Abstract
Oxidative stress is suggested to play a major role in the pathogenesis of Alzheimer’s disease (AD). Among the antioxidants, vitamin C has been regarded as the most important one in neural tissue. It also decreases β-amyloid generation and acetylcholinesterase activity and prevents endothelial dysfunction by regulating nitric oxide, a newly discovered factor in the pathogenesis and progression of AD. However, clinical trials using antioxidants, including vitamin C, in patients with AD yielded equivocal results. The current article discusses the relevance of vitamin C in the cellular and molecular pathogenesis of AD and explores its therapeutic potential against this neurodegenerative disorder.
Keywords: Alzheimer’s disease, vitamin C, oxidative stress
Introduction
In the last decade, the elderly population has expanded rapidly, and the prevalence of neurodegenerative disorders has increased significantly. It has been suggested that the number of people with dementia doubles every 20 years and is projected to be 81.1 million by 2040. 1 In spite of enormous funds being invested in research, definitive disease-modifying drugs have not been discovered, and the exact pathological mechanisms of Alzheimer’s disease (AD) are yet to be revealed. It is becoming clear that oxidative stress plays a major role in the pathogenesis of AD.2–5 Vitamin C is regarded as one of the most important antioxidants that decrease free-radical-mediated damage caused by toxic chain reactions in neural tissue. 6 Moreover, it decreases amyloid β (Aβ) oligomerization, a major cause of neuronal toxicity in AD pathogenesis. 7 Here, we will review the neuroprotective effect and therapeutic efficacy of vitamin C in AD described in the current experimental and clinical literature.
The Metabolism of Vitamin C in the Brain
Vitamin C (ascorbic acid) is a powerful water-soluble antioxidant that protects against oxidative stress and is a cofactor in at least 8 enzymatic reactions. 8 The 6-carbon lactone is synthesized from glucose in most mammalian species except humans, nonhuman primates, and guinea pigs, which lack l-gulonolactone oxidase activity, the final step in ascorbate synthesis. 8
Vitamin C acts as an antioxidant by donating 2 of its electrons, which prevents other compounds from being oxidized. A semidehydroascorbate or ascorbyl radical is formed after loss of 1 electron. 9 However, it is relatively stable compared to other free radicals, and is known as a good free-radical scavenger. Loss of a second electron forms the compound dehydroascorbate (DHA) (Figure 1). 10 Only some of the DHA formed is reduced back to ascorbate, while the rest is metabolized by hydrolysis. 11 One of the metabolites, oxalate, can cause oxalate kidney stones in some individuals.
Figure 1.
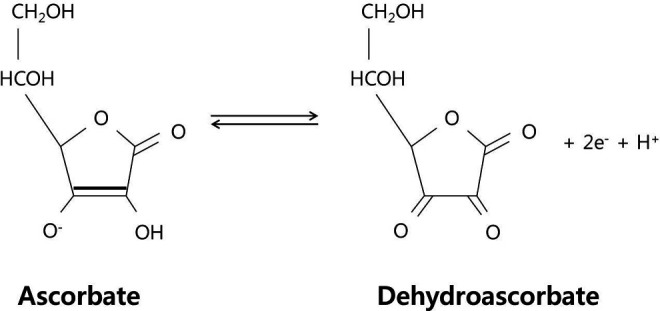
Molecular structure of l-ascorbic acid (the monovalent anion) and dehydroascorbate (oxidation product by the loss of 2 electrons and a proton; adapted from Rice, 2000). 10
Vitamin C homeostasis is highly regulated, with the greatest concentrations found within the brain, spinal cord, and adrenal glands (Figure 2). 10 Vitamin C enters the central nervous system (CNS) via sodium-dependent vitamin C transporter type 2 (SVCT2) from the plasma to the cerebral spinal fluid (CSF) across the epithelium of the choroid plexus. 12 Plasma vitamin C crosses the basolateral membrane into the cells, and intracellular vitamin C then exits the cells into the CSF through the apical membrane of the ependymal cells of the choroid plexus. This transcellular transport system results in a 4-fold plasma to CSF vitamin C gradient in rats (200-400 µmol/L in CSF, 60 µmol/L in plasma). 13 Similarly, the concentration of CSF vitamin C in humans (160 µmol/L) shows a pronounced gradient compared to plasma values of 40 to 60 µmol/L. 13 In addition, DHA can enter the CNS more rapidly than vitamin C via glucose transporter 1 (GLUT1) in the blood–brain barrier (BBB) endothelium. 14
Figure 2.
