Abstract
As well known to the scientific community, Alzheimer’s disease (AD) is an irreversible neurodegenerative disease that ends up with impairment of memory and cognition due to neuronal and synapse loss. Patient’s quality of life can be enhanced by targeting neurogenesis as a therapeutic paradigm. Moreover, several research evidences support the concept that AD is a type of metabolic disorder mediated by impairment in brain insulin responsiveness and energy metabolism. Growing evidence suggests that endogenous peptides such as glucagon-like peptide-1 (GLP-1) and stromal-derived factor-1α (SDF-1α) provide neuroprotection across a range of experimental models of AD. So, preserving functional activity of SDF-1α and GLP-1 by dipeptidyl peptidase-4 inhibition will enhance the homing/recruitment of brain resident and nonresident circulating stem cells/progenitor cells, a noninvasive approach for promoting neurogenesis. So, herewith we provide this in support of dipeptidyl peptidase-4 inhibitors as a new target of attention for treating AD.
Keywords: Alzheimer’s, chemotaxis, insulin resistance, neurogenesis, synaptic plasticity, type 3 diabetes
Introduction
From disease enlightening days, neurodegeneration in Alzheimer’s disease (AD) is dominantly hypothesized with lesions in hippocampus characterized by toxic amyloid beta (Aβ) species and phosphorylated Tau aggregation. Cascade of results from different experiments including clinical studies had provided supportive evidence for the presence of insulin resistance in AD. In AD, brain loses its capacity for energy production by utilizing glucose efficiently due to insulin resistance and lack of signals for various trophic factors such as insulin-like growth factor (IGF). 1 -8 Previously, the brain was considered as an insulin-insensitive organ, but modern scientific research provides evidence for the wide distribution of insulin and its receptors in brain and it’s critical role in maintaining metabolic, neurotrophic, neuromodulatory, and neuroendocrine responses, as well as in memory and learning processes. 9,10
Biochemical and molecular-level consequences that occur because of resistance to insulin and IGF in AD brain are comparable and similar to either extraneuronal organs or tissues. Lack of this proper signaling costs neuronal survival and synaptic plasticity in addition to downregulation of cholinergic function. 10,11 The above-mentioned resistance was manifested by lack of or reduced levels of responsiveness when the respective receptors were stimulated with their particular ligand. 6,12,13 The deficit in the levels of insulin/IGF associates with alter/improper expression of insulin and IGF polypeptides in the brain as well as in cerebrospinal fluid. 3,6,12 Moreover, postmortem brain studies of clinically characterized patients with AD evidenced the deficiency and resistance to insulin/IGF along with impaired functionalities in signal transduction mechanisms. 6,12 These sort of findings had given enough support to the concept which emphasizes chronic impairment in brain insulin signaling can mediate AD pathogenesis. Hyperglycemia and insulin resistance in parallel with hyperinsulinemia are widely accepted and established responsible factors and features of type 2 diabetes mellitus (T2DM), 14 and this insulin resistance might be a forgotten link between AD and T2DM.
The defective insulin signaling might be the cause for increased density of insulin receptors (IRs) in AD brain than with age-matched controls. Insulin-degrading enzyme (IDE) has been reported to be involved in degradation and clearance of Aβ in the brain. 15 Both insulin and Aβ are the substrates of IDE, of which insulin has a high affinity for IDE. Initially, because of feedback mechanism, insulin levels increase during resistance as it can move across the blood–brain barrier (BBB) from the periphery. This leads to a slow accumulation of Aβ. 16,17 Moreover, elevated levels of butyrylcholinesterase may act as a key triggering factor for the initiation of the inflammatory processes observed in T2DM and AD via the downregulation of the cholinergic anti-inflammatory pathway. 18 Oxidative stress in DM affects adenosine triphosphate production by mitochondria, which causes neuronal dysfunction due to lack of energy source. 19
Liu et al also revealed influence of decreased insulin in the brain in promoting AD pathology by enhancing hyperphosphorylation of Tau protein through the downregulation of insulin–phosphoinositide 3-kinase–protein kinase B (AKT) signaling. 20
So, we can say that AD is a type of diabetes, as dysregulation of insulin in brain contributes to AD by promoting accumulation of Aβ and neurofibrillary tangles. Even though T2DM has not yet been concluded as a considerable risk factor for the development of AD, the concept of checking/testing drugs of the prior disease for the later has been aroused.
Epidemiology Between T2DM and AD
The incidence of T2DM and aging population become a persistent global problem. Unexpected increase in T2DM cases may be due to increase in physically inactive life style and obesity. Type 2 diabetes mellitus is no more a disease of old age as it is emerging as serious threat for younger also.
