Abstract
Severe Impairment Battery (SIB) data from the 24-week, randomized, double-blind ACTivities of daily living and cognitION (ACTION) study suggest that patients with severe Alzheimer’s disease (AD) benefit from treatment with 13.3 versus 4.6 mg/24 h rivastigmine patch. The objective of this retrospective analysis was to further examine the cognitive efficacy of 13.3 versus 4.6 mg/24 h rivastigmine patch on individual SIB items, and SIB domains derived using factor analysis of these items. Change from baseline at Week 24 on 9 new factor-defined domains and individual items was calculated and compared using effect sizes (Cohen’s d). Numerically less decline was observed with 13.3 versus 4.6 mg/24 h patch on all domains and the majority of individual items. Largest least squares mean treatment differences were observed on “visuospatial reasoning,” “object naming,” “recognition,” “design copying,” “social agency,” “ideational praxis,” and “comprehension” domains. These findings suggest 13.3 mg/24 h rivastigmine patch demonstrates broad cognitive efficacy across a range of SIB items and domains in patients with severe AD.
Keywords: cholinesterase inhibitor, cognition, randomized clinical trial, rivastigmine patch, severe Alzheimer’s disease, Severe Impairment Battery
Introduction
Assessment scales designed to evaluate the symptomatic efficacy of treatment and changes in cognitive function over time in patients with mild-to-moderate Alzheimer’s disease (AD) are of limited use in patients with severe disease due to the magnitude of the cognitive loss and potential floor effects. 1 Taking this into account, the Severe Impairment Battery (SIB) was specifically developed to assess cognitive function in patients with severe dementia, who may have difficulty with completing more complex standard neuropsychological assessments. 2
The SIB is divided into 6 scorable subscales (attention, orientation, language, memory, visuoperception, and construction). There are also brief evaluations of praxis, the patient’s ability to respond appropriately to their name, and social interaction skills. 1 Scores for each subscale, and the total scale, are calculated based on the sum score of individual items. Items are presented as single verbal or 1-step commands (eg, “please sit here”), which are enhanced through gestures. In this way, the SIB is a performance-based index, rather than a clinical rating derived from observation. 3 The SIB has been shown to be a reliable and valid measure of cognitive function in patients with moderate-to-severe AD. 2,3
In June 2013, the US Food and Drug Administration approved the high-dose 13.3 mg/24 h rivastigmine patch for the treatment of severe AD. 4 Approval for the severe indication was based on the findings of the ACTivities of daily living and cognitION (ACTION) study, a randomized controlled comparison of 13.3 versus 4.6 mg/24 h rivastigmine patch in patients with severe AD. 5
Due to the advanced disease severity of the patient population planned for enrollment into the ACTION study, the SIB and Alzheimer’s Disease Cooperative Study–Activities of Daily Living scale–Severe Impairment Version (ADCS-ADL-SIV) were selected as the coprimary outcome measures to assess the cognitive and functional efficacy of the high-dose 13.3 versus 4.6 mg/24 h rivastigmine patch. 1,5 -7 The version of the SIB used in the ACTION study was scored from 0 to 100, with a higher score indicating better cognitive function, derived from the cumulative sum score of 51 individual items/subitems (49 items/subitems have a maximum score of 2 and 2 items/subitems have a maximum score of 1). Patients treated with 13.3 mg/24 h rivastigmine patch showed significantly less deterioration on the total SIB over the 24-week study period than patients treated with 4.6 mg/24 h rivastigmine patch (P < .0001). 5 The observed greater efficacy of the high-dose 13.3 versus 4.6 mg/24 h rivastigmine patch was not associated with a marked increase in the overall incidence of adverse events (74.6% and 73.3%, respectively). 5
ACTION was the first study of rivastigmine patch in a large sample of patients with severe AD. Further analyses of SIB item data obtained in the ACTION study will enable investigation into whether the observed cognitive efficacy of rivastigmine patch in this population of patients with severe AD was broad or driven predominantly by stabilization or reduced impairment in selected cognitive abilities. In patients with severe AD, where cognitive deficits are marked and progressive, a treatment that stabilizes or reduces broad decline in cognition represents a clinically relevant response. Identifying the specific cognitive domains likely to respond to treatment may aid physicians when monitoring disease progression in patients with severe AD.
