Abstract
It has been postulated that Alzheimer disease (AD) is a systemic process, which involves multiple pathophysiological factors. A combination of pharmacotherapy and nonpharmacological interventions has been proposed to treat AD and other dementia. The nonpharmacological interventions include but are not limited to increasing sensory input through physical and mental activities, in order to modify cerebral blood flow and implementing nutritional interventions such as diet modification and vitamins and nutraceuticals therapy to vitalize brain functioning. This article highlights the recent research findings regarding novel treatment strategies aimed at modifying natural course of the disease and delaying cognitive decline through simultaneous implementation of pharmacological and nonpharmacological modulators as standardized treatment protocols.
Keywords: dementia, Alzheimer disease, depression, cardio- and cerebrovascular disease, nutrition, physical exercises, memory training, nonpharmacological interventions, integrative treatment, naturalistic study
Introduction
Alzheimer disease (AD) is a chronic and progressive neurodegenerative disorder, with multiple pathophysiological mechanisms that affect brain and body functions. 1 The AD and other mixed dementia currently affect more than 5 million individuals in the United States, with ever-growing incidences reported on a daily basis. The AD in particular is characterized by a diverse combination of symptoms reflecting the complexity of vascular, biochemical, and morphological changes in the brain during development and progression. The amyloid cascade hypothesis has now dominated the field of AD for many years, but the intensive research concerning amelioration of protein abnormalities in AD based on the amyloid hypothesis does not have practical value yet. 2 Conventional therapies like monotherapy or combination of multiple medications are also not able to diminish or stop the disease progression.
Based on many years of basic and clinical AD research, we have found that multiple factors should be taken into account to devise treatment strategies for this devastating disease. 3 Among these, hemodynamic and nutritional factors have been extensively studied in clinical studies and animal models that mimic AD-like pathology. 3 The hemodynamic factors, particularly chronic brain hypoperfusion, along with other microcirculatory abnormalities affecting cerebral blood flow (CBF) and brain structural changes have been well documented by our group as well as other researchers. 3–6 A decrease in CBF has also been reported during the progression of dementia. 6 Nutritional deficiencies begin in the early stages of AD with a loss of taste and smell, which interferes with normal digestive processes. This in turn leads to digestive disorders, malnutrition, and weight loss in advanced stages of dementia. 7
Nutrition and other nonpharmacological interventions, especially physical and cognitive activities, have shown promising results in delaying the onset of dementia and could significantly improve the outcome of dementia and/or depression treatment. 8,9 The main driving force for this hypothesis is that combined therapy has an unique influence on brain functions in patients with AD or other dementia/depression, and potentially exerts a synergistic effect, by controlling the rate of cognitive decline and improving the quality of life, thus delaying nursing home placement. Existing clinical research observations have focused on the potential implementation of one of the several modifying factors, with or without medication. Unfortunately, research related to the simultaneous implementation of multiple nonpharmacological interventions and medications is severely limited. The majority of these studies are related to diet, physical, and cognitive activities alone but not in combination. In addition, most of these studies were performed in animal models, so immediate implementation of these results into clinical practices is very difficult. The goal of this review is to highlight the recent clinical and animal studies concerning the possibility of modifying the course of dementia and potentially improving the cognitive functioning through simultaneous implementation of pharmacological and nonpharmacological treatment regimes in the real-life clinically ill patients with dementia and/or depression. 8,9
The Influence of the Vascular Hemodynamics Failure in the Context of Dementia
Effects of CBF in Initiation and Progression of Dementia
The fact that cardio- and cerebrovascular pathology goes along with neurodegenerative processes in dementia is well documented. 10 However, it remains necessary to investigate the interconnections and order of occurrence of these factors (Table 1). The course of dementia is associated with progressive changes in vascular pathology, increase in the number of micro and lacunars infarcts, cerebral atrophy, white matter changes, and signs of demyelination. 10–12
Table 1.
Demographics and Clinical Profile of Patients.a
| Patients | Percentage | |
|---|---|---|
| Men | 79 | 50.64 |
| Women | 77 | 49.36 |
| Mean | Standard Deviation | |
| Age | 73.65 | 5.43 |
| Education, years | 12.71 | 2.91 |
| Depression duration (months) | 39.00 | 22.87 |
| Memory duration, months | 8.00 | 3.20 |
| Diagnosis | Patients | Percentage of group |
| Anxiety | 130 | 83.33 |
| Insomnia | 128 | 82.05 |
| Hypertension | 133 | 85.26 |
| High cholesterol | 112 | 71.79 |
| Coronarv arterv disease | 91 | 58.33 |
| Diabetes | 56 | 35.90 |
| Arthritis | 53 | 33.97 |
| Stroke | 26 | 16.67 |
| Head trauma | 29 | 18.59 |
| Thyroid disorder | 18 | 11.54 |
| Cancer | 18 | 11.54 |
| COPD | 15 | 9.62 |
| Parkinsonism | 10 | 6.41 |
| Renal insufficiency | 10 | 6.41 |
| Anemia | 6 | 3.85 |
| Radiology Findings | Patients | Percentage of Group |
| Normal | 17 | 23.9 |
| Abnormal | 54 | 76.1 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
a Reprinted with permission from Bragin et al. 9
