings E, Reimer MA. Consent and assent to participate in research from people with dementia. Nurs Ethics. 2007;14(1):27–40. [DOI] [PubMed] [Google Scholar]
- 29.Mahoney F, Barthel D. Functional evaluation: the barthel index. Md State Med J. 1965;14:56–61. [PubMed] [Google Scholar]
- 30.Figueiredo D, Sousa L. Easycare: an instrument for assessing the quality of life and well being of the elderly. Easycare: um instrumento de avaliação da qualidade de vida e bem estar do idoso. Ver Port Med Geriatr. 2001;130:41–47. [Google Scholar]
- 31.Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry. 1999;14(11):936–940. [PubMed] [Google Scholar]
- 32.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the barthel index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709. [DOI] [PubMed] [Google Scholar]
- 33.Fowler S. Sensory stimulation: sensory-focused activities for people with physical and multiple disabilities. London, England: Jessica Kingsley Publishers; 2007. [Google Scholar]
- 34.Martin GA, Mockbee D, Alonzo T, Deyo J, Dougherty J, Horton A. Activity programming. In: Martin GA, Sabbagh MN, eds. Palliative Care for Advanced Alzheimer's and Dementia: Guidelines and Standards for Evidence-Based Care. New York, NY: Springer Publishing Company; 2011:153–169. [Google Scholar]
- 35.Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging Ment Health. 2010;14(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansebo G, Kihlgren M. Carers' interactions with patients suffering from severe dementia: a difficult balance to facilitate mutual togetherness. J Clin Nurs. 2002;11(2):225–236. [DOI] [PubMed] [Google Scholar]
- 37.Haidet KK, Tate J, Divirgilio-Thomas D, Kolanowski A, Happ MB. Methods to improve reliability of video-recorded behavioral data. Res Nurs Health. 2009;32(4):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altus DE, Engelman KK, Mathews RM. Finding a practical method to increase engagement of residents on a dementia care unit. Am J Alzheimers Dis Other Demen. 2002;17(4):245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrin T. The positive response schedule for severe dementia. Aging Ment Health. 1997;1(2):184–191. [Google Scholar]
- 40.Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil. 1998;12(3):187–199. [DOI] [PubMed] [Google Scholar]
- 41.Fleiss J. Reliability of measurements. In: Fleiss J, ed. The Design and Analysis of Clinical Experiments. 1st ed. New York, NY: John Wiley & Sons; 1986. [Google Scholar]
- 42.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 43.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. [DOI] [PubMed] [Google Scholar]
- 44.Widmaier EP, Raff H, Strang KT. Vander, Sherman, Luciano's Human Physiology: The Mechanisms of Body Function. 9th ed. Boston, MA: Mcgraw-Hill; 2003. [Google Scholar]
- 45.National Collaborating Centre for Mental Health (UK). Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and Their Carers in Health and Social Care - National Clinical Guidelines N. 42. Leicester, England: British Psychological Society; 2007. [PubMed] [Google Scholar]
- 46.Collier L. Planning and implementing sensory interventions. In: Pool J, ed. The Pool Activity Level (PAL) Instrument for Occupational Profiling: a Practical Resource for Carers of People with Cognitive Impairment. 4th ed. London, England: Jessica Kingsley Publishers; 2012:140–153. [Google Scholar]
- 47.Kovach CR, Magliocco JS. Late-stage dementia and participation in therapeutic activities. Appl Nurs Res. 1998;11(4):167–173. [DOI] [PubMed] [Google Scholar]
- 48.American Psychiatric Association A. Practice Guideline For The Treatment of Patients With Alzheimer’s Disease and Other Dementias. Arlington, VA: American Psychiatric Association; 2007. [PubMed] [Google Scholar]
- 49.Kolanowski A, Litaker M. Social interaction, premorbid personality, and agitation in nursing home residents with dementia. Arch Psychiatr Nurs. 2006;20(1):12–20. [DOI] [PubMed] [Google Scholar]
- 50.LeBlanc LA, Cherup SM, Feliciano L, Sidener TM. Using choice-making opportunities to increase activity engagement in individuals with dementia. Am J Alzheimers Dis Other Demen. 2006;21(5):318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edvardsson D, Fetherstonhaugh D, Nay R. Promoting a continuation of self and normality: person-centred care as described by people with dementia, their family members and aged care staff. J Clin Nurs. 2010;19(17-18):2611–2618. [DOI] [PubMed] [Google Scholar]
- 52.Dobbs D, Munn J, Zimmerman S, et al. Characteristics associated with lower activity involvement in long-term care residents with dementia. Gerontologist. 2005;45(suppl 1):81–86. [DOI] [PubMed] [Google Scholar]
- 53.Conn VS, Algase DL, Rawl SM, Zerwic JJ, Wyman JF. Publishing pilot intervention work. West J Nurs Res. 2010;32(8):994–1010. [DOI] [PubMed] [Google Scholar]