Pancreas of diabetic patients are characterized by deposits of amyloid protein formed due to insulin resistance, which contains islet amyloid polypeptide (IAPP), 21,22 and moreover, it’s the fact that IAPP and amyloid precursor protein (APP) are almost structurally similar (90%). 23
Modern scientific reports disclose the possibility of insulin desensitization in the brain and postanalyzed reports from numerous patient databases linked the T2DM as a possible causative factor for neurodegeneration in AD. 24 And moreover several epidemiological studies (Table 1) were concluded by correlating T2DM with the possibility of developing AD at later stage of life. 14,25 -27 One such study from Mayo clinic had drawn clear correlation between T2DM and AD, in which significant number of patients with AD have T2DM when compared with age-matched control. 28 Propensity of patients with T2DM for AD has been identified by another study. 23 Efficacy and sensitivity of insulin in brain is important to get rid of AD, as insulin signaling desensitization was noticed in Alzheimer’s brains 3,29,30 and also biochemical profile is similar to diabetic brains.
Table 1.
Epidemiological Studies on Type 2 Diabetes Mellitus as a Risk Factor for Alzheimer’s Disease.
| Study Name | Sample Size | Follow-Up Period, years | Prone Factor | Reference |
|---|---|---|---|---|
| Kungsholmen project | 1301 | 6 | T2DM, APOE4 allele | 101 |
| – | 1262 | 4.3 | T2DM | 25 |
| The Honolulu-Asia aging study | 2574 | 25 | T2DM | 102 |
| Rotterdam | 6370 | 3 | T2DM | 103 |
| – | 1455 | 15 | T2DM | 28 |
| Canadian study | 5574 | 5 | T2DM | 104 |
| – | 824 | 9 | T2DM | 105 |
| – | 3774 | 15 and 25 | T2DM | 106 |
| Framingham study | 2210 | 20 | DM | 107 |
| – | 71433 | 11 | DM | 108 |
Abbreviations: T2DM, type 2 diabetes mellitus. APOE4, Apolipoprotein E4.
In a different study, patients with AD have been noticed with downregulation and phosphorylation of IRs, 12 and moreover, internalization of IRs in the neurons and reduced levels of insulin receptor substrate-1 and insulin receptor substrate-2 were noticed in histological studies. 31 So, the possible roles of insulin and its resistance along with receptor action became point of study/research target to understand the T2DM–AD link. Although IR has its niche role in AD pathology, clinical study done by Isik and Bozoglu proved the cognitive improvement in patients with AD, that is, independent of IR, which indicates the involvement of complexity in the pathology of AD and difference in neuronal and peripheral insulin resistance. 32
Dipeptidyl Peptidase-4, Its Inhibitors/Gliptins, and Their Expected Importance for AD
Although improper metabolism of APP is responsible for AD, density of Aβ plaques cannot be correlated with cognitive decline always 33 as there were instances where absence of brain atrophy symptoms in subjects with Aβ plaques 34 and tolerability of highly educated persons to Aβ plaques without considerable cognitive impairment. 35 Therefore, although APP metabolic dysfunction might be necessary/essential, it may not alone be sufficient to cause AD. Strategies like targeting APP through α, β, γ-secretases and various immunotherapies, and so on, have end up with severe adverse events (AEs), and modern research diverts in the promotion of neurogenesis and regain of synaptic plasticity, which is main concern in AD. 36,37 So, dipeptidyl peptidase-4 (DPP4) inhibitors, an Aβ-independent therapeutic strategy, can be a considerable choice as its substrates glucagon-like peptide-1 (GLP-1) and stromal-derived factor-1α (SDF-1α) can provide positive outcome.
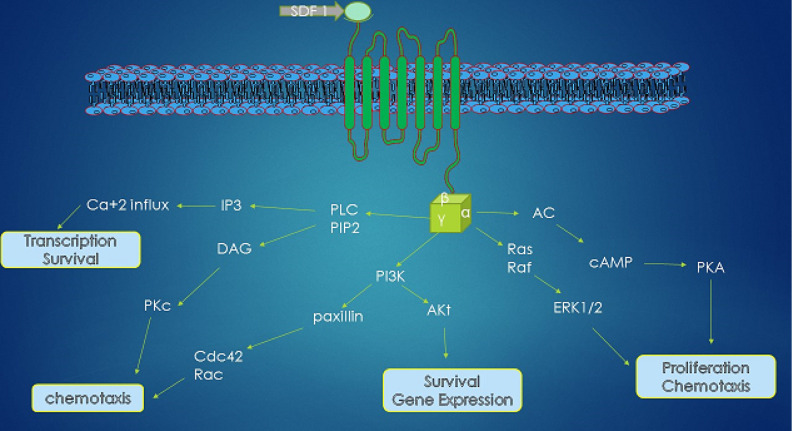
Figure 1.