The objective of this retrospective analysis of the ACTION study was to derive new domains of the SIB, based on factor analysis of individual SIB items, in order to further investigate the specific cognitive efficacy of the high-dose 13.3 mg/24 h rivastigmine patch in patients with severe AD. A similar retrospective analysis was recently conducted8; however, the ACTION study provides a valuable opportunity to explore the cognitive efficacy of high-dose 13.3 mg/24 h rivastigmine patch, rather than oral 3 to 12 mg/d capsules, on newly defined SIB domains in a larger sample of patients with severe AD.
Methods
Study Design and Participants
The ACTION study was a 24-week, prospective, randomized, double-blind, double-dummy trial conducted at 82 centers across the United States, and full details of the study methodology and results have been published previously. 5,6 Patients were men and women, aged 50 years or older, with probable AD (National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association [NINCDS/ADRDA] criteria) 9 and a Mini-Mental State Examination (MMSE) 10 score of 3 to 12, inclusive. 5,6 All patients were required to be able to complete at least 1 item on the SIB, be cooperative, willing to complete all aspects of the study, and capable of doing so with the aid of a responsible caregiver. 6 Exclusion criteria included any advanced, severe, progressive, or unstable disease that may have interfered with efficacy or safety assessments, or any current medical or neurological condition other than AD that could explain the patient’s dementia. 5,6
Eligible patients were randomized (1:1) to 13.3 or 4.6 mg/24 h rivastigmine patch for 24 weeks. 5,6 Patients randomized to 13.3 mg/24 h patch were titrated to the target dose via the 4.6 and 9.5 mg/24 h patch doses (4-week titration steps). 5,6 The 4.6 mg/24 h patch group received this dose throughout. 5,6
The ACTION study was registered (ClinicalTrials.gov identifier: NCT00948766). All patients, or their representative, provided informed written consent before participation. 5 The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. 5
Factor Analysis
In the current analysis, the PROC FACTOR procedure in SAS was applied to the baseline SIB data from the ACTION study to establish a “best fit” for the individual SIB items into new domains. Initial common factor extraction was conducted using the principal component method. Factors with eigenvalues > 0.5 were retained. Estimates of loading were obtained using varimax rotation. Items that loaded on multiple factors were assigned to domains with the highest loadings, that is, the domains were mutually exclusive with respect to the items included. New domain scores were calculated as the total sum of the identified items within each factor. It was planned that the new domains would be named by consensus agreement of the authors, based on the face validity of individual items.
Outcome Measures
Coprimary outcome measures in the ACTION study were the change from baseline at Week 24 on the SIB 1 and the ADCS-ADL-SIV. 5 -7 In the current retrospective analysis, the mean change from baseline at Week 24 on each of the individual SIB items and on each of the newly defined SIB domains was calculated for patients randomized to 13.3 or 4.6 mg/24 h rivastigmine patch.
Statistical Analyses
The current analysis was based on the modified full analysis set (MFAS) and included all randomized patients who received at least 1 dose of study medication and had at least 1 postbaseline assessment on the coprimary efficacy variables (either SIB or ADCS-ADL-SIV). For the factor analysis, no imputation was applied for missing SIB items. Missing data for the newly defined domains were imputed using the last observation carried forward (LOCF) method. A sensitivity analysis, multiple imputation method, was applied to handle the missing data in each domain.
Effect sizes (Cohen’s d) were calculated, based on mean and standard deviation values, to compare the change from baseline at Week 24 on the individual SIB items and newly defined domains in patients randomized to 13.3 or 4.6 mg/24 h rivastigmine patch.
Least squares mean differences on the newly defined SIB domains between the 13.3 and 4.6 mg/24 h patch groups were estimated and 95% confidence intervals (CIs) calculated.
Results
Participants
Of 716 patients randomized, 356 received 13.3 mg/24 h patch and 360 received 4.6 mg/24 h patch. 5 The MFAS comprised 338 patients in the 13.3 mg/24 h patch group and 335 patients in the 4.6 mg/24 h patch group, of which SIB data (baseline and Week 24) were available for 313 and 316 patients, respectively. 5
Baseline and demographic characteristics were generally comparable. 5 The mean (standard deviation) total SIB score at baseline for the MFAS was 69.3 (21.5) and 68.3 (22.8) in the 13.3 mg/24 h (n = 336) and 4.6 mg/24 h (n = 334) patch groups, respectively. 5
Severe Impairment Battery Item Analysis
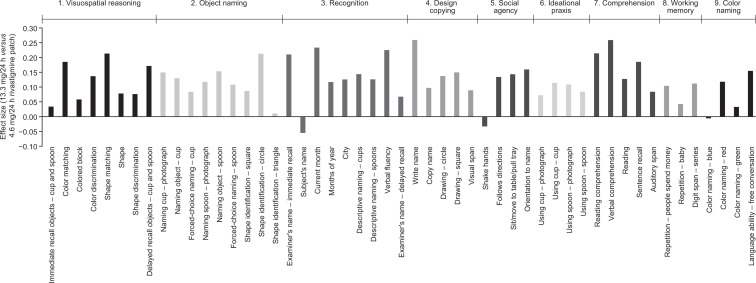
Treatment effect sizes for the majority of SIB items were positive (>0, range: 0.011-0.259), indicating numerically less cognitive decline with the 13.3 versus 4.6 mg/24 h rivastigmine patch at Week 24 (Figure 1).
