Abstract
In Iranian traditional medicine, asafoetida is introduced as a valuable remedy for nervous disorders. Dementia was induced by injection of d-galactose and NaNO2 for 60 consecutive days. Animals were divided into normal control (NC), dementia control (DC), dementia prophylactic (DP), and dementia treated (DT). The learning and memory functions were examined by 1-way active and passive avoidance tests, using a shuttle box device. Avoidance response in training tests and 1 and 3 weeks later was significantly increased in NC, DP, and DT groups compared to the DC group. Step through latency in all groups was significantly greater than the DC group. Total time spent in light room, which shows the memory retention ability, in DP, NC, and DT was significantly greater than the DC group. Our findings indicate that asafoetida could prevent and treat amnesia. These beneficial effects maybe related to some constituent’s effectiveness such as ferulic acid and umbelliferone.
Keywords: asafoetida, Alzheimer’s disease, d-galactose, NaNO2 , shuttle box
Introduction
Alzheimer’s disease (AD) is an age-related, progressive and irreversible neurodegenerative disease, characterized by a gradual and permanent loss of memory and learning abilities 1 associated with the loss of neurons. 2 Although several hypotheses have been put forward, the exact molecular etiology of this devastating disorder remains unknown. Oxidative stress, as one of these hypotheses, is a consequence of an imbalance between pro-oxidant and antioxidant defenses, causing accumulation of reactive oxygen species (ROS) and has important role in impairing cellular function and cellular senescence. 3 Therefore, protection against oxidative stress is critical for delaying brain aging and preventing neurodegenerative disorders, such as AD. d-Galactose is a reducing sugar that can generate ROS during its metabolism in vivo. 4 Rats and mice chronically treated with d-galactose show brain oxidative stress and cognitive dysfunction, accompanied with several hallmarks of age-related neurodegeneration such as cholinergic degeneration, 5 impairment of synaptic plasticity and neurogenesis, 6 altered expression of amyloid-beta metabolism-associated molecules, 7 reactive gliosis, 8 and neuroinflammation. 9 In this regard, it would be important to establish suitable animal models and chronic injection of d-galactose serves as a good animal model for age-related brain oxidative damage and antiaging pharmacology research. Medicinal plants are believed to be beneficial source of new chemical and antioxidant substances with potential therapeutic effects. In the recent years, people recognized and used the medicinal properties of many cultivated or wild plants to fight the disease. Plant drugs are frequently considered to be less toxic than synthetic ones. The genus of Ferula belongs to the family of Apiaceae and includes about 130 species distributed throughout Central Asia and Mediterranean area. 10 Ferula assa-foetida L is one of these species that grows wildly in the central area of Iran. The part used of this plant and several other species of Ferula is an oleo gum resin (asafoetida) that obtained by incision of the stem and root. 11 In Iranian folk medicine, asafoetida is used as an antispasmodic, antihelminthic, carminative, anticonvulsant, and analgesic agent. 12 In Ayurveda, asafoetida is introduced as a valuable remedy for hysteria and nervous disorders. 11 American people orally use it as a stimulant to the brain and nerves. 13 In recent studies, several pharmacological and biological activities of asafoetida have also investigated and have shown this oleo gum resin has antioxidant, antiviral, antifungal, cancer chemopreventive, antidiabetic, antispasmodic, hypotensive, and molluscicidal effects. 11 Beneficial effects of F assa-foetida on learning and memory were investigated by Vijayalakshmi et al. 14 They showed that aqueous extract of F assa-foetida could improve learning and memory compared to normal animals. They concluded that these beneficial effects could be attributed to facilitation of cholinergic transmission due to inhibition of acetylcholinesterase (AchE) in rat brain and partly due to its boosting effect on endogenous antioxidant system. These evidences provided reliable reasons for investigation of the effects of asafoetida on prevention and treatment of Alzheimer’s disorder. The aim of this study is to research the effect of asafoetida on prevention and treatment of Alzheimer induced by d-galactose and NaNO2 in mice.