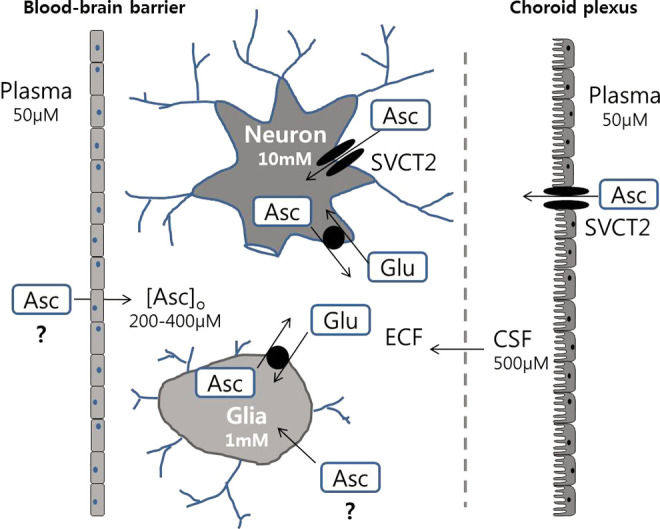
The vitamin C regulation in the central nervous system (CNS). Vitamin C enters from the blood into cerebrospinal fluid (CSF) by sodium-dependent vitamin C transporter type 2 (SVCT2) in the choroid plexus, and extracellular fluid (ECF) by carrier-mediated uptake and simple diffusion across brain capillaries (adapted from Rice, 2000). 10
Vitamin C in the CSF enters the brain interstitium by diffusion, and then enters the neurons and glia by 2 mechanisms. 15 The first, SVCT2 on the plasma membranes transports vitamin C across the membrane. The second, vitamin C becomes oxidized to DHA which then enters the cell via GLUT transporters. Subsequently, DHA is reduced back into vitamin C within the cell. Although neurons may use both the mechanisms, the high intracellular vitamin C concentration (approximately10 µmol/L) is likely maintained by SVCT2 that is located abundantly in the neurons of the cerebral cortex, hippocampus, and cerebellum. 16 However, SVCT2 is only found in neurons and not in astrocytes. This suggests that DHA uptake and reduction is the only mechanism of vitamin C uptake in astrocytes. 17
Vitamin C Against AD
Experimental Studies Supporting the Efficacy of Vitamin C in AD Pathogenesis
Oxidative stress is suggested to play a major role in the pathogenesis of AD. 18 Oxidation of mitochondrial DNA and nuclear DNA has been detected in the parietal cortex of patients with AD, and marked lipid peroxidation has also been observed in the temporal cortex.19,20 In addition, the end products of peroxidation—peroxynitrite, superoxide dismutase 1, and heme oxygenase 1—were identified in the brains of patients with AD, especially within neurofibrillary tangles and senile plaques.21–23 Moreover, heme oxygenase 1, a cellular stress protein expressed in the brain and other tissues in response to oxidative challenges, is overexpressed in neurons and astrocytes of the hippocampus and cerebral cortex. 23
Vitamin C is an essential nutrient for a range of metabolic reactions in all animals and plants and a well-known antioxidant that protects the body against oxidative stress. 24 One study reported lower plasma levels of vitamin C in patients with AD despite adequate dietary intake. 25 Most plasma components in humans are diluted about 100-fold in CSF, whereas vitamin C is highly concentrated, especially in AD. 26 The CSF to serum vitamin C ratio is 3.1 and 5.1 in control participants and patients with AD, respectively. These results are comparable to previous reports of a CSF to serum vitamin C ratio of 4.4 for healthy individuals and 5.8 for neurologically ill patients. 27 The authors hypothesized that the oxidatively stressed AD brain consumes more vitamin C, which leads to its lower plasma levels.