Chemokine receptor 4 (CXCR 4)–mediated signaling pathway. Stromal-derived factor-1 binding activates the G-protein-coupled receptor CXCR 4. Gai protein–mediated signaling involves various downstream pathways activation of calcium efflux, protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K)/AKT pathway. PKC and PI3K are capable of phosphorylation of important focal adhesion components (Pyk-2, p130Cas, paxillin) regulating (i) cell migration and (ii) chemotaxis of stem cells. Signaling through the PI3K-AKT mediates (iii) cell proliferation and (iv) cell survival.
The existence of DPP4, a serine protease that belongs to S9b protein family, was first revealed by Hopsu-Havu and Glenner 38 as a glycylproline β-naphthylamidase. It was expressed on various cell types like epithelium of renal proximal tubules, intestine, corpus luteum, TH cells, and also on the subsets of macrophages and as a soluble form in semen, plasma, and urine. 39 -43 Multiview structure and amino acid arrangement of this enzyme were well established by many crystallographic studies. The active site of the enzyme lies in large inner cavity, which is formed by α/β hydrolase domain and β propeller domain. 44,45 The entry and exit of the ligands to the active site occur through the “side opening” that connects inner cavity with solvent.
Even information regarding any change in the activity and levels of DPP4 during AD needs scientific evaluation, and elevation in its levels has been proved in T2DM. Moreover, one of the important inflammatory mediators, tumor necrosis factor-α, one of the prone factors for AD, has been proved to augment the soluble form of the enzyme (DPP4). 46 The enzyme acts on several natural substrates such as chemokines, cytokines, neuropeptides, circulating hormones, and bioactive peptides, 47 which acts as regulatory switches for peptide hormonal metabolism and amino acid transport. 48 This serine protease causes the activation or inactivation of its substrates by acting at proline or alanine at the N-terminal penultimate position. 49
Glucagon-like peptide-1 and SDF-1 are important substrates of DPP4. So, that circulating time/half-life of these 2 agents drastically reduce by DPP4, which rendered them not to execute their functions. So, by using DPP4 inhibitors, life span of these 2 can be increased.
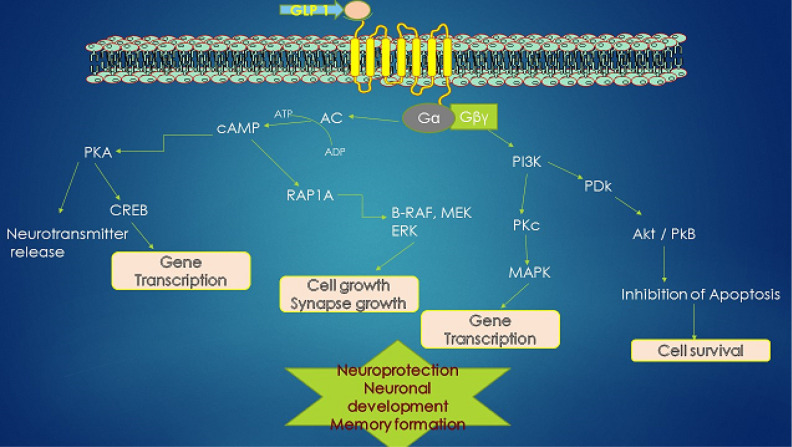
Figure 2.
Glucagon-like peptide 1 (GLP-1) receptor activation in neurons influences various downstream pathways in promoting neuroprotection. Stimulation of the GLP-1 receptor (GLP-1 R) activates adenylyl cyclase, which causes the elevation in Cyclic adenosine monophosphate Pyk-tyrosine kinase (cAMP) that causes the activation of protein kinase A (PKA) and phosphoinositide 3-kinase (PI3K), which in turn activates various downstream signaling pathways through phosphorylation: the mitogen-associated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and PI3K/protein kinase B (AKT) pathways. These activated pathways promote the modulation of intracellular events like activation of calcium channels, protein synthesis, cellular proliferation, and mitochondrial biogenesis and also promote the inhibition of apoptosis, inflammation, and protein aggregation.