Materials and Methods
Animals
Mice were divided randomly and equally into 4 groups (normal control [NC], dementia control [DC], dementia prophylaxis [DP], and dementia treated [DT]) for each test. Dementia was induced by intraperitoneal injection of 120 mg/kg d-galactose (Sinopharm Chemical Reagent Co, China) and 90 mg/kg NaNO2 (Merck, Germany) for 60 consecutive days. 15,16
Treatments
Animals allocated for DP survey received 100 mg/kg/d asafoetida (intraperitonelly [IP]) along with the dementia induction protocol. The DT group received the same amount of asafoetida extract (IP) for 15 consecutive days after induction of dementia. Animals in the NC and DC groups received equivalent volume (10 mL/kg, IP) of distilled water.
Preparation of Asafoetida
Asafoetida was collected from Tabas region (Yazd province, Iran) during the summer, and the plant species were botanically identified by the botanist in Yazd Agricultural Research Center. The dried powder of asafoetida was soaked in distilled water overnight at room temperature, and the yielded suspension was used IP. Concentrations and dosages of the extract were expressed as crude amount of the dried oleo gum resin used in preparation of the stock solution.
Apparatus
The learning and memory functions in mice were examined by the “one-way active & passive avoidance learning and memory” tests in a Shuttle box apparatus. 17,18 The apparatus is a rectangular box with 2 distinct compartments measuring 22 × 21 × 22 cm and separated by a sliding door. The walls and lid of safe compartment were made of white-colored acrylic plates while the shock compartment with the dark ones. Two stainless steel plates (22 × 20 cm) were obliquely embedded in the side walls of each compartment, apart from each other by 20 cm at the top and 1 cm at the bottom. A 10-W red light bulb and a buzzer (70 dB, 760 Hz) were located in the top of front wall of the safe room. The stainless steel plates in the dark compartment were connected to a square-pulse stimulator (Grass model no. S-48, Narko) in series with a constant current unit (Grass model no. CCU-1A, Narko) by which a reproducible, aversive electric foot shock of 1 mA and 1-second duration could be delivered when the mice are in contact with both the plates.
Active Avoidance Test
One-way active avoidance test was performed basically as described by Galindo et al. 17 In training or learning session, each animal was placed in the dark room with its head toward the wall opposite to the sliding door and the lid was closed. Immediately, the door between the compartments was opened and 2 initial conditioned stimuli sound and light flash were applied simultaneously. If the animal crossed the white room during 10 seconds, a “shock-free” trial was scored (avoidance). If the animal failed to cross to the white room within 10 seconds, an electric foot shock (0.4 mA), as an unconditioned stimulus, was continually applied until leaving the dark room and a “shock” trial was assigned (escape). Following the entrance of animal into the safe room, the sliding door was closed, and after 30 seconds rest in this room, the next trial was started. Maximum duration for electric shock delivery was 10 seconds, so if the animal avoids leaving the shock compartment during this period, stimulation was stopped and the animal was manually conducted to the white room to follow a new trial. In this experiment, 90 trials per animal were performed in 3 successive days (3 sessions and 10 trials in each session per day), and the mean number of “shock-free” trials was considered as an index for learning. In the fourth day, all animals were trained to acquire at least 70% of full learning (ie, for each 10 trials at least in 7 trials the animal would leave the dark side without receiving any foot shock). From this point, evaluation of memory retention followed for 3 successive days, by the same procedure used in the training session without any foot shock, after 1 and 3 weeks. In these sessions also the mean number of “avoidance” trials was considered as the index of memory consolidation.