In an amyloid precursor protein/presenilin protein 1 (APP/PSEN1) transgenic and wild-type mouse study, parenterally administered vitamin C did not alter Alzheimer-like neuropathologic features of plaque deposition, oxidative stress, or acetylcholinesterase activity. 28 Therefore, the authors suggested that vitamin C only possessed nootropic properties, whereby an acute elevation of its levels modulate neurotransmitter function. On the other hand, several studies provided evidence to support a therapeutic role of vitamin C in AD.29,30 Orally administered vitamin C reduced oxidative stress and proinflammatory cytokines induced by Aβ peptide injections in the CA1 area of the hippocampus in rat brains. 29 β-Amyloid exposure leads to the apotosis and death of cultured neurons by the generation of reactive oxygen species (ROS).30,31 In a study testing whether vitamin C might protect cultured SH-SY5Y neuroblastoma cells from apoptotic cell death, vitamin C pretreatment prevented apoptosis as well as cell death due to β-amyloid. 30 Additionally, vitamin C-pretreated cells also showed a decrease in basal rates of endogenous β-amyloid generation. Furthermore, vitamin C has been reported to decrease acetylcholinesterase activity in mice, an action analogous to that of the more common antidementic drugs. 32 Vitamin C was also demonstrated to affect the release of acetylcholine from synaptic vesicles. 33
More recently, the nitric oxide (NO)-catalyzed release of anhydromannose (anMan)-containing oligosaccharides from glypican 1-nitrosothiol in the presence of vitamin C has been reported. 34 In in vitro assays, anMan-containing heparan sulfate oligo- and disaccharide preparations suppressed A11 immunoreactivity and the oligomerization of Aβ42 peptides. A temporary interaction between the Aβ domain and small, anMan-containing oligosaccharide may preclude the formation of toxic Aβ oligomers. In addition, Murakami et al revealed that AD mice treated with a vitamin C solution showed mitigation of Aβ oligomer formation and behavioral decline. 7
These results may indicate that an inadequate supply of vitamin C could contribute to the development of sporadic AD.
Epidemiological Evidence Against Vitamin C in AD Pathogenesis
In contrast to the beneficial effects expected from the laboratory investigations outlined above, human epidemiologic evidence for delaying AD onset or progression have been mixed at best.
In one longitudinal study of 3385 Japanese-American men living in Hawaii, both vitamin C and E supplement use showed a significant protective effect in vascular dementia, but not in Alzheimer’s dementia. 35 Another study by Morris et al examined whether the intake of antioxidant nutrients, vitamin E, vitamin C, and β-carotene were associated with the incident AD. The study included 815 participants followed for a mean of 3.9 years from 3 contiguous neighborhoods in the south side of Chicago. 36 They concluded that the intake of vitamin C, β-carotene, and vitamin E supplements were not significantly associated with risk of reduction of AD. Luchsinger et al also investigated the relationship between AD and the intake of carotenes, vitamin C, and vitamin E in 980 elderly participants in the Washington Heights-Inwood Columbia Aging Project. 37 During a 4-year follow-up, there were 242 incident cases of AD in 4023 person-years of follow-up. Neither dietary, supplemental, nor total intake of carotenes and vitamins C and E showed any benefit in AD development.
On a positive note, the Rotterdam Study showed that individuals with a higher intake of antioxidants at baseline had a lower incidence of AD. 38 The study involved 5395 participants who were at least 55 years old, free of dementia, and had a reliable dietary assessment. After a mean follow-up of 6 years, 146 developed AD. Their data revealed an association between high vitamin C and vitamin E intake and a lower risk of AD. In addition, in the Cache County Study intake of both vitamins C and E as dietary supplements was associated with a lower incidence of AD. 39 The cross-sectional prospective study included 4740 county elderly residents and identified 200 prevalent cases of AD from 1995 to 1997. Among the 3227 survivors, 104 incident AD cases developed at follow-up from 1998 to 2000. The study revealed that the use of vitamin E and C supplements in combination was associated with reduced AD prevalence and incidence, but there was no evidence of a protective effect with the use of vitamin E or vitamin C supplements alone.