Role of Chemokines
Chemokines (chemotactic cytokines) are tiny low-molecular heparin-binding proteins that involve in pro-inflammatory mediation and maintenance or development of various tissues. Functional specificity and types are based on their primary structure and divided as α (CXC), β (CC), γ (CX3C), and δ (C). Their classification is mainly based on the number of amino acids separating 2 conserved cysteine residues . 50,51 In the brain, chemokines involve in important roles like formation and maintenance of the neuronal network integrity and supporting synaptic transmission, and their implications were discussed in the pathological profile of different neurodegenerative diseases such as multiple sclerosis or AD. 52 -54
Redundancy in the action of chemokines may be due to binding of specific chemokine to different receptors of chemokine family because of overlap and interaction. 55 Stromal cell–derived factor SDF-1α/CXCL12 is an important chemokine that dragged its attention due to its crucial role in the neurogenesis of the central nervous system (CNS) 56 and binds only to chemokine receptor 4 (CXCR 4) through amino terminus for executing signals, 57 although an orphan receptor, RDC1 was also claimed for binding but its involvement in cell trafficking regulation needs to be confirmed. 58
Two different forms of SDF-1 are SDF-1α and SDF-1β for which major functional difference is not yet claimed but carboxy terminus of later is having 4 extra amino acids. 59,60 But Stumm et al, have showed the contribution of SDF-1β and SDF-1α for leukocyte infiltration and neuronal repair, respectively. 61 CXCL12 involve in various discrete developmental processes that include haematopoiesis, 62 vascular, 63 and neurogenesis, 64 along with maintenance of tissue stem cells as well. 64
Table 2.
Various DPP4 Inhibitors (Natural and Synthetic Source).
| Natural Source | Synthetic Source | |||||
|---|---|---|---|---|---|---|
| Apigenin | Berberine | Caffeic acid | Sitagliptin | Vildagliptin | Saxagliptin | Anagliptin |
| Calebin A | Carnosol | Cirsimaritin | Alogliptin | Retagliptin | Omarigliptin | Gosogliptin |
| Curcumin | Cyanidin | Cyanidin-3-glucoside | Denagliptin | Carmegliptin | Evogliptin | Trelagliptin |
| Epigallocatechin gallate | Eriocdictyol | Eriocitrin | Melogliptin | Dutagliptin | Linagliptin | Teneligliptin |
| Gallic acid | Genistein | hesperetin | Gemigliptin | SSR162369 | TS-021 | ALS 2-0426 |
| Hispidulin | Isoquercitin | Kaempferol | GRC8200 | PF00734200 | SYR472 | TA6666 |
| Luteolin | Malvidin | Naringenin | – | – | – | – |
| Naringin | Quercetin | Resveratrol | – | – | – | – |
| Rosmarinic acid | – | – | – | – | – | – |
Neural progenitor cells (NPCs) and neural stem cells (NSCs) that are present throughout lifetime are responsible for neurogenesis by replenishing neurons and glia during normal and pathological condition 65 through SDF-1/CXCR 4 axis. However, recruitment of these cells at lesion site occurs as a natural phenomenon, which becomes unsatisfactory often in promoting regeneration of damaged site. 66,67 As cell therapy is an efficient and possible approach, consideration of NPCs/precursor cells and NSCs through SDF-1α/CXCR 4 axis will be a good alternative strategy for CNS to get repair during AD without transplantation, as homing and mobilization of these cells toward CNS injury is highly influenced by the expression of CXCR4 and its ligand SDF-1α. 68 As NSCs express CXCR4 upon exposure to SDF-1α, intracellular molecular pathways get activated and causes proliferation and migratory promotion. 69
Functional link between hematopoietic systems and nervous system was established due to the expression of neuronal receptors on human hematopoietic stem cells and progenitor cells (HSPCs). 70 Vasculature became one of the neurogenic contributing factors which involves proliferation, migration, and differentiation of stem cells. In vitro model studies emphasize the importance of vascular secreted factors for neuronal production, self-renewal of NSCs 71 and transdifferentiation of HSCs into neurons. 72
Stromal-derived factor-1α becomes well known for its role in the trafficking of CD34+ cells and also promotes the mobilization of HSPCs by chemotaxis from bone marrow. 73,74 Increasing research evidence emphasizes the role of CD34+ cells in various neuroregenerative procedures, which include differentiation to neural cells and vascular genesis and also their positive impact on cognitive improvement after stroke. 75,76 Capability of progenitor cells in enhancing neurogenesis by stem cells through angiogenesis also has been demonstrated. 71,77
Down the road, we have emphasized the involvement SDF-1 produced by stromal cells of bone marrow and importance of SDF-1α/CXCR 4 axis in promoting neurogenesis by chemotaxis.
Glucagon-Like Peptide-1 and Its Importance
Glucagon-like peptide-1 can be considered as a peptide hormone when released in the gut by L cells of the intestine, and neuropeptide as well when released in the brain. 78,79 Peripherally, it controls glucose levels by promoting/inducing insulin secretion postprandially. So, this incretin hormone is well known for its glucose homeostasis and insulin signaling facilitation. Glucagon-like peptide-1 analogs/DPP-4 inhibitors are currently in use for the treatment of T2DM. 80 Both central and peripheral GLP-1 prefer hepatic glycogen storage to muscle by driving the glucose toward hepatic system, a physiological regulation.