Passive Avoidance Test
One-way passive avoidance test was essentially carried out according to Saxena et al. 19 The test was initiated by acclimatization to the shuttle box by placing each animal to the light room with its head toward the wall opposite to the interconnected door. When the animal turned toward the dark room, the door was opened in coincidence with sound and light flash display. Upon complete entrance to the dark room, the door was closed, and after 30 seconds the animal was returned to its home cage. Adaptation trial was repeated 3 times with 30 minutes intertrial interval. Thirty minutes after the last adaptation trial, the training trial was run similar to the previous except that a mild foot shock (0.5 mA; for 10 seconds) was delivered immediately after the animal arrived to the dark room with closed door. After applying the aversive stimulus, the door was opened so that the harassed animal could escape the electrified compartment and brought to its home cage. Two minutes after the training, trial learning test was carried out by introducing each animal to light room, and the initial time spent to completely enter the dark room (step through latency [STL]) within 2 minutes was recorded. Following this session, another training session was repeated, while the animals remained in the apparatus and received a foot shock upon re-entrance to the dark room. Full training trial was terminated when the mouse achieved full acquisition (ie, when the mouse stayed in the light room for 2 consecutive minutes). The retention assay was performed 24 hours, 1 week, and 1 month after the full training session similar to the learning session. In these sessions, the initial time spent by each animal to completely enter the dark room (retention time [RT]), number of relocating, and total time spent in light (LS) rooms within 5 minutes were recorded. All behavioral tests were performed by blinded experimenters about the animal treatments.
Acute Toxicity
At the end of the experiments, the rats under study were observed for symptoms of short- and long-term toxicity and finally mortality recorded. Then, animals were kept under observation for up to 10 days to rollout the behavioral changes (tremor and paralysis), weight loss, and mortality. 12
Statistical Analysis
In this study, shock-free/avoidance responses in active avoidance test were expressed as mean ± standard error of the mean (SEM). These data were analyzed by 2-way analysis of variance (ANOVA) followed by Bonferroni posttest, repeated measures ANOVA followed by Tukey’s multiple comparisons tests’, and Kruskal-Wallis followed by Dunn’s multiple comparison test. In passive avoidance test, all data are presented as mean ± SEM and were statistically analyzed by Kruskal-Wallis followed by Dunn’s multiple comparison tests. All statistical tests were 2 tailed and P < .05 was considered as the statistically significant level.
Results
Active Avoidance Test
In active avoidance test, the avoidance index in all groups was progressively increased during 3 successive days of training session, except for the DC group, and this factor was significantly increased in NC, DT, and DP groups compared to the DC group (Table 1). In training session, the avoidance response in the DC group significantly diminished when compared to the NC group (12 ± 3 vs 25.1 ± 3.9, P < .001; Figure 1), while in DT and DP groups, training was improved so that there was a significant difference between them and the DC group (22.4 ± 4 and 23.7 ± 5 vs 12 ± 3). There was no difference in avoidance response between DT, DP, and NC groups when compared to each other. One and three weeks after the training session, the memory retention in DC groups was significantly less than the other groups (P < .05, Figures 2 and 3).
Table 1.
The Avoidance Index (Mean ± SEM) in Different Groups During 3 Successive Days of Active Avoidance Training Session.a
| Training Days | Groups | |||
|---|---|---|---|---|
| Dementia Control | Dementia Prophylaxis | Dementia Treated | Normal Control | |
| First day | 12.8 ± 0.6 | 18.2 ± 0.9b | 17.7 ± 0.9b | 19.3 ± 0.4b |
| Second day | 13.1 ± 0.8 | 24.9 ± 0.7b,c | 23.2 ± 0.6b,c | 26.9 ± 1.3b |
| Third day | 11.2 ± 0.7 | 28.2 ± 1.2b,c | 26.3 ± 0.5b,c | 29.2 ± 1.5b,c |
Abbreviations: ANOVA, analysis of variance; SEM, standard error of the mean.
a n = 7.
b The significant differences in avoidance trials when compared to the DC group, respectively, using 2-way ANOVA followed by Bonferroni’s posttest.
c The significant difference in avoidance trials when compared to the first day of training session for each group, using repeated measures ANOVA followed by Tukey’s multiple comparisons tests.
Figure 1.