In the study of dietary supplements usage for cognitive health, however, adults reporting a cognitive problem tended to use supplements more likely than others. 40 They could have a vulnerable brain to dementia and this could partially explain the discrepancies between precise laboratory and vague epidemiologic studies. Moreover, short durations, limited sample sizes, and a focus on younger-old population in prospective or randomized studies than real state could limit the usefulness of the results. 41
Vitamin C and Endothelial Function
Endothelial Dysfunctions in AD
Recent studies suggest a relationship between cerebrovascular and AD pathologies. 42 In epidemiologic studies, the presence of cerebrovascular pathology seems to play an important role in the development of the clinical symptoms of AD.43,44 In a study of 1015 participants of the prospective, population-based Rotterdam study, the presence of silent stroke more than doubled the risk of AD. 45 The risk of either AD or vascular dementia was higher in people with elevated blood pressure, which suggested that artery stiffness and severe atherosclerosis play an important role in AD and vascular dementia. 46 It has been suggested that endothelial dysfunction contributes to increased arterial stiffness in patients with systolic hypertension. 47 Moreover, arterial stiffness is a strong predictor of cognitive decline. 48 The monolayer of brain endothelial cells making up capillaries is the main component of the BBB. 49
In line with these findings, endothelial dysfunction is one of the major factors in the pathogenesis and progression of AD. 42 In a case–control study, the endothelial function, evaluated with flow-mediated dilatation of the brachial artery, was impaired in patients with AD and worse with severe AD. 50 Moreover, Aβ is known to be cleared from the brain across the BBB.51,52 The Aβ concentration in the brain is regulated by its influx into the brain across the BBB via receptor for advanced glycation end products (RAGEs) and clearance across the BBB via low-density lipoprotein (LDL) receptor-related protein 1 (LRP-1). 52 In patients with AD, brain endothelial LRP-1 expression in the BBB is reduced, whereas RAGE expression is increased, which could facilitate Aβ accumulation in the brain.53,54 These data suggested an essential role of the endothelial cell integrity lining the BBB in the onset and progression of AD.
Endothelial Protection by Vitamin C
Many studies reported a correlation between vitamin C and BBB integrity. In in vitro and in vivo studies, vitamin C ameliorated BBB disruption.55,56 The efficacy of vitamin C as a potent ROS scavenger was demonstrated in rat brains as it protected against BBB breakdown caused by cortical compression. 55
Endothelial dysfunction and BBB breakdown occur during ischemic-reperfusion, and reduced bioactivity of NO has been suggested to be a key step in tissue injury during reperfusion. 57 The NO produced by the endothelium is known to inhibit many of the pathogenic mechanisms of atherosclerosis including monocyte adhesion, platelet aggregation, endothelial permeability, and vascular smooth muscle cell proliferation. 58 Interestingly, there have been several suggested mechanisms by which vitamin C might prevent endothelial dysfunction by the generation or metabolism of NO. 59 First, vitamin C prevents endothelial dysfunction by inhibiting LDL oxidation. 60 Oxidized LDL is known to increase the permeability of the vascular endothelium, which is ameliorated by vitamin C. 61 Second, vitamin C could induce the release of NO from S-nitrosothiols in the plasma. 62 Third, vitamin C can reduce nitrite (NO2−) to NO, which may preserve NO in tissues or plasma. 63 Fourth, superoxide generated by endothelial cells reacts with NO to form cytotoxic peroxynitrite. 64 Vitamin C could decrease NO consumption by scavenging superoxide. 65 Fifth, vitamin C regulates endothelial NO synthase as a cofactor or through tetrahydrobiopterin, which results in NO production. 66 In addition to its action on NO, administration of vitamin C prevented the impaired response to the vasodilator acetylcholine (endothelium-dependent agonist) and reduced ROS (eg superoxide) produced by neutrophils. 67
Conclusion
In this review, we examined several aspects of the biological and clinical significance of vitamin C on AD. (1) It is evident that vitamin C has shown protective and therapeutic efficacy against oxidative injury. (2) Its impact on AD pathology has been demonstrated by numerous basic and clinical studies. (3) Moreover, the efficacy of vitamin C on BBB dysfunction has been suggested as a new possible therapeutic mechanism in AD. The Aβ immunotherapy, which is currently being actively pursued, also suggests that the efficacy of Aβ clearance is determined by the levels of cell surface Aβ receptors in the BBB. (4) The clinical impact of antioxidants including vitamin C on AD is yet to be firmly established. Additional clinical investigations examining the dose and duration of vitamin C intake are certainly worthwhile. Combining other antioxidants with vitamin C may prove beneficial for AD prevention by providing a more comprehensive activation of pathways to reduce oxidative stress.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the financial support for the research, authorship, and/or publication of this article: the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2011-0018267, 20120005823).