The CBF changes have been well documented in normal aging, mild cognitive impairment (MCI), and dementia, using different imaging techniques like single-photon emission computed tomography (SPECT), functional magnetic resonance imaging, positron emission tomography, and others. On a regional CBF (rCBF)-SPECT test, people with mild AD showed significant reduction in rCBF in the left parietal cortex during an episodic memory task. 10,13 Based on the rCBF data, there is a correlation between letter fluency and activity in the left prefrontal area. Additionally, category fluency scores correlate with rCBF activity in the left prefrontal as well as left temporal areas. 14 The conversion from MCI to AD, as well as the progression of AD, is associated with CBF changes. 3,10 The lower the patient’s CBF, the faster and more drastic is the decline in Mini-Mental State Examination (MMSE) scores. 15 It has been shown in CBF studies that the first notable changes start in the entorhinal and hippocampal areas of the brain, which gradually expands into the temporal, parietal, and frontal lobes. 16 At the same time, it has been reported that the blood circulation in the sensory motor strip areas and the cerebellum is relatively well preserved in dementia. 17 This observation provides a basis for the preservation of procedural memory in dementia, which is primarily initiated in the sensomotor areas of the brain. 18 This study also suggested that the regulation of CBF, characterized by unequal blood volume distribution in the cortex, is most likely relatively preserved, at least in sensory motor strip and cerebellum in moderate stages of the disease. Another example of preserved CBF in patients with dementia reported from this study indicates that an increased level of CBF was observed in frontal–occipital cortex in patients with mild/moderate AD (7 people) compared to the control group (8 healthy individuals). 19 However, many questions regarding how blood flow changes are involved in the pathobiology of dementia including AD remains to be explored. The exact and unknown factors that control local blood flow requires more extensive studies that will potentially open new knowledge avenues for AD therapeutics. 3,10
The Relationship Between CBF and Cognitive Performance
The relationship between CBF and cognitive performance has been extensively highlighted in recent reports. 3,10 There is a linear positive correlation between mental activities and CBF in healthy individuals, demonstrating that the normal CBF appears to be one of the key components in delaying the onset of dementia. 20 Research related to improving CBF in patients with AD through the use of cognitive activities is yet to be properly investigated. 3,10 Recent reports showed that a program of mental exercises for nursing home residents with mild AD was associated with an improvement in cognitive function after 6 months of its implementation. This program was developed and extensively used, based on a previous research by Kawashima. 21 This brief analysis indicates that physical and mental exercises alone, and especially in combination, are able to improve CBF and therefore cerebral metabolism and decrease the harmful effects and/or consequences of hypoxia and/or ischemia/reperfusion that increases the availability of oxygen and nutrients to brain cellular compartments. Outcome of this effectiveness explores improvement in the mental and cognitive performance. 3,8–10
Influence of the Physical Activities on CBF and Cognitive Performance
The relationship between physical activities and rCBF have been extensively studied in humans (healthy seniors and patients with MCI) as well as animal models that mimic human MCI or mental retardation. 22,23 Physical exercise is considered as a preventive and/or disease-modifying intervention that has significant effects on the brain neuroprotection that decreases with age. 24
The effect of specific trainings and aerobic exercises have been considered as factors that initiate increased functional and biochemical activities affecting the whole body including cardio- and cerebrovasculature and increase the rate of CBF. 25 However, the available literature and our own experiences strongly suggest that aerobic exercises create large scale problems for the “ fragile ” aged populations and/or medically ill patients, especially patients with dementia/depression or AD. The problems include interruption of homeostasis at the systems, organs, cellular, and subcellular levels, which in turn appears to be secondary injury stimulus inducing oxidative stress and formation of unwanted reactive oxygen species (ROS) and reactive nitrogen species (RNS) that cannot be utilized in medically ill patients. 3,10 Moreover, very often these type of activities appear to be the main reason for complication of coexisting diseases in aged populations that make situation even worse. 3,10
Our knowledge and ongoing naturalistic observation demonstrate that hand exercises are more suitable and safer for the fragile patients in all stages of dementia/depression and AD, as they require limited muscle involvement and very minimal level of whole body activation. 8,9 Therefore, we propose that seated and/or lying down positions appear to be more optimal and effective techniques from the practical stand point in the context of home based exercise regimen 8,9 that do not require any special arrangement and/or expensive equipments or trainer. 25 Simple hand movements have been shown to increase CBF in contralateral hemisphere of healthy individuals. 26 In healthy volunteers, meditation with simultaneous chanting and finger movements (dual tasks) has been reported to increase the CBF, which was observed by SPECT. 27 Therefore, local and/or peripheral physical exercises can be considered as a preventive and disease modifying modalities, which can be implicated not only for the brain aging but also as modulating factors for the patients with dementia/depression and AD. 8,9
Nutrition as a Pathogenic Factor in the Development and Maturation of Dementia
In epidemiological studies, nutrition has been given special attention as an important factor that has significant contribution to healthy aging and prevention of dementia and/or other chronic diseases such as arteriosclerosis, high blood pressure, diabetes, arthritis, renal failure, and cancer. 28 Based on the literature evidences and our current knowledge, the nutritional aspect as preventive and treatment options for dementia and AD can be summarized under the following 3 categories.
The first component of this strategy is related to the diet per se. There is currently no consensus regarding diet composition that can at least partially or completely restore and diminish brain metabolism failure that occurs in patients with dementia and/or AD. Growing body of significant attention regarding the well known Mediterranean diet was recently brought to a ketogenic diet. These 2 diets may have a neuroprotective effect on aging and especially during the course of the neurodegenerative diseases, such as AD, Parkinson’s disease (PD), stroke, traumatic brain injury, mitochondrial disorders, epilepsy, amyotrophic lateral sclerosis, and multiple sclerosis. 28 In addition, if we take into consideration that cardiovascular and glucose metabolic abnormalities and failure accompanies the progression of dementia, changes in the dietary content can become an important factor in disease modification in the patients having dementia and/or AD coexisting with cardio- or cerebrovascular disease as well as diabetes and other diseases. 8,9 Therefore, diet modification can be considered an important part of treatment strategies, especially the diet enriched with high-quality fats and devoid of simple carbohydrates is the most suitable and practically acceptable option for patients with dementia and/or AD.