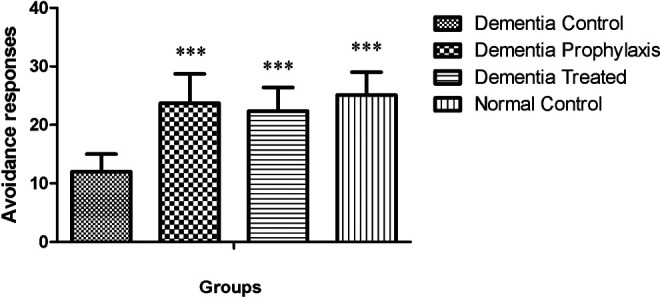
Avoidance responses of different groups in active avoidance training session (n = 7). ** indicates the significant differences in avoidance trials when compared to the DC group, respectively, using repeated measures analysis of variance (ANOVA) followed by Tukey’s multiple comparisons tests.
Figure 2.
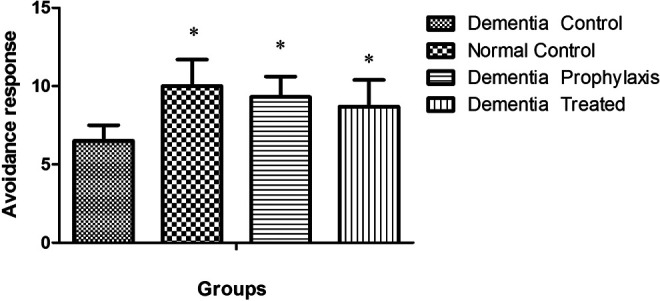
Effect of asafoetida on avoidance response at 1 week after training session. Data indicated mean ± standard error of the mean (SEM). *P < .05 showed significant difference between the normal control (NC), dementia prophylactic (DP), and dementia treated (DT) compared to dementia control (DC).
Figure 3.
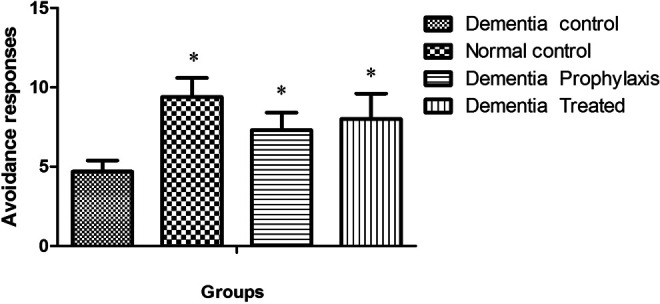
A comparison between the active avoidance responses in different groups 1 month after training session (n = 7). *Indicative of significant difference by P < .05, respectively, as compared with the dementia control (DC) group according to Kruskal-Wallis and Dunn’s multiple comparison tests.
Passive Avoidance Test
In passive avoidance test, animal’s STL, as an index for learning, in all groups was significantly greater than the DC group (P < .05, Figure 4).Total time spent in light room (LS) in the DC group was significantly less than NC, DT, and DP groups at 24 hours, 1 week, and 3 weeks after full training (P < .05), while there was not any significant difference between this index in DP and DT groups as compared with the NC group (Table 2, P < .05). Another index that was evaluated in passive avoidance test was the RLN between light and dark rooms, which in DC group was significantly greater than the DP, DT, and NC groups 24 hours and 1 week after the full training session (Table 3, P < .05). The retention time (RT) as the major index for memory consolidation at 24 hours, 1 week, and 1 month after full training session for different groups is shown in Table 4. There was a significant difference in RT among different groups at 24 hours, 1 week, and 1 month compared to the DC group (P < .05).
Figure 4.
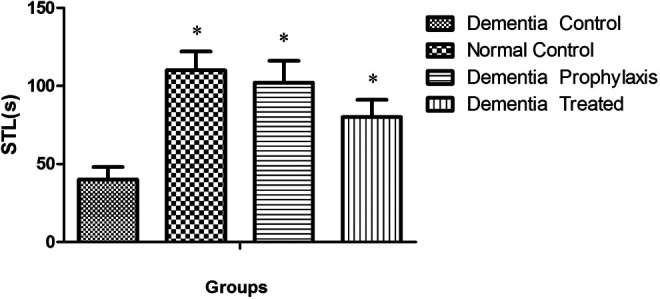
Step through latency (STL) in different groups during passive avoidance learning session (n = 7). *indicates the significant difference (P < .05) as compared with the dementia control (DC) group according to Kruskal-Wallis and Dunn’s multiple comparison tests.