References
- 1.Ferri CP, Prince M, Brayne C, et al. Alzheimer’s disease international: global prevalence of dementia: a delphi consensus study. Lancet. 2005;366(9503):2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Y, Chinnici C, Tang H, Trojanowski JQ, Lee VM, Pratico D. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. J Neuroinflammation. 2004;1(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhry F, Howlett DR, Richardson JC, Francis PT, Williams RJ. Pro-oxidant diet enhances beta/gamma secretase-mediated APP processing in APP/PS1 transgenic mice. Neurobiol Aging. 2012;33(5):960–968. [DOI] [PubMed] [Google Scholar]
- 4.Murakami K, Shimizu T. Cytoplasmic superoxide radical: a possible contributing factor to intracellular Aβ oligomerization in Alzheimer disease. Commun Integr Biol. 2012;5(3):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman GL. Ascorbic acid, cognitive function, and Alzheimer’s disease: a current review and future direction. Biofactors. 2012;38(2):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Wang X. Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev. 2012;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami K, Murata N, Ozawa Y, et al. Vitamic C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;26(1):7–18. [DOI] [PubMed] [Google Scholar]
- 8.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. [DOI] [PubMed] [Google Scholar]
- 9.Nualart FJ, Rivas CI, Montecinos VP, et al. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278(12):10128–10133. [DOI] [PubMed] [Google Scholar]
- 10.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–216. [DOI] [PubMed] [Google Scholar]
- 11.Lewin S. Vitamin C: Its Molecular Biology and Medical Potential. London, England: Academic Press; 1976. [Google Scholar]
- 12.Angelow S, Haselbach M, Galla HJ. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003;988(1-2):105–113. [DOI] [PubMed] [Google Scholar]
- 13.Spector R. Vitamin homeostasis in the central nervous system. N Engl J Med. 1977;296(24):1393–1398. [DOI] [PubMed] [Google Scholar]
- 14.Farrell CL, Yang J, Pardridge WM. GLUT-1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. J Histochem Cytochem. 1992;40(2):193–199. [DOI] [PubMed] [Google Scholar]
- 15.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46(6):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mun GH, Kim MJ, Lee JH, et al. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J Neurosci Res. 2006;83(5):919–928. [DOI] [PubMed] [Google Scholar]
- 17.Castro M, Caprile T, Astuya A, et al. High-affinity sodium-vitamin C co-transporters (SVCT) expression in embryonic mouse neurons. J Neurochem. 2001;78(4):815–823. [DOI] [PubMed] [Google Scholar]
- 18.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621–9. [DOI] [PubMed] [Google Scholar]
- 19.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36(5):747–751. [DOI] [PubMed] [Google Scholar]
- 20.Marcus DL, Thomas C, Rodriguez C, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol. 1998;150(1):40–44. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17(8):2653–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of antioxidant stress in Alzheimer’s disease. Am J Pathol. 1992;140(3):621–628. [PMC free article] [PubMed] [Google Scholar]
- 23.Schipper HM, Cisse S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol. 1995;37():758–768. [DOI] [PubMed] [Google Scholar]
- 24.Naseer MI, Ullah I, Ullah N, et al. Neuroprotective effect of vitamin C against PTZ induced apoptotic neurodegeneration in adult rat brain. Pak J Pharm Sci. 2011;24(3):263–268. [PubMed] [Google Scholar]
- 25.Riviere S, Birlouez-Aragon I, Nourhashemi F, Vellas B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13(11):749–754. [DOI] [PubMed] [Google Scholar]
- 26.Quinn J, Suh J, Moore MM, Kaye J, Frei B. Antioxidants in Alzheimer’s disease-vitamin C delivery to a demanding brain. J Alz Dis. 2003;5(4):309–313. [DOI] [PubMed] [Google Scholar]
- 27.Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin Chim Acta. 1993;217(2):163–173. [DOI] [PubMed] [Google Scholar]
- 28.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009;93(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosales-Corral S, Tan DX, Reiter RJ, et al. Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J Pineal Res. 2003;35(2):80–84. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, May JM. Ascorbic acid protects SH-SY5Y neuroblastoma cells from apoptosis and death induced by beta-amyloid. Brain Res. 2006;1097(1):52–58. [DOI] [PubMed] [Google Scholar]
- 31.Abe T, Tohgi H, Isobe C, Murata T, Sato C. Remarkable increase in the concentration of 8-hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. J Neurosci Res. 2002;70(3):447–450. [DOI] [PubMed] [Google Scholar]
- 32.Dhingra D, Parle M, Kulkarni SK. Comparative brain cholinesterase-inhibitng activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J Med Food. 2006;9(2):281–283. [DOI] [PubMed] [Google Scholar]