It was noticed that pyramidal neurons of the hippocampus and Purkinje cells of cerebellum have expressed with GLP-1 receptor (GLP-1R). 81,82 Several research reports support extrapancreatic actions of GLP-1 and its analogs by crossing BBB, which are independent of its actions on glucose regulation. 83 It exhibits classical type growth effects by influencing the expression of genes that involve in cell growth and repair. 24
This incretin peptide executes actions through its own receptor GLP-1R, which is a transmembrane G-protein-coupled receptor. Protein kinase A and phosphoinositide 3-kinase get activated following the activation of the receptor. Further, it leads to promotion of mitogen-associated protein kinase/extracellular signal-regulated kinase and AKT pathways. Of which GLP-1 influences the downstream signaling of AKT. 83
Glucagon-like peptide-1 mimetics have been proved for their protective action on synapses against toxic effects of Aβ in the hippocampus, 84 and also chronic intraperitoneal injection of the Val(8)GLP-1 rescued synaptic plasticity by preventing synaptic degradation in AD mouse model 85 and prevented the impairment of spatial learning. 86 Iwai et al have demonstrated importance of GLP-1 as an anti-inflammatory against LPS model in which the peptide rescues the reduction in neuronal transmission caused by Interleulin-1 beta. 87 By using Alzheimer mouse models, different studies have claimed capability of incretin analogs in promoting proliferation of neuronal progenitor cells in the brain, which indirectly states their capability in promoting neurogenesis during pathological condition. 88,89 Few studies performed in transgenic models had made us to understand the direct role of GLP-1 and its receptor in cognition. Lack of synaptic transmission was noticed after the deletion of GLP-1R that leads to impaired long-term potentiation in the hippocampus and learning tasks. 90 Learning and cognitive deficits in GLP-1R knockout mice were restored by making gene transfer into the hippocampus. 81 And moreover spatial learning capability of wild rats has been enhanced by administering GLP-1 N-terminal nonapeptide (DPP4 resistant) into the brain. 81
Liraglutide, which is already available in the market for the treatment of T2DM, has been proven for its synaptoprotective action from Aβ, 91 progenitor cell proliferation, and differentiation in wild and APP/PS1 mice. 92 Furthermore, mice with GLP-1R over expression in the hippocampus had enhanced neuronal outgrowth and learning capabilities, and moreover, GLP-1 mimetics demonstrated to induce neurite outgrowth in cell culture. 81,93 D’Amico et al showed anti-AZ potential of sitagliptin, a DPP4 inhibitor in transgenic mice, 94 and moreover in a recent observational study, sitagliptin has been proved for its cognitive improvement action in diabetics with and without AD. 95 In the study by Kosaraju et al, it also has been proven GLP-1 mediated neuroprotection and enhanced learning capabilities in rats by using saxagliptin and vildagliptin against streptozotocin (STZ)-induced Alzheimer model. 96,97 Also, in our previous studies, we had shown the anti-Alzheimer potential of Eugenia jambolana and Pterocarpus marsupium, which was mediated by DPP4 inhibition. 98
Apart from this, neuroprotective action of DPP4 inhibitors against excitotoxicity was also proved in vivo and transforming growth factor β1–mediated neuroprotective actions of DPP4 inhibitors needs further evaluation 99,100
Conclusion
The unexpected growth in the aging population around the globe increases the prevalence and absolute numbers of dementias in which AD becomes prominent. Moreover, AD could be regarded as a brain disorder that has composite features of type 1 (insulin deficiency) and type 2 (insulin resistance) diabetes. Although many scientific studies have considered the diabetes as one of the risk factors for AD, the main lacuna lies in lack of cognitive measuring evaluations in patients with DM. Even though immunotherapy has shown promising results, it ends with severe AEs such as brain edema and hemorrhages. As neuronal and synaptic loss are the major concerns in AD, the concept of promoting neurogenesis has been arisen. Although stem cell therapy can be a good strategy, proliferation of exogenously administered cells, logistic issues, and ethical issues become bar to this implementation. Also, in our previous studies, we had shown DPP4 inhibitory mediated anti-Alzheimer potential of gliptins and herbal extracts.
So, increasing the life span of SDF-1 and GLP-1 in the body by using DPP4 inhibitors can promote neurogenesis, as they were proved for the same. Moreover, with this strategy, problem with BBB will not arise as in the case of peripherally administrable neurotrophic growth factors.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was financially supported by Department of AYUSH-A (EMR scheme), Government of India, New Delhi.