The second components of the diet, which play a crucial role, are the vitamins and nutraceuticals known to have significant effects on the age-associated diseases including dementia and other cognitive conditions. 8,9,29–31 Vitamins and nutrients as a part of cellular metabolic activities are extensively utilized in aged populations as well as those under the chronic oxidative stresses, especially in the conditions that appear to be pathogenic factors for the disease development. 32,33 A recent report demonstrated that the vitamin/nutraceuticals formulation appears to be one of the successful strategies for the treatment of moderate and later stages of AD based on the placebo-controlled pilot study. 34 This study also showed that even single modification in the vitamin/nutraceuticals formulation is capable to stop or completely diminish the memory decline in patients with AD. Moreover, the role of vitamins/minerals and nutritional components with the combination of mitochondrial selective antioxidants has been shown to be a new and more successful treatment strategy in animal models of aging and pathology that mimic AD-like conditions. 3,31,33,35
The pilot study by Remington and coworkers supports the hypothesis that even in patients with severe dementia, modification or addition of vitamins and nutrients as dietary supplements is able to attenuate disease progression. 34,36,37 These vitamin/nutraceutical combinations and medical foods consist of antioxidants, vitamins B, D, E, and other vitamins, and substances essential for energy production and protein synthesis in the body.
It has also been reported that low micronutrient intake may accelerate the age-associated degenerative diseases through allocation of scarce micronutrients by triage. 30 To maximize human health and life span, scientists must abandon outdated models of micronutrients. 31 So, the general recommendation for the supplementation of diet appears to be the diet enriched with antioxidants, especially the precursors for mitochondrial function and membrane phosphatide synthesis, which appears to be major factor for controlling membrane permeability, oxygen consumption, normal mitochondrial function, and their stabilities for the adequate cellular homeostasis. 31,35,38,39 Therefore, this type of clinically oriented research is able to provide not only more detailed information about the pathophysiology of dementia and/or AD but also a better and simplified way of treatment strategies against these incurable diseases. 3,40–42
The third component of the dietary factors that associates with the gastrointestinal system (GIS) appears to be homeostasis and activities of daily life (ADLs). It has been identified that very often the early stages of dementia and its progression frequently coexists or manifests as nutritional disorders such as anorexia, maldigestive symptoms, malnutrition, and weight loss. 43 Moreover, this study also showed that in the early stages of dementia, the loss of taste and smell appeared to result in loss of appetite and disrupt normal GIS function. For example, in the early stages of AD, community-dwelling patients display poor nutritional consumption. 43 Moreover, patients with dementia often forget to eat or drink normally. Patient with dementia loses feeling of thirst and often became dehydrated and agitated. In the advanced stages of dementia, progressive GIS malfunctions coexist with chewing and swallowing problems, dysphagia as well as decreased thirst, which in turn is related to poor digestion and absorption, vitamin deficiencies, decreased immunity, poor balance, falls, and loss of muscle mass that also results in increased frequency of viral and bacterial infections, 7 In addition, weight losses are very often associated with severity and mortality rate of the patients with AD and has been suggested as hallmarks of the protein, energy, vitamin, and nutrient deficiency in these patients. 44 According to this report, in the middle stage of AD, significant weight loss (MMSE—16.6 ± 4.9) was observed in more than 40% of the patients living at home. 44
The presence of maldigestive symptoms and malnutrition in patients with dementia also initiates GIS dysregulation in general, which characterizes change in appetite, weight, and GIS motility, and their future depends on other life-supporting organ dysfunctions such as decrease in the exocrine and endocrine function of the pancreas. Further, exocrine and endocrine pancreatic insufficiencies appear to be permanent features of the GIS in patients with dementia. 45 The most permanent indicator of pancreatic exocrine insufficiency was seen at the level of fecal enzyme elastase 1, as its level decreases progressively with age. In 1 study, pancreatic exocrine insufficiency was found in 21.7% of the people older than 65 years of age without gastrointestinal disorders, surgery, or diabetes. 45
Another report indicates that there is a pancreatic exocrine insufficiency in 32% of the patients with non insulin diabetes and 51% of the patients with insulin-dependent diabetes. 46 Therefore, it is evident that due to pancreatic insufficiency during the aging process and in diabetes and changes in glucose metabolism in dementia, it is quite possible that exocrine pancreatic insufficiency could play a certain role in the digestive malfunctions in dementia and should be taken into account for the treatment plan of patients with dementia and/or AD. 9
Role of Other Factors in the Pathogenesis of Dementia
Pharmacological Factor
The role of pharmacological interventions as a significant factor has been considered as highly modifiable, because the optimal combination of psychopharmacological and other medications is able to effectively diminish most signs and symptoms of the early and moderate stages of the disease progression in patients with dementia and/or AD. 8,9 The medications used in the treatment of AD are derived from almost all of the available psychopharmacological active medications. 8,9
Currently, 2 Food and Drug Administration (FDA) approved classes of medication related to cognitive decline in AD have been extensively used (1) acetylcholinesterase inhibitors and (2) N-methyl-D-aspartate receptor antagonists. 8,9 Combination medication strategies that utilize both classes of antidementia medications have shown to have an advantage over monotherapy in the management of cognitive decline and behavioral problems. 47 There are quite a few studies related to preservation of CBF in patients with AD taking Donepezil for over a year. 48 Similar type of efficiencies are also reported from patients taking Rivastigmine for 2 years. 49 One of the biggest challenges of using acetylcholine esterase inhibitors appeared to be their very limited or sometimes almost negative effects on brain neuroplasticity. 50,51 However, other studies show contradicting effects of the Donepezil’s treatment on the hippocampal volume. 52,53 Memantine, as a glutamate receptor antagonist, has an effect on the glutamatergic transmission, preventing excess influx of calcium into neurons and has been considered as a neuroprotective agent capable of blocking excitotoxicity.