Figure 1.
Calculated effect sizes (Cohen’s d) based on the mean change from baseline at Week 24 on the individual SIB items (MFAS-LOCF). MFAS-LOCF indicates modified full analysis set with missing data imputed using the last observation carried forward method; SIB, Severe Impairment Battery.
Greatest treatment effect sizes (>0.2, range: 0.210-0.259) were observed on “verbal comprehension” and “write name” (both 0.259), “current month” (0.233), “verbal fluency” (0.225), “reading comprehension” (0.214), “shape identification—circle” and “shape matching” (both 0.213), and “examiner’s name—immediate recall” (0.210; Figure 1). Effect sizes ≥0.1 and <0.2 (range: 0.104-0.185) were observed on 25 items, spanning all of the newly defined domains (Figure 1). Effect sizes ≥0 and <0.1 (range: 0.011-0.097) were observed on 15 items, spanning all domains except social agency.
Negative treatment effect sizes (<0, range: −0.005 to −0.055), indicating numerically less cognitive decline with the 4.6 versus 13.3 mg/24 h rivastigmine patch at Week 24, were observed on “subject’s name” (−0.055), “shake hands” (−0.033), and “color naming—blue” (−0.005).
Severe Impairment Battery Domain Analysis
Factor analysis of the SIB derived 9 new domains, named “visuospatial reasoning,” “object naming,” “recognition,” “design copying,” “social agency,” “ideational praxis,” “comprehension,” “working memory,” and “color naming,” based on the items included in each of these domains (Table 1). The individual items allocated to each newly defined domain, and their corresponding previously defined SIB domain, are summarized in Table 2.
Table 1.
Individual SIB Items Assigned to the Newly Defined SIB Domains Derived by Factor Analysis.a
| Item | Factor 1: Visuospatial Reasoning (16.68)b | Factor 2: Object Naming (1.94)b | Factor 3: Recognition (1.53)b | Factor 4: Design Copying (1.12)b | Factor 5: Social Agency (1.07)b | Factor 6: Ideational Praxis (0.82)b | Factor 7: Comprehension (0.72)b | Factor 8: Working Memory (0.66)b | Factor 9: Color Naming (0.55)b |
|---|---|---|---|---|---|---|---|---|---|
| Shake hands | 0.24 | 0.12 | 0.11 | 0.05 | 0.41 | 0.03 | 0.12 | 0.13 | 0.03 |
| Follows directions | 0.15 | 0.11 | 0.03 | 0.11 | 0.70 | 0.10 | 0.07 | 0.09 | 0.05 |
| Sit/move to table/pull tray | 0.19 | 0.14 | −0.02 | 0.12 | 0.68 | 0.11 | 0.07 | 0.04 | 0.06 |
| Examiner’s name—immediate recall | 0.18 | 0.20 | 0.22 | 0.13 | 0.21 | 0.03 | 0.22 | 0.20 | −0.03 |
| Subject’s name | 0.16 | 0.13 | 0.24 | 0.17 | 0.13 | 0.10 | 0.02 | 0.18 | 0.05 |
| Write name | 0.19 | 0.10 | 0.32 | 0.70 | 0.14 | 0.16 | 0.14 | 0.11 | 0.04 |
| Copy name | 0.18 | 0.13 | 0.20 | 0.70 | 0.14 | 0.12 | 0.18 | 0.08 | 0.07 |
| Current month | 0.11 | 0.01 | 0.32 | 0.10 | 0.04 | 0.15 | 0.10 | −0.01 | 0.03 |
| Months of year | 0.12 | 0.11 | 0.54 | 0.30 | 0.05 | 0.13 | 0.15 | 0.20 | 0.07 |
| City | 0.12 | 0.11 | 0.38 | 0.06 | 0.01 | 0.07 | −0.02 | 0.12 | 0.06 |
| Descriptive naming—cups | 0.13 | 0.34 | 0.59 | 0.04 | 0.03 | 0.14 | 0.07 | 0.08 | 0.02 |