References
- 1. Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4(2):147–152. [DOI] [PubMed] [Google Scholar]
- 2. Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J Neural Transm (Vienna). 2002;109(3):341–360. [DOI] [PubMed] [Google Scholar]
- 3. Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490(1-3):115–125. [DOI] [PubMed] [Google Scholar]
- 4. Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20(3):723–736. [DOI] [PubMed] [Google Scholar]
- 5. Neumann KF, Rojo L, Navarrete LP, Farías G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5(5):438–447. [DOI] [PubMed] [Google Scholar]
- 6. Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–268. [DOI] [PubMed] [Google Scholar]
- 7. Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101(9):3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009;42(8):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(1):35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10(10):1049–1060. [PMC free article] [PubMed] [Google Scholar]
- 12. Steen E, Terry BM, J Rivera E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes?. J Alzheimers Dis. 2005;7(1):63–80. [DOI] [PubMed] [Google Scholar]
- 13. Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haan MN. Therapy insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2(3):159–166. [DOI] [PubMed] [Google Scholar]
- 15. Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–178. [DOI] [PubMed] [Google Scholar]
- 16. Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272(31):19547–19553. [DOI] [PubMed] [Google Scholar]
- 17. Frölich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna). 1998;105(4-5):423–438. [DOI] [PubMed] [Google Scholar]
- 18. Sridhar GR, Thota H, Allam AR, Suresh Babu C, Siva Prasad A, Divakar Ch. Alzheimer’s disease and Type 2 diabetes mellitus: the cholinesterase connection? Lipids Health Dis. 2006;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Opie EL. The relation Oe diabetes mellitus to lesions of the Pancreas. Hyaline degeneration of the Islands Oe Langerhans. J Exp Med. 1901;5(5):527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosselman S, Höppener J, Zandberg J, et al. Islet amyloid polypeptide: identification and chromosomal localization of the human gene. FEBS Lett. 1988;239(2):227–232. [DOI] [PubMed] [Google Scholar]
- 23. Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–481. [DOI] [PubMed] [Google Scholar]
- 24. Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221(1): T31–T41. [DOI] [PubMed] [Google Scholar]
- 25. Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63(7):1187–1192. [DOI] [PubMed] [Google Scholar]
- 26. Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl). 2004;82(8):510–529. [DOI] [PubMed] [Google Scholar]
- 27. Strachan MW. Insulin and cognitive function in humans: experimental data and therapeutic considerations. Biochem Soc Trans. 2005;33(pt 5):1037–1040. [DOI] [PubMed] [Google Scholar]
- 28. Leibson CL, Rocca WA, Hanson V, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145(4):301–308. [DOI] [PubMed] [Google Scholar]
- 29. Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur J Pharmacol. 2004;490(1-3):127–133. [DOI] [PubMed] [Google Scholar]
- 30. Carro E, Torres-Aleman I. Insulin-like growth factor I and Alzheimer’ s disease: therapeutic prospects?. Expert Rev Neurother. 2004;4(1):79–86. [DOI] [PubMed] [Google Scholar]
- 31. Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31(2):224–243. [DOI] [PubMed] [Google Scholar]
- 32. Isik AT, Bozoglu E. Acetylcholinesterase inhibition and insulin resistance in late onset Alzheimer’s disease. Int Psychogeriatr. 2009;21(06):1127–1133. [DOI] [PubMed] [Google Scholar]
- 33. Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. [DOI] [PubMed] [Google Scholar]
- 34. Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–2309. [DOI] [PubMed] [Google Scholar]
- 35. Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11–labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65(11):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacLeod R, Hillert EK, Cameron RT, Baillie GS. The role and therapeutic targeting of α-, β-and γ-secretase in Alzheimer’s disease. Future Sci OA. 2015;1(3):FSO11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease—the challenge of adverse effects. Nat Rev Neurol. 2012;8(8):465–469. [DOI] [PubMed] [Google Scholar]
- 38. Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-β-naphthylamide. Histochemie. 1966;7(3):197–201. [DOI] [PubMed] [Google Scholar]
- 39. Hartel S, Gossrau R, Hanski C, Reutter W. Dipeptidyl peptidase (DPP) IV in rat organs. Histochemistry. 1988;89(2):151–161. [DOI] [PubMed] [Google Scholar]
- 40. McCaughan GW, Wickson JE, Creswick PF, Gorrell MD. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990;11(4):534–544. [DOI] [PubMed] [Google Scholar]
- 41. Bühling F, Kunz D, Reinhold D, et al. Expression and functional role of dipeptidyl peptidase IV (CD26) on human natural killer cells. Natl Immun. 1993;13(5):270–279. [PubMed] [Google Scholar]
