Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by beta-amyloid plaques accumulation and cognitive impairment. Both environmental factors and heritable predisposition have a role in AD. Histamine is a biogenic monoamine that plays a role in several physiological functions, including induction of inflammatory reactions, wound healing, and regeneration. The Histamine mediates its functions via its 4 G-protein-coupled Histamine H1 receptor (H1R) to histamine H1 receptor (H4R). The histaminergic system has a role in the treatment of brain disorders by the development of histamine receptor agonists, antagonists. The H1R and H4R are responsible for allergic inflammation. But recent studies show that histamine antagonists against H3R and regulation of H2R can be more efficient in AD therapy. In this review, we focus on the role of histamine and its receptors in the treatment of AD, and we hope that histamine could be an effective therapeutic factor in the treatment of AD.
Keywords: histamine, Alzheimer’s disease, histamine H1 receptor, histamine H2 receptor
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the brain that is characterized by the presence of beta-amyloid (βA) plaques in the brain. 1 An increased blood-to-brain influx and decreased brain-to-blood efflux across the blood–brain barrier (BBB) can cause the accumulation of βA plaques. 2 The first case of AD was identified by Alois Alzheimer in 1906 in Germany. 3 Four pathological mechanisms for AD are postulated (1) extracellular deposition of βA plaques, (2) formation of tau-positive intracellular neurofibrillary tangles, (3) neuronal loss in hippocampus and cerebral cortex, (4) lesions of cholinergic neurons. 4 It has been proven the synaptic changes such as the accumulation of Aβ together with a loss of synoptic functional and neuritic dystrophy can affect learning and memory. 5 Three mechanisms have been described to increase the brain levels of βA plaques: (1) increased influx, (2) decreased efflux, and (3) increased neuronal production. 2 The peroxisome proliferator-activated receptor-γ (PPAR-γ), a member of nuclear receptors family, can regulate the transcription of proinflammatory molecules, such as inducible nitric oxide synthase. It has been demonstrated that the activation of PPAR-γ can reduce Aβ levels in both cell culture and animal models of AD. 6 Immunological factors such as cytotoxic molecules and proinflammatory cytokines that are produced by microglia and astrocytes have an important role in neuroinflammation observed in AD. 7 Activated microglia causes Aβ phagocytosis, degradation, and clearance. Moreover, microglia produces a number of soluble factors such as the glia-derived neurotrophic factor that play an important role in the survival of neurons. 6 A significant increase in a number of cytokines and chemokines such as interleukin 1β (IL-1β), IL-6, tumor necrosis factor α (TNF-α), IL-8, transforming growth factor β, and macrophage inflammatory protein 1α has been shown in AD. 6 Furthermore, a reduced number of B cells and T cells in AD have been reported. 8 Histamine is a biogenic monoamine that affects NK cells, epithelial cells, T, and B lymphocytes. Moreover, it plays a role in several physiological functions, such as cell proliferation, wound healing, and regeneration and modulates the Th1-Th2 balance and hematopoiesis. 9,10 It has been demonstrated that histamine has a major regulatory role in experimental allergic encephalomyelitis, the autoimmune model of multiple sclerosis. 11 Histamine performs all its functions through 4 histamine, that is, H1, H2, H3 and H4, cell surface receptors (H1R-H4R). All 4 histamine receptors belong to G-protein-coupled receptors (GPCRs). The human histamine H3 receptor (H3R) has more than 40% homology to the human H4R. 12 Histamine is produced by mast cells, circulating basophils and neurons in response to immunoglobulin E. 10,13 Histamine and the histaminergic system play a major role in several physiological processes in rat brain. 14 Histamine induces the progression of allergic-inflammatory responses by increasing the production of proinflammatory cytokines such as IL-1α, IL-1β, and IL-6 and chemokines such as RANTES or IL-8, in different cell types and tissues. 15 It has been recently demonstrated that H4R can recruit eosinophil. Thus, it can be as an eosinophil chemoattractant. 15 In addition, H4R can determine the frequency of T regulatory (T(R)) cells in secondary lymphoid tissues, and control T(R) cell chemotaxis and suppressor activity. 