Abstract
Background:
Stabilizing/reducing decline in the ability to perform activities of daily living (ADLs) is important in management of Alzheimer’s disease (AD).
Methods:
Post hoc analysis of OPtimizing Transdermal Exelon In Mild-to-moderate Alzheimer’s disease (OPTIMA), a double-blind trial comparing 13.3 and 9.5 mg/24 h rivastigmine patch in patients with AD demonstrating functional and cognitive decline with 9.5 mg/24 h patch. Efficacy on Alzheimer’s disease Cooperative Study-instrumental ADL (ADCS-IADL) items, higher level function (HLF), and autonomy factors was assessed.
Results:
The ADCS-IADL, HLF, and autonomy factors favored 13.3 mg/24 h patch at all time points, reaching significance from weeks 16 to 48, 24 to 48, and 32 to 48, respectively. Higher dose patch demonstrated significantly greater efficacy on 10 of 17 ADCS-IADL items at 1 or more time points (P < .05 vs 9.5 mg/24 h patch). More adverse events were observed with higher dose patch; study discontinuations were similar between the doses.
Conclusions:
Greater efficacy of 13.3 versus 9.5 mg/24 h patch on ADL, including autonomy and HLF factors, supports this additional dosing option to prolong patients’ independence.
Keywords: rivastigmine patch, efficacy, higher dose, activities of daily living
Introduction
Alzheimer’s disease (AD) is characterized clinically by deterioration in the ability to perform activities of daily living (ADLs), cognitive impairments, and behavioral disturbances. 1 Progressive loss of independence during the course of the disease leads to a greater need for caregiver assistance, increased health care costs, and increased likelihood of patient institutionalization. 2 Therefore, stabilization or improvements in the ability to perform ADL are important therapeutic goals in the optimal management of patients with AD, in order to enhance outcomes and reduce health care costs. 3–5
As AD progresses, functional impairments become more pronounced and dependence on caregivers increases. 5 The importance of using a functional assessment to monitor disease progression and efficacy of treatment is increasingly recognized. Guidelines now recommend that for monitoring progression and symptomatic treatment of AD, a reliable and sensitive measurement of functional impairment (ie, ADL), as well as cognitive and behavioral symptoms, should be utilized. 5 Scales commonly used to assess function in patients with AD include the Alzheimer’s Disease Cooperative Study-ADL (ADCS-ADL) scale, the Disability Assessment for Dementia, the Progressive Deterioration Scale, and the Bristol Activities of Daily Living scale. 6–8 The ADCS-ADL scale is used in clinical studies to assess patients’ performance of both basic ADL (BADL) and instrumental ADL (IADL). 8 The BADLs include general mobility and self-maintenance skills and typically become impaired in later stages of the disease; items 1 to 6 of the ADCS-ADL scale form the BADL domain (eating, walking, toileting, bathing, grooming, and dressing). 9 The IADL items require complex thinking to perform and generally demonstrate impairment in mild-to-moderate disease. 10 Using the results of a factor analysis, 11 2 further factors of the ADCS-ADL were defined to allow more specific analysis of functional ability. Items of the ADCS-IADL were divided into those requiring “higher level functioning” (HLF) and those that affect the patients' ability to live independently, the “autonomy” factor. 11
Currently, cholinesterase inhibitors (ChEIs; rivastigmine, donepezil, and galantamine) are the first-line treatment for providing symptomatic relief for patients with mild-to-moderate AD. 1 Efficacy of treatment on both cognitive function and ability to perform ADLs have been assessed in randomized controlled trials with ChEIs. 7,11–18 Efficacy of cholinesterase inhibition is dose dependent, and a higher ChEI dose has been investigated for all compounds as a means to achieve additional efficacy. However, tolerability of these drugs has been found to decrease with increasing dose, limiting this strategy. 12,14 Rivastigmine is the only approved ChEI available in patch formulations for the symptomatic treatment of mild-to-moderate AD. 19
The efficacy of oral rivastigmine has been shown to be dose dependent on measures of ADL, cognition, and global functioning. 17 However, rivastigmine capsules (12 mg/d) are associated with greater incidence of gastrointestinal adverse events (AEs) compared with the 9.5 mg/24 h (10 cm2) rivastigmine transdermal patch. 12 The 9.5 mg/24 h (10 cm2) rivastigmine patch displays similar efficacy to 12 mg/d capsules on the ability to perform ADL in patients with mild-to-moderate AD 12 and demonstrates significantly greater efficacy over placebo on the IADL domain and HLF and autonomy factors. 11 In addition, the rivastigmine transdermal patch formulation offers a once-daily dosing regimen and continuous and consistent drug delivery over a 24-hour period. 18 Improved tolerability of patch versus capsules allows administration of a higher dose rivastigmine patch (13.3 mg/24 h) that has been recently approved for use in the United States, 20 providing access to additional efficacy for patients with AD.