Finally, antidepressants have also been considered as a treatment option for patients with dementia/depression. Most likely, antidepressants act via the stimulation and/or suppression of the brain receptor networks (serotonin, norepinephrine, and dopamine) and thus can be considered as a potential medication that is able to change the course of AD, especially when dementia coexists with depression, anxiety, fear, and insomnia. 8,9
Emotional Factor
The emotional factor is another modifiable factor of dementia having direct relation to stress response, which interferes negatively with cognitive performance, especially attention, concentration, and working memory that is very often observed in patients with dementia and depression. 8,9 Increased levels of cortisol and activity of the hypothalamic pituitary axis are seen with rapid cognitive decline in dementia. 54
Among the various emotional problems, depression, anxiety, anger, apathy, and fear are commonly encountered in patients with dementia and depression. Therefore, if we take into account how emotional factors appear to be a very important pathogenetic factor for the disease progression, then we will be able to improve the quality of life in patients with dementia/depression or AD.
Coexisting Medical Comorbidities Factors
Aging is characterized with slowdown of the organ and tissue function. These effects are much more visible on highly metabolic organs like brain, heart, and other life supporting systems. 3 It has been well established that in most of the cases, neurodegenerative diseases coexist with other medical illness (Table 1). 9 In addition, comorbidities have significant and very often accelerating effects on the highly metabolic active organs and systems such as brain. 3 The metabolic misbalance and/or symptoms that were seen in the patients with obesity, high blood pressure, significantly high cholesterol levels, hypertension, diabetes, cardiac failure, and liver dysfunction showed that correction of the deficiency of vitamins B12, D2, and D3 levels has a positive impact on cognitive performances. 9 Moreover, correction of the vision and hearing loss not only helps the patients in restoring specific function of these organs but also seems to have positive effects on the restoration of the sensory information input to audiovisual centers of the brain in patients with dementia. 9 These findings indicate that the treatment of comorbidities has a positive impact on the cognitive performance of the patients with dementia/depression and/or AD.
An Integrative Treatment Strategy as an Unique and Successful Option for the Treatment of the Patients With Dementia and AD
Foundation for Integrative Treatment Strategy in the Context of Dementia and AD
Oxidative stress in the cardio- and cerebrovascular system, central nervous system, and brain parenchymal cells results in accumulation of ROS and RNS, thus promoting cellular hypoperfusion and leukocyte adhesion and thus increasing endothelial permeability. 3,10 The resulting chronic injury stimulus results in progressive cellular and subcellular hypometabolism. Recently, we proposed that hypometabolism coupled with oxidative stress is responsible for most cases of AD and cerebrovascular accidents and appears to be a central initiating factor for vascular abnormalities, mitochondrial damage, and an imbalance in the activity of vasoactive substances, such as different isoforms of nitric oxide synthase (NOS), endothelin 1 (ET-1), oxidative stress markers, mitochondrial DNA (mtDNA), and mitochondrial enzymes in the vascular wall and brain parenchymal cells. 3,10 At higher concentrations, ROS induces cell injury and death, which occurs during the aging process, where accelerated generation of ROS and a gradual decline in cellular antioxidant defense mechanisms occurs, especially in the neuronal and vascular endothelial mitochondrion. Because the vascular endothelial and neuronal mitochondria are especially vulnerable to oxidative stress due to their role in energy supply and use, it can cause a cascade of debilitating factors such as the production of giant and/or vulnerable young mitochondrion who’s DNA has been compromised. Therefore, mtDNA abnormalities such as overproliferation and/or deletion can be used as a key marker for disease differentiation and effectiveness of the treatment. 3,10 We hypothesize that mitochondrial specific antioxidants such as acetyl-l-carnitine and R-α lipoic acid seem to be potential treatment options for AD. 31,33 They target the factors that damage mitochondria and reverse their effects, thus eliminating the imbalance seen in energy production and restore the normal cellular functions, making these antioxidants very powerful alternate strategies for the treatment of cardio- and cerebrovascular as well as neurodegenerative diseases including AD. In addition, future potential exploration using mtDNA markers can be considered more accurate hallmarks for diagnosis and monitoring the treatment of human diseases. 3,10 Moreover, age-related dementias such as AD and vascular dementia have been linked to vascular disorders like hypertension, diabetes, and atherosclerosis. 3,10 These risk factors cause ischemia, inflammation, oxidative damage, and consequently reperfusion, which is largely due to ROS that are believed to induce mitochondrial damage. 31–33 The integrative treatment strategies, which target the factors that initiate complex failure-manifesting dementia and mental retardation are able to reverse its effect, thus eliminating the imbalance seen in energy production and amyloid β-oxidation and making these antioxidants very powerful alternate strategies for the treatment of AD. 31,33 Therefore, using combination therapy including pharmacological and nonpharmacological modulators can provide ample opportunities for the treatment of the patients with dementia and AD. 3,10,55
Animal Models
Brain energy disorders can be present in aged men as well as animals. In this respect, the mitochondrial and free radical theory of aging postulates that age-associated brain energy disorders are caused by an imbalance between pro- and antioxidants that can result in oxidative stress. Our recent study was designed to investigate brain energy metabolism and the activity of endogenous antioxidants during the life span in 2- and 3-vessel occlusion models of brain ischemia/reperfusion and ApoE transgenic mice that mimics MCI and AD-like pathology in humans. 4–6,23,31,33–34,56 These reports indicate that the level of enzymatic antioxidant catalase, as well as other biomarkers, is significantly increased in the brain tissue, in the peripheral blood of aged animals as well as in a different models, which in turn can be diminished using selective or combination of the antioxidants with multivitamins and other ingredient (NOS inhibitors, ET-1 receptor antagonists, etc). We hypothesize that mitochondrial dysfunction and oxidative inactivation of endogenous enzymes, especially during brain hypoperfusion and hypometabolism, may participate in age-related disorders of brain energy metabolism dysregulation that manifests as dementia and cognitive failure, the clinical signs of AD. 3 Therefore, further research on animal models may provide crucial information that can be used as a basis for the clinical trials and treatment against these devastating diseases.