| Descriptive naming—spoons | 0.14 | 0.33 | 0.58 | 0.03 | 0.01 | 0.19 | 0.06 | 0.06 | 0.09 |
| Reading comprehension | 0.20 | 0.14 | 0.17 | 0.16 | 0.13 | 0.19 | 0.56 | 0.04 | 0.10 |
| Verbal comprehension | 0.27 | 0.09 | 0.09 | 0.05 | 0.28 | 0.09 | 0.44 | 0.23 | 0.14 |
| Reading | 0.23 | 0.20 | 0.07 | 0.21 | 0.13 | 0.07 | 0.61 | 0.03 | 0.18 |
| Sentence recall | 0.19 | 0.17 | 0.24 | 0.21 | 0.17 | 0.10 | 0.47 | 0.18 | 0.06 |
| Repetition—people spend money | 0.24 | 0.22 | 0.25 | 0.19 | 0.11 | 0.14 | 0.20 | 0.51 | 0.06 |
| Repetition—baby | 0.18 | 0.25 | 0.10 | 0.04 | 0.19 | 0.09 | 0.06 | 0.45 | 0.10 |
| Digit span—series | 0.25 | 0.20 | 0.17 | 0.19 | 0.17 | 0.13 | 0.12 | 0.55 | 0.10 |
| Verbal fluency | 0.20 | 0.21 | 0.58 | 0.15 | 0.10 | 0.14 | 0.04 | 0.06 | 0.06 |
| Examiner’s name—delayed recall | 0.04 | 0.02 | 0.40 | 0.07 | 0.04 | 0.03 | 0.11 | 0.03 | 0.07 |
| Naming cup—photograph | 0.14 | 0.69 | 0.21 | 0.12 | 0.11 | 0.08 | 0.10 | 0.06 | 0.06 |
| Using cup—photograph | 0.15 | 0.22 | 0.35 | 0.08 | 0.11 | 0.59 | 0.13 | 0.07 | 0.11 |
| Naming object—cup | 0.16 | 0.69 | 0.17 | 0.06 | 0.13 | 0.20 | 0.02 | 0.20 | 0.11 |
| Using cup—cup | 0.17 | 0.26 | 0.17 | 0.17 | 0.21 | 0.55 | 0.14 | 0.10 | 0.07 |
| Forced-choice naming—cup | 0.24 | 0.53 | 0.03 | 0.03 | 0.22 | 0.14 | 0.06 | 0.26 | 0.17 |
| Naming spoon—photograph | 0.13 | 0.70 | 0.28 | 0.12 | 0.09 | 0.14 | 0.16 | 0.04 | 0.13 |
| Using spoon—photograph | 0.19 | 0.18 | 0.28 | 0.13 | 0.04 | 0.67 | 0.13 | 0.11 | 0.05 |
| Naming object—spoon | 0.16 | 0.69 | 0.23 | 0.15 | 0.09 | 0.20 | 0.19 | 0.14 | 0.05 |
| Using spoon—spoon | 0.25 | 0.24 | 0.23 | 0.15 | 0.12 | 0.64 | 0.07 | 0.12 | 0.08 |
| Forced-choice naming—spoon | 0.25 | 0.55 | 0.09 | 0.11 | 0.14 | 0.21 | 0.19 | 0.20 | 0.10 |
| Immediate recall objects—cup and spoon | 0.50 | 0.13 | 0.21 | 0.15 | 0.26 | 0.10 | 0.11 | −0.05 | 0.01 |
| Color naming—blue | 0.23 | 0.30 | 0.19 | 0.14 | 0.24 | 0.06 | 0.09 | 0.08 | 0.36 |
| Color matching | 0.53 | 0.23 | 0.11 | 0.20 | 0.15 | 0.15 | 0.18 | 0.20 | 0.11 |
| Colored block | 0.59 | 0.13 | 0.14 | 0.13 | 0.12 | 0.16 | 0.11 | 0.12 | 0.12 |
| Color discrimination | 0.64 | 0.21 | 0.17 | 0.07 | 0.17 | 0.15 | 0.10 | 0.17 | 0.13 |
| Color naming—red | 0.27 | 0.31 | 0.08 | 0.17 | 0.16 | 0.12 | 0.12 | 0.04 | 0.36 |
| Color naming—green | 0.26 | 0.33 | 0.12 | 0.21 | 0.17 | 0.13 | 0.13 | 0.03 | 0.46 |
| Shape identification—square | 0.30 | 0.32 | 0.26 | 0.31 | 0.04 | 0.08 | 0.15 | 0.17 | 0.32 |
| Shape matching | 0.63 | 0.17 | 0.16 | 0.15 | 0.15 | 0.10 | 0.18 | 0.32 | 0.15 |
| Shape | 0.62 | 0.16 | 0.19 | 0.13 | 0.12 | 0.12 | 0.17 | 0.23 | 0.14 |
| Shape discrimination | 0.64 | 0.21 | 0.17 | 0.11 | 0.12 | 0.14 | 0.16 | 0.18 | 0.14 |
| Shape identification—circle | 0.32 | 0.36 | 0.13 | 0.29 | 0.09 | 0.07 | 0.20 | 0.17 | 0.31 |
| Shape identification—triangle | 0.18 | 0.30 | 0.23 | 0.12 | 0.01 | 0.05 | 0.18 | 0.05 | 0.28 |
| Drawing—circle | 0.28 | 0.21 | 0.13 | 0.40 | 0.11 | 0.11 | 0.17 | 0.18 | 0.30 |
| Drawing—square | 0.29 | 0.16 | 0.16 | 0.50 | 0.04 | 0.16 | 0.22 | 0.11 | 0.32 |
| Auditory span | 0.20 | 0.21 | 0.18 | 0.26 | 0.08 | 0.19 | 0.28 | 0.25 | 0.05 |