Use of the higher dose (13.3 mg/24 h) rivastigmine patch was investigated in the OPtimizing Transdermal Exelon In Mild-to-moderate Alzheimer’s disease (OPTIMA) trial, a 72- to 96-week multicenter study, composed of a 24- to 48-week initial open-label phase with rivastigmine 9.5 mg/24 h (10 cm2) patch followed by a 48-week randomized, double-blind (DB), and parallel-group phase. 21 Patients displaying functional and cognitive decline during the initial open-label phase entered the DB phase, which compared the efficacy of the higher dose 13.3 mg/24 h (15 cm2) rivastigmine patch with that of the 9.5 mg/24 h (10 cm2) patch. 21 To further examine the efficacy of the newly approved higher dose (13.3 mg/24 h [15 cm2]) rivastigmine patch on the ability to perform ADL, we performed a post hoc analysis of the OPTIMA study on the individual items of the ADCS-IADL domain and the autonomy and HLF factors.
Materials and Methods
Study Population
This was a post hoc exploratory analysis of data from the OPTIMA study (Clinicaltrials.gov identifier NCT00506415), full details of which have been published previously. 21 Briefly, eligible patients were aged 50 to 85 years with a diagnosis of probable mild-to-moderate AD, according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association and a Mini-Mental State Examination (MMSE) score of ≥10 and ≤24. 21 Exclusion criteria included dementia or medical or neuropsychiatric conditions other than AD which could interfere with the evaluation of patient’s response to study medication; sensitivity to cholinergic compounds, or skin lesion/disorder that would prevent transdermal patch use; history or current diagnosis of cerebrovascular disease; and use of ChEIs or other approved AD treatments for 2 weeks prior to study enrollment, with the exception of stable memantine (if taken for more than 3 months prior to study entry). 21 The study was designed and implemented in accordance with Good Clinical Practice, and the local regulations and ethical principles laid down in the Declaration of Helsinki. All patients, or a legally acceptable representative, and caregivers provided written informed consent prior to participating in the study.
Study Design
Patients were enrolled into the initial 24- to 48-week open-label phase with 9.5 mg/24 h (10 cm2) rivastigmine patch. Those who met prespecified functional (assessed by investigator) and cognitive decline criteria (≥2-point decline in MMSE from previous visit or ≥3-point decline from baseline) were then randomized (1:1) to 48 weeks of DB treatment with 13.3 mg/24 h (15 cm2) patch or 9.5 mg/24 h (10 cm2) patch. The change from DB-baseline to week 48 on the ADCS-IADL scale was a coprimary outcome. Safety and tolerability assessments included the frequency of AEs and serious AEs (SAEs), the discontinuation rate due to AEs, the monitoring of vital signs, and the 12-lead electrocardiogram.
Outcomes
The current analysis compared the change from DB-baseline during the 48-week DB phase on the 17 items of the ADCS-IADL domain and on the previously defined HLF and autonomy factors 11 for 9.5 mg/24 h (10 cm2) and 13.3 mg/24 h (15 cm2) patch groups. The HLF items of the ADCS-IADL domain included using the telephone, watching the television, paying attention to conversation, finding personal belongings, keeping appointments, talking about current events, reading, writing, and participation in pastimes, hobbies, or games. Autonomy items of the ADCS-IADL included obtaining a beverage, making a meal or snack, traveling outside of the home, shopping, ability to be left alone, and using household appliances.
Statistical Analysis
Data were analyzed according to the randomized treatment. Efficacy analysis was based on the intent-to-treat population in the DB phase (ITT-DB) using a last observation carried forward imputation (ITT [DB]-LOCF). The ITT-DB population included all patients who received at least 1 dose of study drug and had at least 1 postrandomization assessment for the coprimary efficacy variables during the DB phase. Analysis was also conducted using observed cases (OCs) only without imputing any missing derived scores, based on the ITT-DB population (ITT [DB]-OC). The safety population during the DB phase consisted of all randomized patients who received at least 1 dose of study drug and who had at least 1 postrandomization safety assessment during the DB phase. Difference of least square mean (DLSM), 95% confidence intervals (CIs), and P values comparing the 13.3 mg/24 h (15 cm2) and 9.5 mg/24 h (10 cm2) patch groups were based on an analysis of covariance model adjusted for country and corresponding baseline scores. As a post hoc analysis in a select population, this study was not powered to detect specific differences between ADCS-IADL items. No adjustment for multiplicity was carried out due to the exploratory nature of the analysis.
Results
Study Participants and Disposition
Of 1584 patients enrolled into the initial open-label phase, 567 met the prespecified cognitive and functional decline criteria and were randomized into the DB phase; 280 to the 13.3 mg/24 h (15 cm2) patch and 287 to the 9.5 mg/24 h (10 cm2) patch. The DB-baseline demographics and characteristics were comparable between the treatment groups. 21
During the DB phase, patient disposition was well balanced between the 13.3 mg/24 h (15 cm2) and the 9.5 mg/24 h (10 cm2) patch groups. 21 In the 13.3 mg/24 h (15 cm2) patch group, 73.9% completed the DB phase compared with 70.7% of the 9.5 mg/24 h (10 cm2) patch group. 21 In both the groups, the most common reasons for discontinuation were AEs (13.3 mg/24 h [15 cm2] patch group, n = 28; 9.5 mg/24 h [10 cm2] patch group, n = 33). 21
Alzheimer’s Disease Cooperative Study-IADL Total Scores
As described previously, both the 9.5 mg/24 h (10 cm2) and the 13.3 mg/24 h (15 cm2) patch groups demonstrated functional decline from DB-baseline over the duration of the 48-week DB phase due to disease progression. 21 Overall, the between-group differences in change from baseline on the ADCS-IADL were numerically less with the 13.3 mg/24 h (15 cm2) patch compared with the 9.5 mg/24 h (10 cm2) at all time points and were statistically significant in favor of the 13.3 mg/24 h (15 cm2) patch compared with the 9.5 mg/24 h (10 cm2) patch at weeks 16 to 48 for the ITT [DB]-LOCF and ITT [DB]-OC populations 21 (Table 1).