Clinical Findings
The encouraging outcome of different animal models has been extensively implicated for the clinical research including randomized-controlled clinical trials (RCCTs). In this section, we will summarize the possible effectiveness of combined integrative treatment in medically ill patients showing clinical signs of dementia/depression and AD. 3,8,9
There is a recent report on the efficiency of the nonnutrition-related, nonpharmacological therapies (NPTs) analysis based on 179 RCCTs. The substantial increase in the number of RCCTs over the past 30 years indicates a significant growing interest among researchers toward NPT. 3,8,9 Moreover, in most of the cases, NPTs appeared to be heavily cost-effective and useful therapeutic modality for patients and their caregivers. Therefore, this aspect appeared to be the main problem in the large-scale clinical trials and monitoring, because the delay in institutionalization of patients with dementia got associated with multimodal interventions of the caregiver. 57
Another report observed from 22 RTCs of physical exercises or cognitive intervention for all stages of dementia and social interventions for mild to moderate dementia showed that the baseline MMSE scores in all studies related to physical and cognitive activities were significantly increased from 8.8 to 24.5. 58 Moreover, the physical activity trials showed improvement in cognition and ADL in 6 of the 8 trials. In the remaining 2 trials with severe dementia (initial MMSE—8.8 and 10.7, respectively), physical performance was visibly improved after the observation period. Nine cognitive activity (intervention) trials demonstrated cognitive improvement in 5 studies (initial MMSE was 14.8-24.53) and stabilization of cognition in 4 studies. 58 However, in our judgment, the weakness of this report lies in the absence of any data regarding how physical and mental activities play one of the key components in decreasing cognition, behavior, and/or ADLs.
It has been well documented that the brain-activating rehabilitation is able to improve the orientation and diminish the level of withdrawal without any changes in cognition in mid 80s elders with moderate dementia living in residential care settings. 59 The implementation of nutraceuticals in the RTC format showed the stabilization of cognitive functions in all stages of dementia. 34,60 However, there is a lack of research on the long-term effects of simultaneous implementation of medications and all currently available nonpharmacological modalities such as sensory stimulations (physical and mental activities, music, light, and others) as well as diet, vitamins, and nutraceuticals. 3
Integrative Treatment Protocols in the Context of Dementia and AD: Success of 5 Years Naturalistic Observation
The design of an integrative treatment as a successful strategy for the treatment of patients with dementia/depression and AD with chronic illness requires taking into consideration all of the above-mentioned factors having significant outcome on the treatment results (Table 1). It has been well documented that aged individuals and/or patients are more vulnerable and very often prone to any possible drug–drug interaction or synergistic effects between different modalities. 3
We for the first time developed and clinically implemented the combined treatment as a successful and effective treatment strategy for the patients with dementia/depression and AD with coexisting illness. 8,9 The first and foremost basis for our protocol takes into account the modification of CBF in the brain by implementing all existing sensory modalities. Sensory activations mainly consist of a combination of physical and mental exercises and other sensory activities such as listening to music, singing, and using light-inducing changes in the CBF. 8,9 Moreover, breathing exercises and stress management techniques also appear to be an important sensory activation stimulus. 8,9,25 The second important factor that has been taken into the account is the use of combined medication strategies (antidementia and antidepressant medications) that are aimed at potentially partial or complete restoration of neurotransmitter deficiencies, as well as medication for accompanying symptoms, such as insomnia, pain, depression, and so on. 8,9 The third basis in our protocols is the nutritional factor that can be applied not only for normalizing the GIS function but also for increasing the efficiency of enzymatic systems and therefore accelerating digestive processes (pancreatic and liver support). This finally affects the delivery of necessary nutrients and gas into the cellular and subcellular compartments, especially highly metabolic organs such as brain, heart, and others and also inducing removal of oxidants and their derivatives and normalizing normal energy function that can lead to an improvement in the mental and cognitive function. 3,8,9
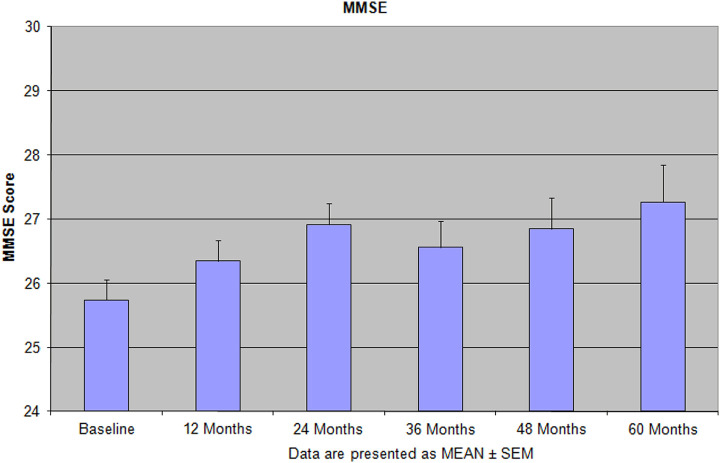
Implementation of this model that includes disease specific medications, vitamins and supplements, and physical and mental activities showed not only improvement in the patients cognitive and mental function but also improved their ADL. 8,9 Especially under this protocol, we have found that the patients treated with the combination of memantine, new antidepressants (escitalopram and duloxetine), and vitamins and supplements showed more reliable improvement than single administration any of these pharmacological and nonpharmacological medication or supplements (Figures 1 –4). 8,9 During 5 years of treatment, the new medications, memantine, escitalopram, duloxetine, vitamin D, lovaza, methylfolate, and axona, were used for the treatment. The novel approach of our treatment protocols is the notion of ongoing sensory activity and stimulation at home-based programs that does not require any additional interruption on the patient’s life style. 8,9 The unique feature of our treatment protocols and observational naturalistic study is the use of individually tailored treatment, based on the clinical symptoms and patients’ physical and mental capacities that takes into account all the issues in aged demented/depressed and AD populations 8,9 (Table 1; Figures 1 –4). This study has been organized as a proof of conception to verify our working hypothesis about the possibility of preserving cognitive functions in golden age (Figures 1 –4). Yearly cognitive assessment for the entire period of active treatment in our center demonstrated success of this strategy. In another 60-month study, we found that the performance on cognitive tests was preserved for the entire period of observation, and some tests (the MMSE, attention and judgment on The Neurobehavioral Cognitive Status Examination, and Total Unique Designs on Ruff Figural Fluency Test) showed an increase in scores above the baseline level at the end of treatment (Figures 1 –4). 9
Figure 1.