- 33.Kuo CH, Hata F, Yoshida H, Yamatodani A, Wada H. Effect of ascorbic acid on release of acetylcholine from synaptic vesicles prepared from different species of animals and release of noradrenaline from synaptic vesicles of rat brain. Life Sci. 1979;24(10):911–915. [DOI] [PubMed] [Google Scholar]
- 34.Cheng F, Cappai R, Ciccotosto GD, et al. Suppression of amyloid beta A11 antibody immunoreactivity by vitamin C: possible role of heparin sulfate oligosaccharides derived from glypican-1 by ascorbate-induced, nitric oxide (NO)-catalyzed degradation. J Biol Chem. 2011;286(31):27559–27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54(6):1265–1272. [DOI] [PubMed] [Google Scholar]
- 36.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230–3237. [DOI] [PubMed] [Google Scholar]
- 37.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. [DOI] [PubMed] [Google Scholar]
- 38.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2001;287(24):3223–3229. [DOI] [PubMed] [Google Scholar]
- 39.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in uvsers of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61(1):82–88. [DOI] [PubMed] [Google Scholar]
- 40.Laditka JN, Laditka SB, Tait EM, Tsulukidze MM. Use of dietary supplements for cognitive health: results of a national survey of adults in the United States. Am J Alzheimers Dis Other Demen. 2012;27(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704–16. [DOI] [PubMed] [Google Scholar]
- 42.Bomboi G, Castello L, Cosentino F, Giubilei F, Orzi F, Volpe M. Alzheimer’s disease and endothelial dysfunction. Neurol Sci. 2010;31(1):1–8. [DOI] [PubMed] [Google Scholar]
- 43.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 44.Pasquier F, Henon H, Leys D. Relevance of white matter changes to pre- and poststroke dementia. Ann N Y Acad Sci. 2000;903:466–469. [DOI] [PubMed] [Google Scholar]
- 45.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. [DOI] [PubMed] [Google Scholar]
- 46.Qiu C, Winblad B, Viitanen M, Fratigliioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community based, longitudinal study. Stroke. 2003;34(3):594–549. [DOI] [PubMed] [Google Scholar]
- 47.Wallace SM, Yasmin McEniery CM, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50(1):228–233. [DOI] [PubMed] [Google Scholar]
- 48.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–1040. [DOI] [PubMed] [Google Scholar]
- 49.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dede DS, Yavuz B, Yavuz BB, et al. Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? Geriatr Soc. 2007;55(10):1613–1617. [DOI] [PubMed] [Google Scholar]
- 51.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8(1):16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MC, Tavares R, Johanson CE, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112(4):405–415. [DOI] [PubMed] [Google Scholar]
- 55.Lin JL, Huang YH, Shen YC, Huang HC, Liu PH. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J Cereb Blood Flow Metab. 2010;30(6):1121–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun. 2011;404(2):701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao PS, Aoki N, Lefer DJ, Johnson III G, Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990;82(4):1402–1412. [DOI] [PubMed] [Google Scholar]
- 58.Wever R, Stroes E, Rabelink TJ. Nitric oxide and hypercholesterolemia: a matter of oxidation and reduction? Atherosclerosis. 1998;137(suppl):S51–S60. [DOI] [PubMed] [Google Scholar]
- 59.May JM. How does ascorbic acid prevent endothelial dysfunction. Free Radic Biol Med. 2000;28(9):1421–1429. [DOI] [PubMed] [Google Scholar]
- 60.Kaneko T, Kaji K, Matsuo M. Protective effect of lipophilic derivatives of ascorbic acid on lipid peroxide-induced endothelial injury. Arch Biochem Biophys. 1993;304(1):176–180. [DOI] [PubMed] [Google Scholar]
- 61.May JM, Qu ZC. Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res. 2010;44(11):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scorza G, Pietraforte D, Minetti M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-gluthathione in human plasma. Free Radic Biol Med. 1997;22(4):633–642. [DOI] [PubMed] [Google Scholar]
- 63.Millar J. The nitric oxide/ascorbate cycle: how neurons may control their own oxygen supply. Med Hypotheses. 1995;45(1):21–26. [DOI] [PubMed] [Google Scholar]
- 64.Ischiropoulos H. Biological tyrosine nitrationa pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356(1):1–11. [DOI] [PubMed] [Google Scholar]
- 65.Bendich A, Machlin LJ, Scandurra O, Burton GW, Wayner DM. The antioxidant role of vitamin C. Adv Free Radic Biol Med. 1986;2:419–444. [Google Scholar]
- 66.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–47. [DOI] [PubMed] [Google Scholar]
- 67.Pleiner J, Schaller G, Mittermayer F, et al. Intra-arterial vitamin C prevents endothelial dysfunction caused by ischemia-reperfusion. Atherosclerosis. 2008;197(1):383–391. [DOI] [PubMed] [Google Scholar]