- 42. Iwaki-Egawa S, Watanabe Y, Kikuya Y, Fujimoto Y. Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem. 1998;124(2):428–433. [DOI] [PubMed] [Google Scholar]
- 43. Gorrell MD, Wickson J, McCaughan GW. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell Immunol. 1991;134(1):205–215. [DOI] [PubMed] [Google Scholar]
- 44. Longenecker KL, Stewart KD, Madar DJ, et al. Crystal structures of DPP-IV (CD26) from rat kidney exhibit flexible accommodation of peptidase-selective inhibitors. Biochemistry. 2006;45(24):7474–7482. [DOI] [PubMed] [Google Scholar]
- 45. Bjelke JR, Christensen J, Branner S, et al. Tyrosine 547 constitutes an essential part of the catalytic mechanism of dipeptidyl peptidase IV. J Bio Chem. 2004;279(33):34691–34697. [DOI] [PubMed] [Google Scholar]
- 46. Röhrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front Immunol. 2015;6:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambeir AM, Pereira JF, Chacon P, et al. A prediction of DPP IV/CD26 domain structure from a physico-chemical investigation of dipeptidyl peptidase IV (CD26) from human seminal plasma. Biochim Biophys Acta. 1997;1340(2):215–226. [DOI] [PubMed] [Google Scholar]
- 48. Hildebrandt M, Reutter W, Petra A, Matthias R, Klapp BF. A guardian angel: the involvement of dipeptidyl peptidase IV in psychoneuroendocrine function, nutrition and immune defence. Clin Sci. 2000;99(2):93–104. [PubMed] [Google Scholar]
- 49. Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept. 1999;85(1):9–24. [DOI] [PubMed] [Google Scholar]
- 50. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. [DOI] [PubMed] [Google Scholar]
- 51. Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12(4):313–335. [DOI] [PubMed] [Google Scholar]
- 52. Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR 4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia. 2002;16(10):1992–2003. [DOI] [PubMed] [Google Scholar]
- 53. Burwinkel M, Riemer C, Schwarz A, et al. Role of cytokines and chemokines in prion infections of the central nervous system. Int J Dev Neurosci. 2004;22(7):497–505. [DOI] [PubMed] [Google Scholar]
- 54. González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. [DOI] [PubMed] [Google Scholar]
- 55. Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30(3):459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu Y, Murakami F. Chemokine CXCL12 and its receptors in the developing central nervous system: emerging themes and future perspectives. Dev Neurobiol. 2012;72(10):1349–1362. [DOI] [PubMed] [Google Scholar]
- 57. Ratajczak M, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1–CXCR 4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. [DOI] [PubMed] [Google Scholar]
- 58. Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. [DOI] [PubMed] [Google Scholar]
- 59. Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28(3):495–500. [DOI] [PubMed] [Google Scholar]
- 60. Dunussi-Joannopoulos K, Zuberek K, Runyon K, et al. Efficacious immunomodulatory activity of the chemokine stromal cell–derived factor 1 (SDF-1): local secretion of SDF-1 at the tumor site serves as T-cell chemoattractant and mediates T-cell–dependent antitumor responses. Blood. 2002;100(5):1551–1558. [PubMed] [Google Scholar]
- 61. Stumm RK, Rummel J, Junker V, et al. A dual role for the SDF-1/CXCR 4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22(14):5865–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Link DC. Targeted Therapy of Acute Myeloid Leukemia. In: Andreeff M, ed. Regulation of Hematopoiesis by CXCL12/CXCR4 Signaling. New York: Springer; 2015:593–605. [Google Scholar]
- 63. Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105(8):3155–3161. [DOI] [PubMed] [Google Scholar]
- 64. Cui L, Qu H, Xiao T, Zhao M, Jolkkonen J, Zhao C. Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia. Restor Neurol Neurosci. 2013;31(3):239–251. [DOI] [PubMed] [Google Scholar]
- 65. Barkho B, Zhao X. Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther. 2011;6(4):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102(5):1459–1465. [DOI] [PubMed] [Google Scholar]
- 67. Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76(2):223–231. [DOI] [PubMed] [Google Scholar]
- 68. Jiang Z, Zhou W, Guan S, Wang J, Liang Y. Contribution of SDF-1α/CXCR4 signaling to brain development and glioma progression. Neurosignals. 2012;21(3-4):240–258. [DOI] [PubMed] [Google Scholar]
- 69. Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kalinkovich A, Spiegel A, Shivtiel S, et al. Blood-forming stem cells are nervous: direct and indirect regulation of immature human CD34+ cells by the nervous system. Brain, Behav Immun. 2009;23(8):1059–1065. [DOI] [PubMed] [Google Scholar]
- 71. Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. [DOI] [PubMed] [Google Scholar]
- 72. Kuci S, Kuci Z, Schmid S, et al. Efficient in vitro generation of adult multipotent cells from mobilized peripheral blood CD133+ cells. Cell Prolif. 2008;41(1):12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354–3360. [DOI] [PubMed] [Google Scholar]