Features of the Mini-Mental State Examination (MMSE) score during 60 months of the integrative treatment protocols. Reprinted with permission from Bragin et al. 9
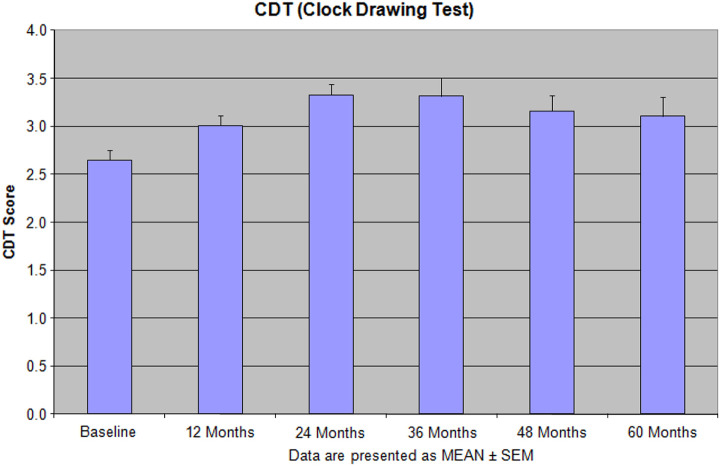
Figure 2.
The effectiveness of the integrative treatment protocols on the clock drawing test (CDT) in the patients with depression, dementia, and Alzheimer’s disease (AD). Reprinted with permission from Bragin et al. 9
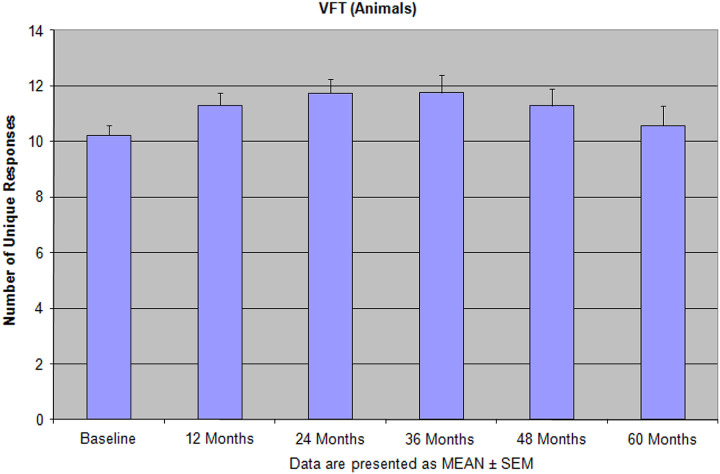
Figure 3.
The number of unique response (verbal fluency test [VFT]—animals) during 60 months of the integrative treatment shows not only the sufficient improvements but also never return back to the baseline. Reprinted with permission from Bragin et al. 9
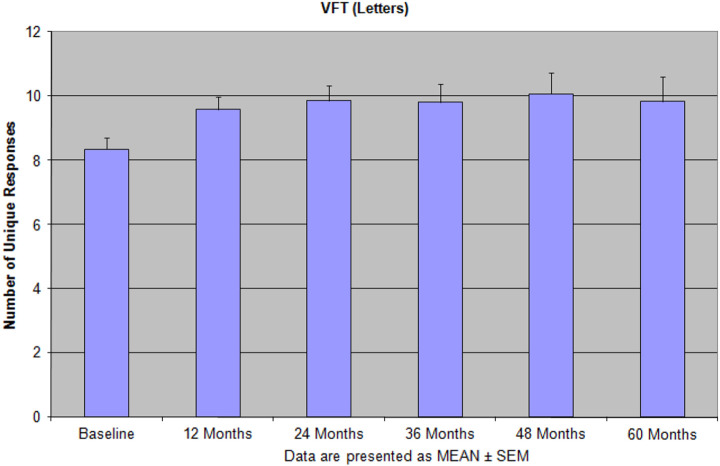
Figure 4.
The pattern of verbal fluency test (VFT; letters) performance after 60 months of the integrative treatment indicates that patients were able to gain significant improvement, especially by the end of long-term treatment period. Reprinted with permission from Bragin et al. 9
Therefore, our study that has been presented in this article demonstrates that integrative treatment can be a potential strategy to improve the ADL and cause long-term remission of clinical patients with depression/dementia and/or AD. Further, large-scale clinical study is the next desirable step in this research avenue. 3,8,9,61
Conclusions
In summary, the literature evidences and our research findings presented in this review indicate that the decreased CBF, difficulty in the absorption and digestion of nutrition, and gastrointestinal malfunction have direct influence on the availability of nutrients and oxygen to the brain. This in turn increases vulnerability of the highly metabolic active neurons in the brain that plays as an initiator of the progression and worsening of dementia and other neurodegenerative diseases. Therefore, disease-modifying treatment strategies offer a promising potential strategy for the preservation of cognitive functions in dementia and depression or other altered cognitive conditions. The multilevel and multilayer implementation of nutritional and other modifiable factors within an integrated treatment model is not only able to temporarily normalize metabolic imbalance in dementia and/or AD but can also become a basis for the development of comprehensive therapy for the treatment of dementia in general. Moreover, we are in the process of designing long-term studies, in which all available treatment modalities can be simultaneously implemented to develop valuable clinical practices.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the GALLY International Biomedical Research Consulting LLC, San Antonio, TX, USA.