| Visual span | 0.31 | 0.19 | 0.19 | 0.37 | 0.08 | 0.17 | 0.34 | 0.11 | 0.12 |
| Delayed recall objects—cup and spoon | 0.54 | 0.11 | 0.19 | 0.17 | 0.24 | 0.12 | 0.15 | −0.07 | 0.06 |
| Orientation to name | 0.09 | 0.07 | 0.08 | −0.02 | 0.52 | 0.06 | 0.19 | 0.14 | 0.39 |
| Language ability—free conversation | 0.15 | 0.09 | 0.18 | 0.08 | 0.39 | 0.07 | 0.17 | 0.18 | 0.41 |
Abbreviation: SIB, Severe Impairment Battery.
a Scores were derived using initial common factor extraction and the principal component method and were based on data from 629 patients. Estimates of loadings were obtained using varimax rotation. SIB items were allocated to factors on the basis of highest score. Items allocated to each factor are shown in bold.
b Eigenvalues.
Table 2.
Allocation of the Individual Items of the SIB Into the Newly Defined 9 Domains.a
| New Subscale | SIB Item | SIB Domain 2 |
|---|---|---|
| Factor 1: Visuospatial reasoning | Immediate recall objects—cup and spoon | Memory |
| Color matching | Visuospatial perception | |
| Colored block | Memory | |
| Color discrimination | Visuospatial perception | |
| Shape matching | Visuospatial perception | |
| Shape | Memory | |
| Shape discrimination | Visuospatial perception | |
| Delayed recall of objects—cup and spoon | Memory | |
| Factor 2: Object naming | Naming cup and spoon—photograph | Language |
| Naming cup and spoon—object | Language | |
| Forced-choice naming—cup and spoon | Language | |
| Shape identification—square, circle, and triangle | Language | |
| Factor 3: Recognition | Examiner’s name—immediate recall | Memory |
| Subject’s name | Orientation | |
| Current month | Orientation | |
| Months of year | Language | |
| City | Orientation | |
| Descriptive naming—cups and spoons | Language | |
| Verbal fluency | Language | |
| Examiner’s name—delayed recall | Memory | |
| Factor 4: Design copying | Write name | Language |
| Copy name | Language | |
| Drawing—circle and square | Construction | |
| Visual span | Attention | |
| Factor 5: Social agency | Shake hands | Social skills |
| Follows directions | Social skills | |
| Sit/move to table/pull tray | Social skills | |
| Orientation to name | Orientation to name | |
| Factor 6: Ideational praxis | Using cup—photograph and cup | Praxis |
| Using spoon—photograph and spoon | Praxis | |
| Factor 7: Comprehension | Reading comprehension | Language |
| Verbal comprehension | Language | |
| Reading | Language | |
| Sentence recall | Memory | |
| Auditory span | Attention | |
| Factor 8: Working memory | Repetition—people spend money and baby | Language |
| Digit span—series | Attention | |
| Factor 9: Color naming | Color naming—blue, red, and green | Language |
| Language ability—free conversation | Language |
Abbreviation: SIB, Severe Impairment Battery.
a SIB domain allocation refers to the 9 domains of the SIB, to which each of the items have been previously assigned. New subscales relate to the newly defined Factors generated from factor analysis of the 40 individual SIB items.