Table 2.
A Comparison Between the Time Spent in Light Rooma (LS) in different Groups 24 hours, 1 Week, and 1 Month After Full Training Session of Passive Avoidance Test.b,c
| Sessions | Groups | |||
|---|---|---|---|---|
| Dementia Control | Dementia Prophylaxis | Dementia Treated | Normal Control | |
| LS 24 hour | 185 ± 13.5 | 236.6 ± 23.8a | 245.8 ± 26.4a | 228 ± 21.4a |
| LS 1 week | 110 ± 14.3 | 229 ± 21.7a | 236.3 ± 22.3a | 234 ± 25.6a |
| LS 1 month | 70 ± 10.7 | 127.5 ± 19.5a | 132.5 ± 17.9a | 146.5 ± 18.9a |
Abbreviations: DC, dementia control; SEM, standard error of the mean.
a Indicates the significant difference (P < .05) as compared with the DC group according to Kruskal-Wallis and Dunn’s multiple comparison tests.
b n = 7.
c Data are presented as means ± SEM.
Table 3.
A Comparison Between the Number of Relocating (RLN), Between Light and Dark Rooms, indifferent Groups 24 hours, 1 Week, and 1 Month After Full Training Session of Passive Avoidance test.a,b
| Sessions | Groups | |||
|---|---|---|---|---|
| Dementia Control | Dementia Prophylaxis | Dementia Treated | Normal Control | |
| RLN 24th hour | 4.8 ± 0.9 | 1.3 ± 0.3c | 2.3 ± 0.8c | 0.8 ± 0.1c |
| RLN 1 week | 5.5 ± 1.1 | 2.7 ± 0.7c | 2.2 ± 0.6c | 2.1 ± 0.8c |
| RLN 1 month | 7.1 ± 1.4 | 6.3 ± 0.9 | 5.9 ± 1.5 | 6.1 ± 1.8 |
Abbreviations: DC, dementia control; SEM, standard error of the mean.
a n = 7.
b Data are presented as means ± SEM.
c Indicates the significant difference (P < .05) as compared with the DC group according to Kruskal-Wallis and Dunn’s multiple comparison tests.
Table 4.
A Comparison Between the Retention Time (RT) in Different Groups 24 hours, 1 week, and 1 month After Full Training Session of Passive Avoidance Test.a,b
| Sessions | Groups | |||
|---|---|---|---|---|
| Dementia Control | Dementia Prophylaxis | Dementia Treated | Normal Control | |
| RT 24th hour | 15.9 ± 3.8 | 183.6 ± 26.3c | 161 ± 0.8c | 198.8 ± 0.1c |
| RT 1 week | 79 ± 11.1 | 104 ± 10.7c | 172.8 ± 16.6c | 156 ± 14.8c |
| RT 1 month | 62.1 ± 9.4c | 89.3 ± 8.9c | 79.3 ± 7.5c | 75.1 ± 8.8c |
Abbreviations: DC, dementia control; SEM, standard error of the mean.
a n = 7.
b Data are presented as means ± SEM.
c Indicates the significant difference (P < .05) as compared with the DC group according to Kruskal-Wallis and Dunn’s multiple comparison tests.
Acute Toxicity Study
Asafoetida in concentrations used did not show any short- or long-term toxic effect. This was evidenced by the absence of tremor, paralysis, weight loss, and autonomic behavioral changes when compared to the control group. Also, there was no mortality in treated animals during 6 weeks of observation.