References
- 1. Aliev G. Oxidative stress induced-metabolic imbalance, mitochondrial failure, and cellular hypoperfusion as primary pathogenetic factors for the development of Alzheimer disease which can be used as a alternate and successful drug treatment strategy: past, present and future. CNS Neurol Disord Drug Targets. 2011;10(2):147–148. [DOI] [PubMed] [Google Scholar]
- 2. Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99(3):689–707. [DOI] [PubMed] [Google Scholar]
- 3. Aliev G. The Role of Oxidative Stress, Mitochondria Failure, and Cellular Hypoperfusion in the Context of Alzheimer Disease: Past, Present and Future. Monograph Book. New York: Nova Science Publishers, Inc; 2013:1–426. ISBN: 978-1-61942-878-2. [Google Scholar]
- 4. Vančová O, Bačiak L, Kašparová S, et al. In vivo and in vitro assessment of brain bioenergetics in aging rats. J Cell Mol Med. 2010;14(11):2667–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horecký J, Baciak L, Kasparová S, Pacheco G, Aliev G, Vancová O. Minimally invasive surgical approach for three-vessel occlusion as a model of vascular dementia in the rat-brain bioenergetics assay. J Neurol Sci. 2009;283(1-2):178–181. [DOI] [PubMed] [Google Scholar]
- 6. Aliev G, Gasimov E, Obrenovich ME, et al. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels: implication in the pathogenesis of Alzheimer's disease. Vasc Health Risk Manag. 2008;4(3):721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pivi GA, Bertolucci PH, Schultz RR. Nutrition in severe dementia. Curr Gerontol Geriatr Res. 2012;983056:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bragin V, Chemodanova M, Dzhafarova N, Bragin I, Czerniawski JL, Aliev G. Integrated treatment approach improves cognitive function in demented and clinically depressed patients. Am J Alzheimers Dis Other Demen. 2005;20(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bragin V, Chemodanova M, Bragin I, et al. A 60-month follow-up of a naturalistic study of integrative treatment for real-life geriatric patients with depression, dementia and multiple chronic illnesses. Open J Psychiatry. 2012;2(2):129–140. [Google Scholar]
- 10. Aliev G, Li Y, Palacios HH, Obrenovich ME. Oxidative stress induced mitochondrial DNA deletion as a hallmark for the drug development in the context of the cerebrovascular diseases. Recent Pat Cardiovasc Drug Discov. 2011;6(3):222–241. [DOI] [PubMed] [Google Scholar]
- 11. Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80(spec 2):S160–S167. [DOI] [PubMed] [Google Scholar]
- 12. Maksimovich IV. Vascular factors in Alzheimer’s disease. Health. 2012;4(9A):735–742. [Google Scholar]
- 13. Sundström T, Elgh E, Larsson A, Näsman B, Nyberg L, Riklund KA. Memory-provoked rCBF-SPECT as a diagnostic tool in Alzheimer's disease? Eur J Nucl Med Mol Imaging. 2006;33(1):73–80. [DOI] [PubMed] [Google Scholar]
- 14. Kitabayashi Y, Ueda H, Tsuchida H, et al. Relationship between regional cerebral blood flow and verbal fluency in Alzheimer's disease. Psychiatry Clin Neurosci. 2001;55(5):459–463. [DOI] [PubMed] [Google Scholar]
- 15. Nagahama Y, Nabatame H, Okina T, et al. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer's disease. Eur Neurol. 2003;50(1):1–9. [DOI] [PubMed] [Google Scholar]
- 16. Silverman DH, Mosconi L, Ercoli L, Chen W, Small GW. Positron emission tomography scans obtained for the evaluation of cognitive dysfunction. Semin Nucl Med. 2008;38(4):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta L, Thomas S. The role of PET in dementia diagnosis and treatment. Appl Radiol. 2012;41(5):8–15. [Google Scholar]
- 18. van Halteren-van Tilborg IA, Scherder EJ, Hulstijn W. Motor-skill learning in Alzheimer's disease: a review with an eye to the clinical practice. Neuropsychol Rev. 2007;17(3):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grady CL, Haxby JV, Horwitz B, et al. Activation of cerebral blood flow during a visuoperceptual task in patients with Alzheimer-type dementia. Neurobiol Aging. 1993;14(1):35–44. [DOI] [PubMed] [Google Scholar]
- 20. Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Front Hum Neurosci. 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawashima R. Mental exercises for cognitive function: clinical evidence. J Prev Med Public Health. 2013;46(suppl 1):S22–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. [DOI] [PubMed] [Google Scholar]
- 23. de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab. 2005;25(6):663–672. [DOI] [PubMed] [Google Scholar]
- 24. Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bragin V. How to Activate Your Brain. Bloomington, IN: Authorhouse; 2007. [Google Scholar]
- 26. Roland PE, Meyer E, Shibasaki T, Yamamoto YL, Thompson CJ. Regional cerebral blood flow changes in cortex and basal ganglia during voluntary movements in normal human volunteers. J Neurophysiol. 1982;48(2):467–480. [DOI] [PubMed] [Google Scholar]
- 27. Khalsa DS, Amen D, Hanks C, Money N, Newberg A. Cerebral blood flow changes during chanting meditation. Nucl Med Commun. 2009;30(12):956–961. [DOI] [PubMed] [Google Scholar]
- 28. Stafstrom CE, Rho JM. The Ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3(59):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. [DOI] [PubMed] [Google Scholar]
- 30. McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90(4):889–907. [DOI] [PubMed] [Google Scholar]