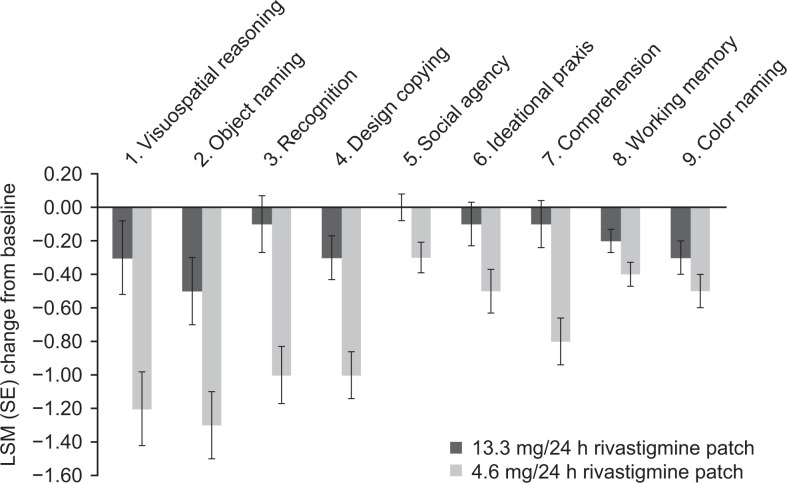
Positive treatment effect sizes (range: 0.125-0.327) were observed on all of the newly defined domains, indicating numerically less cognitive decline with the 13.3 versus 4.6 mg/24 h patch (“visuospatial reasoning,” 0.204; “object naming,” 0.206; “recognition,” 0.327; “design copying,” 0.234; “social agency,” 0.144; “ideational praxis,” 0.135; “comprehension,” 0.294; “working memory,” 0.125; and “color naming,” 0.127).
Based on the calculated least squares mean differences, patients receiving 13.3 mg/24 h patch experienced less decline versus 4.6 mg/24 h patch on the following domains: “visuospatial reasoning,” 0.9, 95% CI: 0.3 to 1.5; “object naming,” 0.7, 95% CI: 0.2 to 1.3; “recognition,” 0.9, 95% CI: 0.5 to 1.4; “design copying,” 0.6, 95% CI: 0.3 to 1.0; “social agency,” 0.3, 95% CI: 0.0 to 0.5; “ideational praxis,” 0.4, 95% CI: 0.0 to 0.7; and “comprehension,” 0.7, 95% CI: 0.3 to 1.1 (Figure 2). In 4 of these domains, patients receiving 13.3 mg/24 h patch showed little to no deterioration from baseline over 24 weeks: “recognition,” −0.1 points; “ideational praxis,” −0.1 points; “comprehension,” −0.1 points; and “social agency,” 0.0 points (all changes are least squares means). The observed treatment differences were relatively smaller on “working memory” and “color naming” (0.2 and 0.2, 95% CI: −0.0 to 0.4 and −0.0 to 0.5, respectively; Figure 2) compared with other domains. Multiple imputation and LOCF results were similar.
Figure 2.
Least squares mean change from baseline at Week 24 on the newly defined SIB domains (MFAS-LOCF). LSM indicates least squares mean; MFAS-LOCF, modified full analysis set with missing data imputed using the last observation carried forward method; SE, standard error; SIB, Severe Impairment Battery.
Discussion
The objective of the current retrospective analysis was to investigate whether the greater efficacy of 13.3 versus 4.6 mg/24 h rivastigmine patch on the SIB observed in the ACTION study 5 was driven by reduced decline in select cognitive functions or a broad effect on a range of SIB items and domains. To address this question, a factor analysis was performed in order to allocate the individual items of the SIB to domains, named based on the predominant cognitive symptoms assessed by these domains. This conventional approach has been used in previous exploratory analyses of clinical data from studies of rivastigmine in patients with AD. 8,11,12 From a practical clinical perspective, a more detailed factor analysis of SIB performance and improvement may help to better contextualize treatment expectations for patients with severe AD and their caregivers, as well as help clinicians monitor clinical progression.