Table 1.
Difference of Least Square Means Change From Baseline on the ADCS-IADL Domain During the 48-Week Double-Blind Phase Between the 13.3 mg/24 h (15 cm2) and 9.5 mg/24 h (10 cm2) patch groups; (A) ITT [DB]-LOCF and (B) ITT [DB]-OC.a
| A | |||||
| Week | DLSM | 95% CI | P value | ||
|---|---|---|---|---|---|
| 8 | 0.8 | −0.2, 1.9 | .114 | ||
| 12 | 0.7 | −0.5, 1.8 | .252 | ||
| 16 | 1.3 | 0.2, 2.5 | .025b | ||
| 24 | 1.7 | 0.5, 2.9 | .005b | ||
| 32 | 2.1 | 0.9, 3.4 | <.001b | ||
| 48 | 2.2 | 0.8, 3.6 | .002b | ||
| B | |||||
| Week | 13.3 mg/24 h (15 cm2) patch, n | 9.5 mg/24 h (10 cm2) patch, n | DLSM | 95% CI | P value |
| 8 | 257 | 261 | 0.8 | −0.2, 1.9 | .122 |
| 12 | 250 | 259 | 0.8 | −0.4, 2.0 | .174 |
| 16 | 237 | 243 | 1.7 | 0.5, 2.9 | .006b |
| 24 | 232 | 243 | 2.3 | 1.0, 3.6 | <.001b |
| 32 | 221 | 222 | 2.5 | 1.1, 3.9 | <.001b |
| 48 | 209 | 198 | 2.5 | 0.8, 4.1 | .004b |
Abbreviations: ADCS-IADL, Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living; CI, confidence interval; DLSM, difference of least square means; ITT [DB]-LOCF, intent-to-treat DB population with a last observation carried forward imputation; ITT [DB]-OC, intent-to-treat DB population with an observed cases imputation.
a n, number of patients with an assessment at baseline (last assessment in the initial open-label phase) and the corresponding visit; N, number of patients with an assessment at baseline (last assessment in the initial open-label phase) and with at least 1 postbaseline assessment. N = 265 for 13.3 mg/24 h (15 cm2) patch group and N = 271 for the 9.5 mg/24 h (10 cm2) patch group. DLSM are calculated for 13.3 mg/24 h (15 cm2) compared with 9.5 mg/24 h (10 cm2) patch groups. 95% CI and P value are based on an analysis of covariance model adjusted for country and baseline item score.
b P ≤ .05 for 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 h (10 cm2) patch.
Alzheimer’s Disease Cooperative Study-IADL Factor Analysis
Higher Level Functioning
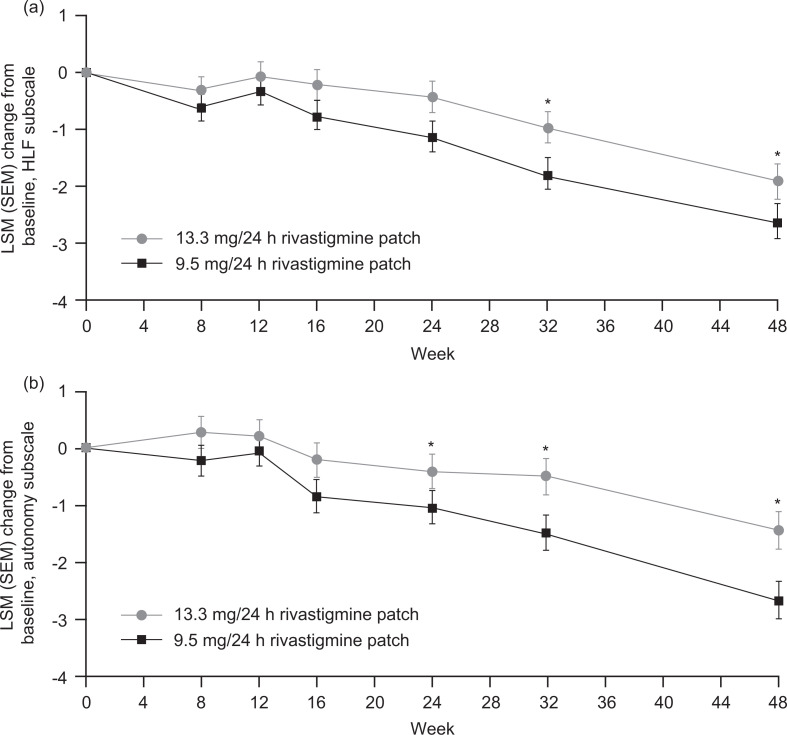
Numerically less decline was displayed by the 13.3 mg/24 h (15 cm2) compared with the 9.5 mg/24 h (10 cm2) patch at all time points during the study on the ADCS-ADL HLF factor. Statistical significance was reached at weeks 32 (DLSM 1.0; 95% CI 0.3, 1.7; P = .006) and 48 (1.2; 0.5, 2.0; P < .001; Figure 1A).