- 31. Aliev G, Liu J, Shenk JC, et al. Neuronal mitochondrial amelioration by feeding acetyl-l-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13(2):320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ames BN. Increasing longevity by tuning up metabolism. Proc Natl Acad Sci USA. 2006;103(47):17589–17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM, Ames BN, Lamanna JC, Aliev G. The effect of acetyl-l-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer's disease. J Neurol Sci. 2009;283(1-2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutraceutical formulation for moderate-stage to later-stage Alzheimer's disease: a placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horecký J, Gvozdjáková A, Kucharská J, et al. Effects of coenzyme Q and creatine supplementation on brain energy metabolism in rats exposed to chronic cerebral hypoperfusion. Curr Alzheimer Res. 2011;8(8):868–875. [DOI] [PubMed] [Google Scholar]
- 36. Mizrahi EH, Jacobsen DW, Debanne SM, et al. Plasma total homocysteine levels, dietary vitamin B6 and folate intake in AD and healthy aging. J Nutr Health Aging. 2003;7(3):160–165. [PubMed] [Google Scholar]
- 37. Mizrahi EH, Bowirrat A, Jacobsen DW, et al. Plasma homocysteine, vitamin B12 and folate in Alzheimer's patients and healthy Arabs in Israel. J Neurol Sci. 2004;227(1):109–113. [DOI] [PubMed] [Google Scholar]
- 38. Pocernich CB, Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer's disease. Curr Alzheimer Res. 2011;8(5):452–469. [DOI] [PubMed] [Google Scholar]
- 39. Kamphuis PJ, Wurtman RJ. Nutrition and Alzheimer's disease: pre-clinical concepts. Danone research-centre for specialised nutrition, Wageningen, The Netherlands. Eur J Neurol. 2009;16(1):12–18. [DOI] [PubMed] [Google Scholar]
- 40. Smith MA, Petot GJ, Perry G. Diet and oxidative stress: a novel synthesis of epidemiological data on Alzheimer's disease. J Alzheimers Dis. 1999;1(4-5):203–236. [DOI] [PubMed] [Google Scholar]
- 41. Perry G, Nunomura A, Raina AK, et al. A metabolic basis for Alzheimer disease. Neurochem Res. 2003;28(10):1549–1552. [DOI] [PubMed] [Google Scholar]
- 42. Petot GJ, Friedland RP. Lipids, diet and Alzheimer disease: an extended summary. J Neurol Sci. 2004;226(1-2):31–33. [DOI] [PubMed] [Google Scholar]
- 43. Shea TB, Rogers E, Remington R. Nutrition and dementia: are we asking the right questions? J Alzheimers Dis. 2012;30(1):27–33. [DOI] [PubMed] [Google Scholar]
- 44. Gillette-Guyonnet S, Nourhashemi F, Andrieu S, et al. Weight loss in Alzheimer disease. Am J Clin Nutr. 2000;71(2):637S–642S. [DOI] [PubMed] [Google Scholar]
- 45. Herzig KH, Purhonen AK, Räsänen KM, et al. Fecal pancreatic elastase-1 levels in older individuals without known gastrointestinal diseases or diabetes mellitus. BMC Geriatr. 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hardt PD, Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nobili F, Vitali P, Canfora M, et al. Effects of long-term Donepezil therapy on rCBF of Alzheimer's patients. Clin Neurophysiol. 2002;113(8):1241–1248. [DOI] [PubMed] [Google Scholar]
- 49. Lipczyńska-Łojkowska W, Ryglewicz D, Jedrzejczak T, et al. The effect of Rivastigmine on cognitive functions and regional cerebral blood flow in Alzheimer's disease and vascular dementia: follow-up for 2 years. Neurol Neurochir Pol. 2004;38(6):471–481. [PubMed] [Google Scholar]
- 50. Alcántara-González F, Mendoza-Perez CR, Zaragoza N, et al. Combined administration of cerebrolysin and Donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse. 2012;66(11):938–949. [DOI] [PubMed] [Google Scholar]
- 51. Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27(52):14442–14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does Donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 2005;162(4):676–682. [DOI] [PubMed] [Google Scholar]
- 53. Wang L, Harms MP, Staggs JM, et al. Donepezil treatment and changes in hippocampal structure in very mild Alzheimer disease. Arch Neurol. 2010;67(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Csernansky JG, Dong H, Fagan AM, et al. Plasma cortisol and progression of dementia in DAT subjects. Am J Psychiatry. 2006;163(12):2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hill KD, LoGiudice D, Lautenschlager NT, Said CM, Dodd KJ, Suttanon P. Effectiveness of balance training exercise in people with mild to moderate severity Alzheimer's disease: protocol for a randomized trial. BMC Geriatr. 2009;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obrenovich ME, Smith MA, Siedlak SL, et al. Overexpression of GRK2 in Alzheimer disease and in a chronic hypoperfusion rat model is an early marker of brain mitochondrial lesions. Neurotox Res. 2006;10(1):43–56. [DOI] [PubMed] [Google Scholar]
- 57. Olazarán J, Reisberg B, Clare L, et al. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–178. [DOI] [PubMed] [Google Scholar]
- 58. Ruthirakuhan M, Luedke AC, Tam A, Goel A, Kurji A, Garcia A. Use of physical and intellectual activities and socialization in the management of cognitive decline of aging and in dementia: a review. J Aging Res. 2012;2012:384875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamagami T, Takayama Y, Maki Y, Yamaguchi H. A randomized controlled trial of brain-activating rehabilitation for elderly participants with dementia in residential care homes. Dement Geriatr Cogn Dis Extra. 2012;2(1):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chan A, Paskavitz J, Remington R, Rasmussen S, Shea TB. Efficacy of a vitamin/nutraceutical formulation for early-stage Alzheimer's disease: a 1-year, open-label pilot study with an 16-month caregiver extension. Am J Alzheimers Dis Other Demen. 2008;23(6):571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palacios HH, Yendluri BB, Parvathaneni K, et al. Mitochondrion-specific antioxidants as drug treatments for Alzheimer disease. CNS Neurol Disord Drug Targets. 2011;10(2):149–162. [DOI] [PubMed] [Google Scholar]