Discussion
Alzheimer’s disease is a neurodegenerative disorder characterized by the deposition of amyloid beta peptide (Aβ) and inducing oxidative stress in the brain. 20 In this study, our findings indicated that chronic administration of asafoetida in both active and passive avoidance tests could prevent and treat the learning impairment and memory deficit induced by d-galactose and NaNO2 in adult male mice. Although, based on our literature review, this is for the first time that the prolonged systemic administration of asafoetida is used to evaluate its prophylaxis and therapeutic effect on a model of chemically induced cognitive deficit, its findings globally support previous studies regarding enhancing effect of asafoetida and its major constituents on learning and memory consolidation processes. Phytochemestry of asafoetida showed that this oleo gum resin contains about 40% to 64% resin, 25% endogeneousgum, 10% to 17% volatile oil, and 1.5% to 10% ash. Its resin fraction consists of ferulic acid esters, free ferulic acid, umbelliferone, and coumarin derivatives such as foetidin and kamolonol and farnesiferoles A, B, and C. The compositions of its gum fraction are known to be glucose, galactose, l-arabinose, rhamnose, and glucuronic acid. 11 The volatile part contains sulfur-containing compounds and volatile terpenoids. 21 Although the exact molecular etiology of AD remains unknown, there are several hypotheses about the AD of which cholinergic, oxidative stress, and deposition amyloid beta-peptide hypotheses are most important. 22 –24 The cholinergic hypothesis is currently regarded as the most widely accepted theory, and it is based on the reports about loss of acetylcholine (Ach) synthesis in patients with AD. 25 The AchE is an enzyme responsible for hydrolysis of acetylcholine in neuronal tissues in the brain, and its inhibition is one of the most important strategies for improving cholinergic functions in neurodegenerative diseases such as AD. 26 Some species of Ferula were traditionally used for enhancing memory or treatment of neurological disorders. 11 In a previous report, researchers showed that Ferula gummosa has AchE inhibition potential. 27 In vitro and in vivo findings indicated that 2 natural compounds of some Ferula species, namely, umbelliferone, eugenol, limonene, and ferulic acid, act as inhibitors of AchE. 28 Methanol extracts of F assa-foetida were subjected to in vitro assay against cycloxygenase 1 enzymes and indicated that F assa-foetida exhibited inhibitory effect on AChE. 29 Among the compounds of asafoetida, ferulic acid is a phenolic compound and a major constituent of asafoetida that showed the protective effect on Aβ-induced neurotoxicity and oxidative stress. 30,31 It is worth noting that anticholinesterase mechanisms, inhibition of amyloid toxicity through oxidative stress, and neuroprotection are some of the key validated targets for Alzheimer’s therapy that are all likely to be modulated by asafoetida. In conclusion, this study showed that asafoetida has protective and treatment effects on impairment of memory induced by d-galactose and NaNo2 in mice. Although the mechanism of action of asafoetida in the prevention and treatment of dementia is unknown, it can be concluded that these beneficial effects maybe due to the presence of biologically active compounds such as sulfur-containing and sesquiterpene coumarins. 32
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from Shahid Sadoghi University of Medical Sciences Research Council. This research was supported by the Foundation of Shahid Sadoughi University of medical sciences and health services, Yazd, Iran.
References
- 1. Price DL, Struble RG, Whitehouse PJ, et al. Alzheimer’s disease: a multisystem disorder. Res Publ Assoc Res Nerv Ment Dis. 1986;64:209–214. [PubMed] [Google Scholar]
- 2. Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19 (10):1008–1018. [DOI] [PubMed] [Google Scholar]
- 3. Dröge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. [DOI] [PubMed] [Google Scholar]
- 4. Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid amelioratescognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol. 2007;74 (7):1078–1090. [DOI] [PubMed] [Google Scholar]
- 5. Lei M, Su Y, Hua X, et al. Chronic systemic injection of d-galactose impairs the septohippocampal cholinergic system in rats. Neuroreport. 2008;19 (16):1611–1615. [DOI] [PubMed] [Google Scholar]