Figure 1.
Least square means change from baseline on the (A) HLF and (B) autonomy factors of the ADCS-IADL domain in the DB phase by treatment group (ITT [DB]-LOCF). Error bars represent the SEM. The difference of LSM and P values are based on an analysis of covariance model adjusted for country and corresponding factor baseline score. 13.3 mg/24 h (15 cm2) patch group, n = 265; 9.5 mg/24 h (10 cm2) patch group, n = 271. *P ≤ .05 for 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 h (10 cm2) patch. ADCS-IADL indicates Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living; DB, double-blind; ITT [DB]-LOCF, intent-to-treat double-blind population with a last observation carried forward imputation; LSM, least square means; SEM, standard error of the mean; HLF, higher level function.
Autonomy
The 13.3 mg/24 h (15 cm2) patch also displayed numerically less decline compared with the 9.5 mg/24 h (10 cm2) patch at all time points on the ADCS-ADL autonomy factor, reaching statistical significance at weeks 24 (DLSM 0.7; 95% CI 0.1, 1.3; P = .025), 32 (0.8; 0.2, 1.4; P = .009), and 48 (0.7; 0.0, 1.4; P = .041; Figure 1B).
Alzheimer’s Disease Cooperative Study-IADL Item Analysis
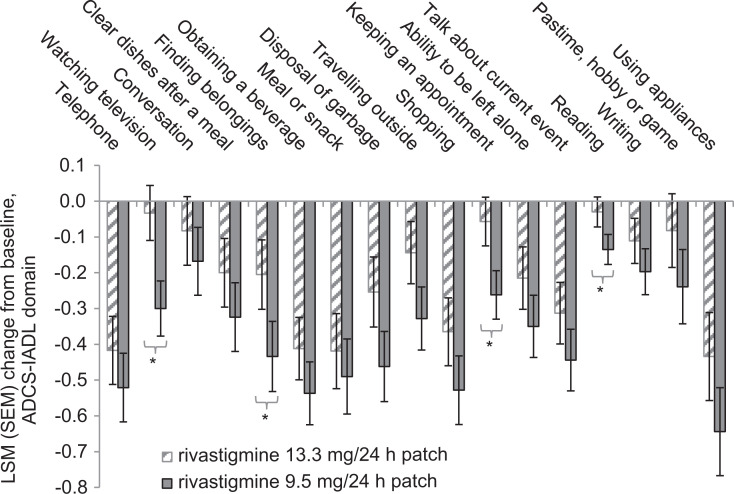
During the 48-week DB phase of this study, numerically less decline was displayed by 13.3 mg/24 h (15 cm2) compared with 9.5 mg/24 h (10 cm2) patch at all time points for 12 of 17 individual ADCS-IADL domain items. The observed treatment differences reached significance (P < .05) in favor of 13.3 mg/24 h (15 cm2) patch for 10 of 17 individual items at 1 or more time points (week 8, 12, 16, 24, 32, and/or 48). At week 24, patients receiving the 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 h (10 cm2) patch experienced numerically less decline from DB-baseline on 15 of the 17 items, reaching significance for 7 items (watching television, P = .034; finding personal belongings, P = .007; disposing of garbage, P < .001; clearing dishes after a meal, P = .005; reading, P = .018; traveling outside of the home, P = .020; and shopping, P = .029). At week 48, decline from DB-baseline was numerically less for all 17 items in patients randomized to receive the 13.3 mg/24 h (15 cm2) patch compared with the 9.5 mg/24 h (10 cm2) patch (Table 2), with statistical superiority observed on 4 items, watching television (P = .002), finding belongings (P = .037), keeping an appointment (P = .008), and reading (P = .028; Figure 2 and Table 2).
Table 2.