In this population with severe AD, the maximum effective dose of 13.3 mg/24 h rivastigmine patch demonstrated greater efficacy than 4.6 mg/24 h patch on the majority of the newly defined SIB domains and numerically less decline on most individual items, spanning each of the domains. Overall, these results suggest that high-dose 13.3 mg/24 h rivastigmine patch is effective in reducing impairment across a broad range of cognitive aspects assessed by the SIB. Negative treatment effect sizes were observed on 3 SIB items, indicating numerically less cognitive decline with the 4.6 versus 13.3 mg/24 h rivastigmine patch. This was considered most likely attributable to data fluctuation as there was no overlap with the few items suggesting better performance in the placebo group than rivastigmine-treated patients in the previously published SIB factor analysis. 8
The greatest treatment effects with 13.3 versus 4.6 mg/24 h patch were observed on the “comprehension” and “recognition” domains, presumably driven by effect sizes >0.2 on the items “reading comprehension” and “verbal comprehension” within the “comprehension” domain and “examiner’s name—immediate recall,” “current month,” and “verbal fluency” within the “recognition” domain. When looking more broadly at the items that comprise these domains, it is noted that “recognition” includes items from the previously defined memory, orientation, and language subscales, whereas “comprehension” includes items from the language, memory, and attention subscales. 2 Therefore, the extent of treatment-induced change in the cognitive functions assessed using these domains may not have been apparent when using the previously defined SIB subscales. In patients with severe AD, the clinical relevance of these effects may lead to more meaningful interpersonal communication between patients and caregivers.
The greatest between-group differences observed in our current study were on the SIB domains assessing “visuospatial reasoning,” “object naming,” “recognition,” and “design copying.” This is consistent with findings from the previous SIB factor analysis, where the working memory/memory subscale (comprising >50% of items included in the newly defined domains noted above) was the only factor out of 5 that showed significantly less decline with rivastigmine versus placebo. 8 It is also well established that it is relatively easier to perform well on recognition versus working memory tasks, 13 which likely contributed to the differential treatment effect between these 2 domains.
When considering the individual items that comprise the previously defined language subscale, it becomes apparent that 6 of the 9 newly defined domains contain at least 2 items from this subscale. 2,8 It has previously been acknowledged that the language subscale contains items that assess more than just language. 8 The allocation of items within this subscale across multiple domains supports this observation; however, the impact on the ability to detect clinically meaningful treatment effects remains unclear. Although there were differences between our analyses and the previous allocation of the SIB items to subscales, some clear overlap was also observed, for example, the items allocated to “social agency” and “ideational praxis” closely reflect the subscales of social interaction and praxis. 8
Previously, it has been suggested that allocation of items into domains may be influenced by the patient population under study. 12 Although this statement relates to the Alzheimer’s Disease Assessment Scale–cognitive subscale, it may extend to the SIB. Previous factor analyses or principal component analyses of the SIB in patients with severe AD have been reported in the literature, and one such analysis derived 4 factors: a cognitive factor, a praxis and visuospatial functions factor, the reactivity to external stimuli factor, and the social aptitudes factor. 14 Ferris et al.’s previous analysis derived 5 domains (visual, language, working memory/memory, praxis and social skills, and naming). 8 Despite a demographically similar population in terms of age and MMSE at baseline between the latter and the ACTION study, the current analysis derived 9, rather than 5, SIB domains. This may be attributed to other differences in the study populations—a randomized placebo-controlled Spanish study of 3 to 12 mg/d oral rivastigmine compared with a randomized active-controlled study of 13.3 mg/24 h rivastigmine patch conducted in the United States. 5,15 Even so, in both studies, rivastigmine demonstrates broad cognitive efficacy in patients with severe AD. 8 Of note, the sample size in the current study was almost 3-fold that of the previously reported SIB factor analysis (n = 629 vs 210), 8 providing a more comprehensive database for robust observations. It would be interesting to investigate the efficacy of rivastigmine patch in the ACTION study on SIB domains derived by alternate analysis in order to ascertain whether rivastigmine patch demonstrates broad cognitive efficacy in this patient population regardless of the definition of the domain under study. However, it should be noted that the ACTION study was not powered to investigate the efficacy of rivastigmine patch on the domains and individual items of the SIB; therefore, any analyses of this nature are purely intended to be for hypothesis generation.