- 74. Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. [DOI] [PubMed] [Google Scholar]
- 75. Reali C, Scintu F, Pillai R, et al. Differentiation of human adult CD34+ stem cells into cells with a neural phenotype: role of astrocytes. Exp Neurol. 2006;197(2):399–406. [DOI] [PubMed] [Google Scholar]
- 76. Zangiacomi V, Balon N, Maddens S, et al. Cord blood-derived neurons are originated from CD133+/CD34 stem/progenitor cells in a cell-to-cell contact dependent manner. Stem Cells Dev. 2008;17(5):1005–1016. [DOI] [PubMed] [Google Scholar]
- 77. Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holst JJ. Glucagon-like peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994;107(6):1848–1855. [DOI] [PubMed] [Google Scholar]
- 79. Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16(2):75–85. [DOI] [PubMed] [Google Scholar]
- 80. Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287(2):E199–E206. [DOI] [PubMed] [Google Scholar]
- 81. During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–1179. [DOI] [PubMed] [Google Scholar]
- 82. Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20(13):1161–1166. [DOI] [PubMed] [Google Scholar]
- 83. Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21(5):802–818. [DOI] [PubMed] [Google Scholar]
- 84. Gault VA, Hölscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol. 2008;587(1):112–117. [DOI] [PubMed] [Google Scholar]
- 85. Gengler S, McClean PL, McCurtin R, Gault VA, Hölscher C. Val (8) GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging. 2012;33(2):265–276. [DOI] [PubMed] [Google Scholar]
- 86. Wang MD, Huang Y, Zhang GP, et al. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience. 2012;226:388–396. [DOI] [PubMed] [Google Scholar]
- 87. Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka JI. Glucagon-like peptide-1 inhibits LPS-induced IL-1β production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–360. [DOI] [PubMed] [Google Scholar]
- 88. Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res. 2011;89(4):481–489. [DOI] [PubMed] [Google Scholar]
- 89. Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205(1):265–271. [DOI] [PubMed] [Google Scholar]
- 91. McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31(17):6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Parthsarathy V, Hölscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PloS One. 2013;8(3):e58784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-β peptide (Aβ) levels and protects hippocampal neurons from death induced by Aβ and iron. J Neurosci Res. 2003;72(5):603–612. [DOI] [PubMed] [Google Scholar]
- 94. D’Amico M, Di Filippo C, Marfella R, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol. 2010;45(3):202–207. [DOI] [PubMed] [Google Scholar]
- 95. Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract. 2017;123:192–198. [DOI] [PubMed] [Google Scholar]
- 96. Kosaraju J, Murthy V, Khatwal RB, et al. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol. 2013;65(12):1773–1784. [DOI] [PubMed] [Google Scholar]
- 97. Kosaraju J, Gali CC, Khatwal RB, et al. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology. 2013;72:291–300. [DOI] [PubMed] [Google Scholar]
- 98. Kosaraju J, Madhunapantula SV, Chinni S, et al. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behavi Brain Res. 2014;267:55–65. [DOI] [PubMed] [Google Scholar]
- 99. Wu YQ, Limburg DC, Wilkinson DE, et al.Neuroprotective effects of inhibitors of dipeptidyl peptidase-IV in vitro and in vivo. In: Back N, Cohen IR, Kritchevsky D, Lajtha A, Paoletti R, eds. Dipeptidyl Aminopeptidases in Health and Disease. Boston, MA: Springer; 2004:351–355. [Google Scholar]
- 100. Reinhold D, Bank U, Bühling F, et al. Inhibitors of dipeptidyl peptidase IV induce secretion of transforming growth factor-β1 in PWM-stimulated PBMC and T cells. Immunology. 1997;91(3):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu W, Qiu CX, Wahlin Å, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project A 6-year follow-up study. Neurology. 2004;63(7):1181–1186. [DOI] [PubMed] [Google Scholar]
- 102. Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. Diabetes. 2002;51(4):1256–1262. [DOI] [PubMed] [Google Scholar]
- 103. Ott A, Stolk R, Hofman A, van Harskamp F, Grobbee D, Breteler M. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39(11):1392–1397. [DOI] [PubMed] [Google Scholar]
- 104. MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14(2):77–83. [DOI] [PubMed] [Google Scholar]
- 105. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61(5):661–666. [DOI] [PubMed] [Google Scholar]
- 106. Curb J, Rodriguez BL, Abbott RD, et al. Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology. 1999;52(5):971–971. [DOI] [PubMed] [Google Scholar]
- 107. Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63(11):1551–1555. [DOI] [PubMed] [Google Scholar]
- 108. Huang CC, Chung CM, Leu HB, et al. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PloS One. 2014;9(1):e87095. [DOI] [PMC free article] [PubMed] [Google Scholar]