Mean ADCS-IADL Item Scores at DB Baseline and Change From Baseline at Week 48 of the DB Phase (ITT [DB]-LOCF population).a
| Item | Score range | 13.3 mg/24 h (15 cm2) patch | 9.5 mg/24 h (10 cm2) patch | DLSM (95% CI) | P value |
|---|---|---|---|---|---|
| 7. Using the telephone | 0-5 | ||||
| Baseline | 2.2 | 2.0 | |||
| Change at week 48 | −0.4 | −0.5 | 0.1 (−0.1; 0.3) | .337 | |
| 8. Watching television | 0-3 | ||||
| Baseline | 1.0 | 1.0 | |||
| Change at week 48 | −0.0 | −0.3 | 0.3 (0.4; 0.4) | .002b | |
| 9. Paying attention to conversation | 0-3 | ||||
| Baseline | 2.0 | 1.8 | |||
| Change at week 48 | −0.1 | −0.2 | 0.1 (−0.1; 0.3) | .427 | |
| 10. Clearing dishes after a meal | 0-3 | ||||
| Baseline | 2.1 | 2.0 | |||
| Change at week 48 | −0.2 | −0.3 | 0.1 (−0.1; 0.3) | .250 | |
| 11. Finding personal belongings | 0-3 | ||||
| Baseline | 1.8 | 1.7 | |||
| Change at week 48 | −0.2 | −0.4 | 0.2 (0.0, 0.4) | .037b | |
| 12. Obtaining a beverage | 0-3 | ||||
| Baseline | 1.7 | 1.7 | |||
| Change at week 48 | −0.4 | −0.5 | 0.1 (−0.1; 0.3) | .201 | |
| 13. Making a meal or snack | 0-4 | ||||
| Baseline | 1.6 | 1.5 | |||
| Change at week 48 | −0.4 | −0.5 | 0.1 (−0.2; 0.3) | .553 | |
| 14. Disposal of garbage | 0-3 | ||||
| Baseline | 2.1 | 2.0 | |||
| Change at week 48 | −0.3 | −0.5 | 0.2 (−0.0; 0.4) | .061 | |
| 15. Traveling outside of the home | 0-4 | ||||
| Baseline | 2.3 | 2.2 | |||
| Change at week 48 | −0.1 | −0.3 | 0.2 (−0.0; 0.4) | .063 | |
| 16. Shopping | 0-4 | ||||
| Baseline | 1.6 | 1.5 | |||
| Change at week 48 | −0.4 | −0.5 | 0.2 (−0.0; 0.4) | .131 | |
| 17. Keeping appointments | 0-3 | ||||
| Baseline | 1.2 | 1.2 | |||
| Change at week 48 | −0.3 | −0.3 | 0.2 (0.1; 0.4) | .008b | |
| 18. Ability to be left alone | 0-3 | ||||
| Baseline | 1.7 | 1.6 | |||
| Change at week 48 | −0.4 | −0.4 | 0.1 (−0.1; 0.3) | .167 | |
| 19. Talking about current events | 0-3 | ||||
| Baseline | 1.1 | 1.0 | |||
| Change at week 48 | −0.4 | −0.4 | 0.1 (−0.1; 0.3) | .179 | |
| 20. Reading | 0-2 | ||||
| Baseline | 0.4 | 0.3 | |||
| Change at week 48 | −0.1 | −0.1 | 0.1 (0.0; 0.2) | .028b | |
| 21. Writing | 0-3 | ||||
| Baseline | 0.8 | 0.9 | |||
| Change at week 48 | −0.2 | −0.2 | 0.1 (−0.1; 0.2) | .233 | |
| 22. Participation in pastimes, hobbies, or games | 0-3 | ||||
| Baseline | 1.6 | 1.5 | |||
| Change at week 48 | −0.2 | −0.2 | 0.2 (−0.1; 0.4) | .177 | |
| 23. Using household appliance | 0-4 | ||||
| Baseline | 2.2 | 2.0 | |||
| Change at week 48 | −0.6 | −0.6 | 0.2 (−0.1; 0.5) | .130 |
Abbreviations: ADCS-IADL, Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living; CI, confidence interval; DB, double-blind; DLSM, difference of least square means; ITT [DB]-LOCF, intent-to-treat DB population with a last observation carried forward imputation.
a N, number of patients with an assessment at baseline (last assessment in the initial open-label phase and at least 1 postbaseline assessment). The IADL items include items 7 to 23 of the ADCS activities of daily living scale. At week 48, N = 209 for 13.3 mg/24 h (15 cm2) patch group and N = 198 for the 9.5 mg/24 h (10 cm2) patch group. 95% CI and P value are based on an analysis of covariance model adjusted for country and baseline item score. No adjustment for multiplicity was carried out due to the exploratory nature of the analysis.
b P ≤ .05 for 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 h (10 cm2) patch.
Figure 2.
Least square mean change from baseline to week 48 of the DB phase on individual items of the ADCS-IADL domain (ITT [DB]-LOCF). Error bars represent the SEM. The P values are based on an analysis of covariance model adjusted for country and corresponding baseline item score. 13.3 mg/24 h (15 cm2) patch group, n = 209; 9.5 mg/24 h (10 cm2) patch group, n = 198. *P ≤ .05 for 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 h (10 cm2) patch. ADCS-IADL indicates Alzheimer’s Disease Cooperative Study-Instrumental Activities of Daily Living domain; DB, double-blind; ITT [DB]-LOCF, intent-to-treat DB population with a last observation carried forward imputation; LSM, least square mean; SEM, standard error of the LSM.