In conclusion, the high-dose 13.3 mg/24 h rivastigmine patch appears to broadly reduce cognitive decline in patients with severe AD. Understanding the underlying treatment effect profile of rivastigmine in patients with severe AD across items and domains of the SIB may help clinicians to manage treatment expectations and monitor disease progression.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
Author’s Note: Data were previously presented as a poster at the US Psychiatric and Mental Health Congress, Las Vegas, NV, USA, September 30 to October 3, 2013, the 66th American Academy of Neurology Annual Meeting, Philadelphia, PA, USA, April 26 to May 3, 2014, and the Joint Congress of European Neurology, Istanbul, Turkey, May 31 to June 3, 2014. The edited manuscript was reviewed by the authors, and the editing process did not change the original scientific intent of the authors in any meaningful way.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Richard S. Isaacson has served as a scientific advisor/consultant for Novartis and Accera in the past year. Steven Ferris has served as a scientific consultant to companies marketing, developing, or contributing to the development of treatments for cognition, including Accera, Baxter, Bristol-Myers Squibb, Cebria, Dart Neuroscience, Eisai, Janssen AI, Eli Lilly, Lundbeck, Lupin, MedAvante, Merck, Novartis, Neuronix, Neurotrack, Targacept, and United Biosource. His institution has received grant/contract support for clinical trials from Accera, Dart Neuroscience, Eisai, Eli Lilly, Forum, GE Healthcare, Genentech, Janssen, Janssen AI, Lundbeck, Merck, Roche, Neuronix, Takeda, and Bristol-Myers Squibb. He also has stock options from Accera, Intellect Neurosciences, and MedAvante and stock in Lexicon Pharmaceuticals. Drew M. Velting and Xiangyi Meng are full-time employees and stock holders of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ACTION study was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Medical writing and editorial assistance in the development of this manuscript were provided by Katy Tucker at Fishawack Communications Ltd, Oxford, United Kingdom, and this service was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
References
- 1. Saxton J, McGonigle-Gibson K, Swihart A, Miller M, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess. 1990;2(3):298–303. [Google Scholar]
- 2. Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51(1):41–45. [DOI] [PubMed] [Google Scholar]
- 3. Schmitt FA, Ashford W, Ernesto C, et al. The severe impairment battery: concurrent validity and the assessment of longitudinal change in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S51–S56. [PubMed] [Google Scholar]
- 4. Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA Exelon Patch® (rivastigmine tartrate) prescribing information. 2013. Web site: http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf. Accessed July 9, 2014.
- 5. Farlow MR, Grossberg GT, Sadowsky CH, Meng X, Somogyi M. A 24-week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/24 h in severe Alzheimer’s dementia. CNS Neurosci Ther. 2013;19(10):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farlow MR, Grossberg G, Gauthier S, Meng X, Olin JT. The ACTION study: methodology of a trial to evaluate safety and efficacy of a higher dose rivastigmine transdermal patch in severe Alzheimer’s disease. Curr Med Res Opin. 2010;26(10):2441–2447. [DOI] [PubMed] [Google Scholar]
- 7. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 8. Ferris S, Karantzoulis S, Somogyi M, Meng X. Rivastigmine in moderately severe-to-severe Alzheimer’s disease: severe impairment battery factor analysis. Alzheimer’s Res Ther. 2013;5(6):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 11. Alva G, Isaacson R, Sadowsky C, Grossberg G, Meng X, Somogyi M. Efficacy of higher-dose 13.3 mg/24 h (15 cm2) rivastigmine patch on the Alzheimer’s disease assessment scale-cognitive subscale: domain and individual item analysis. Int J Geriatr Psychiatry. 2014;29(9):920–927. [DOI] [PubMed] [Google Scholar]
- 12. Weintraub D, Somogyi M, Meng X. Rivastigmine in Alzheimer’s disease and Parkinson’s disease dementia: an ADAS-cog factor analysis. Am J Alzheimer’s Dis Other Dement. 2011;26(6):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atkinson RC, Shiffrin RM. Human memory: a proposed system and its control processes. In: Spence KW, Spence JT, eds. Psychology of Learning and Motivation. New York: Elsevier; 1968:89–195. [Google Scholar]
- 14. Pelissier C, Roudier M, Boller F. Factorial validation of the severe impairment battery for patients with Alzheimer’s disease. A pilot study. Dement Geriatr Cogn Disord. 2002;13(2):95–100. [DOI] [PubMed] [Google Scholar]
- 15. Lopez-Pousa S, Vilalta-Franch J, Hermandez B, Papatz G. Efficacy of rivastigmine in patients with severe Alzheimer’s disease: a double-blind, randomized pilot study. Brain Aging. 2004;4(2):26–34. [Google Scholar]