- 6. Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF. Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem. 2008;90 (1):19–27. [DOI] [PubMed] [Google Scholar]
- 7. Luo Y, Niu F, Sun Z, et al. Altered expression of Abeta metabolism-associated molecules from d-galactose/AlCl(3) induced mouse brain. Mech Ageing Dev. 2009;130 (4):248–252. [DOI] [PubMed] [Google Scholar]
- 8. Lei M, Hua X, Xiao M, Ding J, Han Q, Hu G. Impairments of astrocytes areinvolved in the d-galactose-induced brain aging. Biochem Biophys Res Commun. 2008;369 (4):1082–1087. [DOI] [PubMed] [Google Scholar]
- 9. Shan Q, Lu J, Zheng Y, et al. Purple sweet potato color ameliorates cognition deficits and attenu-ates oxidative damage and inflammation in aging mouse brain induced byd-galactose. J Biomed Biotechnol. 2009;2009:564737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48 (3):242–246. [DOI] [PubMed] [Google Scholar]
- 11. Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gumresin)- a review. J Ethnopharmacol. 2011;134 (1):1–10. [DOI] [PubMed] [Google Scholar]
- 12. Bagheri SM, Dashti-RM H, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. RPS. 2014;9 (3):207–212. [PMC free article] [PubMed] [Google Scholar]
- 13. Eigner D, Scholz D. Das zauberbchelin der Gyani Dolma. Pharmazie in Unserer Zeit. 1990;19 (4):141–152. [DOI] [PubMed] [Google Scholar]
- 14. Vijayalakshmi, Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S. Evaluation of the effect of Ferula asafetida Linn. Gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44 (1):82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hua X, Lei M, Zhang Y, et al. Long-term d-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer’s disease. Life Sci. 2007;80 (20):1897–1905. [DOI] [PubMed] [Google Scholar]
- 16. Zhang D, Liu G, Shi J, Zhang J. Coeloglossumviride var. bracteatum extract attenuatesD-galactose and NaNO2 induced memory impairment in mice. J Ethnopharmacol. 2006;104 (1-2):250–256. [DOI] [PubMed] [Google Scholar]
- 17. Galindo LE, Garin-Aguilar ME, Medina AC, Serafín N, Quirarte GL, Prado-Alcalá RA. Acquisition and retention of enhanced active avoidance are unaffected by interferencewith serotonergic activity. Behav Brain Res. 2008;195 (1):153–158. [DOI] [PubMed] [Google Scholar]
- 18. Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res. 2006;173 (1):112–115. [DOI] [PubMed] [Google Scholar]
- 19. Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581 (3):283–289. [DOI] [PubMed] [Google Scholar]
- 20. Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36 (12):1307–1313. [DOI] [PubMed] [Google Scholar]
- 21. Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: distribution, biosynthesis and analytical methods. J Essent Oil Res. 2012;24 (4):393–434. [Google Scholar]
- 22. Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and alzheimer’s disease related cognitive deficits: recent challenges and their implications for novel drugdevelopment. J Pharmacol Exp Ther. 2003;306 (3):821–827. [DOI] [PubMed] [Google Scholar]
- 23. Hardy J., Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297 (5580):353–356. [DOI] [PubMed] [Google Scholar]
- 24. Olanow CWA. Radical hypothesis for neurodegeneration. Trends Neurosci. 1993;16 (11):439–444. [DOI] [PubMed] [Google Scholar]
- 25. Shvaloff A, Neuman E, Guez D. Lines of therapeutics research in alzheimer’s disease. Psychopharmacol Bull. 1996;32 (3):343–352. [PubMed] [Google Scholar]
- 26. Grutzendler J, Morris JC. Cholinesterase inhibitors for alzheimer’s disease. Drugs. 2001;61 (1):41–52. [DOI] [PubMed] [Google Scholar]
- 27. Adhami HR, Farsam H, Krenn L. Screening of medicinal plants from Iranian traditional medicine for acetylcholinesterase inhibition. Phytother Res. 2011;25 (8):1148–1152. [DOI] [PubMed] [Google Scholar]
- 28. Karimi G, Iranshahi M, Hosseinalizadeh F, Riahi B, Sahebkar A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula . Pharmacologyonline. 2010;1:566–574. [Google Scholar]
- 29. Shereen KA, Ahmed RH, Maha MS, et al. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement Altern Med. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. [DOI] [PubMed] [Google Scholar]
- 31. Yan JJ, Cho JY, Kim HS, et al. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001;133 (1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25 (3):315–323. [DOI] [PubMed] [Google Scholar]