Safety and Tolerability
Full details of the safety and tolerability findings from the OPTIMA study have been described previously. 21 Treatment with rivastigmine patch was well tolerated in both the treatment groups. Overall, the incidence of AEs during the DB phase was greater with 13.3 mg/24 h (15 cm2) patch than with 9.5 mg/24 h (10 cm2) patch (75.0% vs 68.2%, respectively) and was predominantly cholinergic in nature. The most frequently reported AEs in descending order in the 13.3 mg/24 h (15 cm2) patch group were nausea (13.3 mg/24 h [15 cm2], 12.1%; 9.5 mg/24 h [10 cm2], 4.9%), vomiting (10.4% vs 4.6%, respectively), and falls (7.5% vs 6.0%, respectively). Incidence rates of AEs declined during the DB phase, with higher levels of incidences reported between weeks 0 to 24 compared with weeks 24 to 48 for nausea (13.3 mg/24 h [15 cm2], 9.6% vs 4.1%; 9.5 mg/24 h [10 cm2], 3.5% vs 1.6%), vomiting (13.3 mg/24 h [15 cm2], 8.9% vs 2.5%; 9.5 mg/24 h [10 cm2], 2.8% vs 2.4%), and falls (13.3 mg/24 h [15 cm2], 4.3% vs 3.7%; 9.5 mg/24 h [10 cm2], 3.5% vs 2.8%). The incidence of SAEs and deaths in the DB phase was similar between the 13.3 mg/24 h (15 cm2) and 9.5 mg/24 h (10 cm2) patch groups (SAEs: 15.7% vs 15.5%, respectively; deaths: 1.1% vs 1.8%, respectively). No deaths were considered to be related to the study medication. Discontinuations due to AEs or SAEs were fewer with the 13.3 mg/24 h (15 cm2) patch versus the 9.5 mg/24 h (10 cm2) patch (AEs leading to discontinuation: 9.6% vs 12.7%, respectively; SAEs leading to discontinuation: 4.3% vs 6.4%, respectively).
Discussion
Reduced functional ability is an impactful aspect of AD progression with respect to patients’ ability to live independently and the degree of support required from their caregiver. Current guidelines include monitoring of functional decline as an essential part of the diagnostic criteria for AD, evaluated by the patients’ ability to perform both BADL and IADL. 7 In addition, the European Medicines Agency guidelines now require the assessment of ADL, as well as cognition, when evaluating a drug for approval in the symptomatic treatment of AD. 5
The relationship between cognition and function is complex; however, improved overall cognitive ability is generally associated with better day-to-day functioning. 22 Pooled analyses show a relationship between declining executive functioning and impaired functioning in ADL. 23 Many cognitive measures commonly used in clinical trials, including the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), do not assess executive function and correlation between the ADAS-cog, and the ability to perform ADL is generally weak. 24,25 In contrast, tools with measures of executive function, such as the Dementia Rating Scale, 26 show strong correlations between the cognition and the ability to perform ADL. 25,27 An increase in pathological burden, such as neurofibrillary tangles and neuritic plaques, is also associated with deterioration in the ability to perform BADL in patients with AD. 28 This supports a relationship between the ADL measures and the neurobiology of AD.
Efficacy of rivastigmine (12 mg/d capsules and 9.5 mg/24 h [10 cm2] patch) over placebo has been demonstrated previously on cognition (ADAS-cog) and function (ADCS-IADL domain). 12,21 In the OPTIMA study, additional improvements in cognition and function were observed following treatment with the higher dose (13.3 mg/24 h [15 cm2]) patch, compared with the 9.5 mg/24 h (10 cm2) patch in patients with AD who had previously demonstrated functional and cognitive decline during initial open-label treatment with the 9.5 mg/24 h (10 cm2) patch. Less decline was displayed by the 13.3 mg/24 h (15 cm2) than the 9.5 mg/24 h (10 cm2) patch group during the 48-week study, with differences reaching statistical significance at 24 weeks for function and cognition, and at 48 weeks (study end point) for function (ADCS-IADL) but not cognition (ADAS-cog). In the current subanalysis of the OPTIMA study, the 13.3 mg/24 h (15 cm2) patch demonstrated a statistically significant (P ≤ .05) greater efficacy than the 9.5 mg/24 h (10 cm2) patch over the 48-week duration of the DB phase on both the HLF (weeks 32 and 48) and the autonomy (weeks 24, 32, and 48) factors of the ADCS-IADL domain. Higher level and autonomy functions affect daily activities and patient independence; therefore, reduced decline or stabilization of these functions has direct implications for both the patient and their caregiver. 11
Analysis of rivastigmine treatment effects on the ability to perform individual ADL has previously shown significant benefits of rivastigmine over placebo on several ADCS-BADL and IADL domain items, including bathing, clearing dishes after a meal, obtaining a beverage, disposal of garbage, traveling outside of the home, shopping, talking about current events, writing, and using household appliances. 29 In this study, the number of individual items displaying statistical significance varied over the DB treatment period. For example, 1 IADL item at week 8, 7 IADL items at week 24, and 4 IADL items at week 48 displayed significantly less decline in the 13.3 mg/24 h (15 cm2) patch group compared with the 9.5 mg/24 h (10 cm2) patch group. Of the 7 items showing significant benefit at week 24, 3 continued to show significant benefit at week 48, (reading, finding belongings, and watching television). Numerically less decline was displayed with the 13.3 mg/24 h (15 cm2) compared with 9.5 mg/24 h (10 cm2) patch and was maintained for the majority of items across the 48-week duration of the DB phase of this trial, suggesting a consistent and long-lasting effect on many items.
This and the previous analyses of the OPTIMA study demonstrate increased efficacy of 13.3 mg/24 h (15 cm2) patch compared with 9.5 mg/24 h (10 cm2) with an associated increase in the incidence of cholinergic AEs but not SAE. 21 Incidence of AEs decreased during the course of the study with similar incidences reported between the patch treatment groups of most AEs during the second half of the study (weeks 25-48). 21 Discontinuation rates during the 48-week DB phase were lower for 13.3 mg/24 h (15 cm2) patch compared with 9.5 mg/25 h (10 cm2), suggesting that the higher dose 13.3 mg/24 h (15 cm2) patch is well tolerated by the patients.
The observed findings indicate that the 13.3 mg/24 h (15 cm2) rivastigmine patch has improved efficacy compared with the 9.5 mg/24 h (10 cm2) patch and offer clinically meaningful benefits for patients and caregivers on key ADL. These are exploratory hypotheses generating observations that require further study and confirmation.
Functional decline is an important predictor of caregiver burden 30 and a strong risk factor for institutionalization. 31–33 Furthermore, the main factor affecting patient health-related quality of life is their dependence on others to perform ADL. 4 These outcomes on ADL support the 13.3 mg/24 h (15 cm2) patch as an important option for physicians to help preserve functional abilities in their patients with AD.
Conclusion
This analysis demonstrated that the high dose 13.3 mg/24 h (15 cm2) rivastigmine patch offers greater efficacy than the 9.5 mg/24 h (10 cm2) patch on total IADL score, HLF and autonomy IADL factors, and many individual items of the ADCS-IADL domain. The improved efficacy with the higher dose patch on these indicators of everyday function could further reduce the burden of the disease on caregivers and extend independence for the patient by improving or stabilizing their ability to perform daily functions.
Footnotes
Authors’ Note: This study was presented in poster format at the Alzheimer’s Association International Congress, July 14-19, 2012; Vancouver, Canada and at the 16th Congress of the European Federation of Neurological Societies, September 8-11, 2012; Stockholm, Sweden.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GG serves as a consultant to Accera, Baxter Bioscience, Forest Laboratories, Janssen, Eli Lilly, Lundbeck, Novartis, Otsuka, and Pfizer. His department receives research funding from Baxter Bioscience, Forest Laboratories, Janssen, Novartis, Pfizer and NIH. GG serves on 2 Safety Monitoring Committees for Merck.
JC has provided consultation to Abbott, Acadia, ADAMAS, Anavex, Astellas, Avanir, Avid, Baxter, Bayer, Bristol-Myers Squibb, Eisai, Elan, EnVivo, Forest, GE Healthcare, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Lundbeck, MedAvante, Medtronics, Merck, Neurokos, Neuronix, Neurotrax, Novartis, Otsuka, Pain Therapeutics, Pfizer, Plexxicon, Prana, QR, Sanofi, Sonexa, Takeda, Toyama, and UBC pharmaceutical companies. JC owns stock in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, Neurokos, and QR pharma. He has participated as a speaker/lecturer for Eisai, Forest, Janssen, Novartis, Pfizer, and Lundbeck. JC owns the copyright of the Neuropsychiatric Inventory. JC has provided expert witness consultation regarding olanzapine and ropinerol.
LF has received fees for board membership from Eisai, Elan/Wyeth, Eli Lilly, GE Healthcare, Janssen-Cilag, Lundbeck, Merz Pharma, Novartis, Pfizer, and Schering-Plough. LF has provided consultation to AstraZeneca, Merz Pharma, Myriad, Neurochem, Novartis, Pfizer, and Schering-Plough. He has provided an expert testimony to Apotex Inc. LF has also received grants or has grants pending from Novartis and Pfizer. He has received honoraria from Allergan, Eisai, Elan/Wyet, Janssen-Cilag, Lundbeck, Merz Pharma, Neurochem Inc., Novartis, and Pfizer. LF has received payment for the development of educational presentations including service on speakers’ bureau from Novartis. He has also received travel/accommodation expenses from Merz Pharma.
GB has participated as a speaker/lecturer for Novartis, Pfizer, Eli Lilly, and Lundbeck.
JLM has provided scientific advice or has been an investigator or data monitoring board member or received consultancy fees from Bayer, Bristol-Myers Squibb, Eisai, GE Healthcare, GlaxoSmithKline, Innogenetics, Janssen-Cilag, Lundbeck, MSD, Merz Pharma, Novartis, Pfizer, and Roche.
TK and CS are employees of Novartis Pharma AG, Basel, Switzerland.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The OPTIMA study and the current analyses were funded by Novartis Pharma AG, Basel, Switzerland. Editorial assistance in the development of this article was provided by Fishawack Communications Ltd, Oxford, UK; this service was funded by Novartis Pharma AG, Basel, Switzerland.
References
- 1. Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer's disease. Curr Neuropharmacol. 2010;8(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersen CK, Lauridsen J, Andersen K, Kragh-Sorensen P. Cost of dementia: impact of disease progression estimated in longitudinal data. Scand J Public Health. 2003;31(2):119–125. [DOI] [PubMed] [Google Scholar]
- 3. Potkin SG, Anand R, Hartman R, Veach J, Grossberg G. Impact of Alzheimer's disease and rivastigmine treatment on activities of daily living over the course of mild to moderately severe disease. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(4):713–720. [DOI] [PubMed] [Google Scholar]
- 4. Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sorensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Medical Agency. Guideline on medicinal products for the treatment of Alzheimer's disease and other dementias; 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003562.pdf. Accessed October 16, 2012.
- 6. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14(1):e1–e26. [DOI] [PubMed] [Google Scholar]
- 7. Hort J, O'Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010;17(10):1236–1248. [DOI] [PubMed] [Google Scholar]
- 8. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;(11 suppl 2):S33–S39. [PubMed] [Google Scholar]
- 9. Gauthier S, Bodick N, Erzigkeit E, et al. Activities of daily living as an outcome measure in clinical trials of dementia drugs. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Dis Assoc Disord. 1997;(11 suppl 3):6–7. [PubMed] [Google Scholar]
- 10. Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. Int J Geriatr Psychiatry. 2011;26(4):356–363. [DOI] [PubMed] [Google Scholar]
- 11. Grossberg G, Meng X, Olin JT. Impact of rivastigmine patch and capsules on activities of daily living in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2011;26(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer's disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007;22(5):456–467. [DOI] [PubMed] [Google Scholar]
- 13. Gauthier S, Lopez OL, Waldemar G, et al. Effects of donepezil on activities of daily living: integrated analysis of patient data from studies in mild, moderate and severe Alzheimer's disease. Int Psychogeriatr. 2010;22(6):973–983. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson D, Murray J. Galantamine: a randomized, double-blind, dose comparison in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2001;16(9):852–857. [DOI] [PubMed] [Google Scholar]
- 15. Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57(3):489–495. [DOI] [PubMed] [Google Scholar]
- 16. Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52(7):1070–1076. [DOI] [PubMed] [Google Scholar]
- 17. Anand R, Messina J, Hartman R. Dose–response effect of rivastigmine in the treatment of Alzheimer's disease. Int J Geriatr Psychopharmacol. 2000;2(2):68–72. [Google Scholar]
- 18. Kurz A, Farlow M, Lefevre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: a review. Int J Clin Pract. 2009;63(5):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Exelon® US prescribing information; 2010. http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf. Accessed June 19, 2012.
- 20. Exelon US prescribing information; 2012. http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf. Accessed June 20 2012.
- 21. Cummings J, Froelich L, Black S, et al. Randomized, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm2) in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(5):341–353. [DOI] [PubMed] [Google Scholar]
- 22. Burton CL, Strauss E, Hultsch DF, Hunter MA. Cognitive functioning and everyday problem solving in older adults. Clin Neuropsychol. 2006;20(3):432–452. [DOI] [PubMed] [Google Scholar]
- 23. Martyr A, Clare L. Executive function and activities of daily living in Alzheimer's disease: a correlational meta-analysis. Dement Geriatr Cogn Disord. 2012;33(2-3):189–203. [DOI] [PubMed] [Google Scholar]
- 24. Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, Dartigues JF. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40(11):1129–1134. [DOI] [PubMed] [Google Scholar]
- 25. Fields JA, Machulda M, Aakre J, et al. Utility of the DRS for predicting problems in day-to-day functioning. Clin Neuropsychol. 2010;24(7):1167–1180. [DOI] [PubMed] [Google Scholar]
- 26. Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 27. Greenaway MC, Duncan NL, Hanna S, Smith GE. Predicting functional ability in mild cognitive impairment with the Dementia Rating Scale-2. Int Psychogeriatr. 2012;24(6):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):56–59. [DOI] [PubMed] [Google Scholar]
- 29. Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. Int J Geriatr Psychiatry. 2011;26(4):356–363. [DOI] [PubMed] [Google Scholar]
- 30. Razani J, Kakos B, Orieta-Barbalace C, et al. Predicting caregiver burden from daily functional abilities of patients with mild dementia. J Am Geriatr Soc. 2007;55(9):1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wattmo C, Wallin AK, Londos E, Minthon L. Risk factors for nursing home placement in Alzheimer's disease: a longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist. 2011;51(1):17–27. [DOI] [PubMed] [Google Scholar]
- 32. Hatoum HT, Thomas SK, Lin SJ, Lane R, Bullock R. Predicting time to nursing home placement based on activities of daily living scores—a modelling analysis using data on Alzheimer's disease patients receiving rivastigmine or donepezil. J Med Econ. 2009;12(2):98–103. [DOI] [PubMed] [Google Scholar]
- 33. Wattmo C, Wallin AK, Londos E, Minthon L. Long-term outcome and prediction models of activities of daily living in Alzheimer disease with cholinesterase inhibitor treatment. Alzheimer Dis Assoc Disord. 2011;25(1):63–72. [DOI] [PubMed] [Google Scholar]