11 Furthermore, the lack of H4R causes an impairment in anti-inflammatory response, including a decrease in the number of T(R) cells in the central nervous system (CNS) and an increase in the proportion of Th17 cells. 11 Both H1R and H2R antagonists are thought to treat allergy and excess gastric acid secretion. The H3R ligands are useful for the treatment of obesity and neurological diseases. The H4R that has been recently found can play a role in immune functions and in the pathogenesis of inflammatory diseases. 16 Histamine is a key allergic mediator, so that both H1R and H4R may play a major role in allergic inflammation. 17 Brain histamine dysfunction has not been reported to play a role in CNS disease, until now. The histaminergic system has been thought to play a role in the treatment of brain disorders by the development of histamine receptor agonists, antagonists. Moreover, the H3R is identified as a therapeutic target for neuropathic pain, sleep–wake disorders, such as narcolepsy, and cognitive impairment in Alzheimer's, and Parkinson's disease. 18 The H4R can perform many histamine-induced cellular functions of leukocytes, such as cell migration and cytokine production. It has been recently identified that histamine signaling through the H4R is both antipruritic and antinociceptive. Thus, targeting the H4R could be a new therapeutic approach for several chronic inflammatory diseases. 19 The striatum expresses a high density of histamine receptors. Thus, it has been thought that the released histamine can affect striatal function. The thalamostriatal synapses dynamic is controlled by histamine that causes the thalamic input facilitation. 20 Recent studies indicate that the memory deficit caused by early postnatal maternal deprivation in rats can be the result of an impairment of histamine-mediated mechanisms in the cornu ammonis 1 region of the rat hippocampus. 21
Alzheimer
It has been reported that TNF-α, interferon γ (IFN-γ)-inducible protein 10, monocyte chemotactic protein 1, and IL-8, are increased in AD and in mild cognitive impairment. Furthermore, some modifications such as extracellular βA plaques and intracellular neurofibrillary tangles occur in the initial stages of the disease. Thus, a failure of trials with anti-inflammatory agents in patients with Alzheimer has been observed. 22 Recent studies show that the cyclooxygenase 2 (COX-2) G/G genotype is related to AD, and it can support the involvement of COX-2 in AD etiology. 23 It has been proven that the deficiency in CD45 activity may lead to brain accumulation of neurotoxic Aβ oligomers and neuronal loss in AD. 24 Neuroinflammation can exacerbate the fundamental pathology by producing inflammatory cytokines, neurotoxic compounds, and toxic free radicals. Microglia attacks the pathological entities, but this can severely damage host neurons. 25 Recent data indicate that IL-21- and IL-9-producing Aβ-induced CD4(+) T cells, monocytes producing IL-23 and IL-6, and CD4(+) T cells synthesizing both related orphan receptor γ and nuclear factor of activated T-cell c1 transcriptional factors are increased, but monocytes that produce IL-10 are decreased in AD. 26 The level of IL-11 has been increased in AD and the highest peaks are reported in patients with a less severe degree of cognitive impairment. Thus, IL-11 has been expected to play a role in the initial phases of neurodegeneration. 27 Interleukin 15 synthetized by activated monocytes, macrophages, and glial cells can play a role in the pathophysiology of AD and frontotemporal dementia. 28 Interleukin 32 can induce the production of matrix metalloprotein 9 (MMP-9) in rat primary astrocytes and activate 2 major regulators of proinflammatory signaling in rat astrocytes by phosphorylation. Furthermore, it can regulate neuroinflammatory responses in AD (Figure 1). 29 Interleukin 33 can only express in vascular capillaries of the brain. Furthermore, IL-33 overexpression in cellular models can cause a decrease in the secretion of the Aβ(40) peptides, the main cerebral amyloid angiopathy component. Thus, it can be expected that genetic variants in IL-33 gene can lead to a decrease in AD risk. 30 The IL-18, a major mediator of inflammation and immune response, plays a role in physiopathological processes of the brain and affect the integrity of neurons. Thus, it can be important in the pathogenesis of AD. 31
Figure 1.
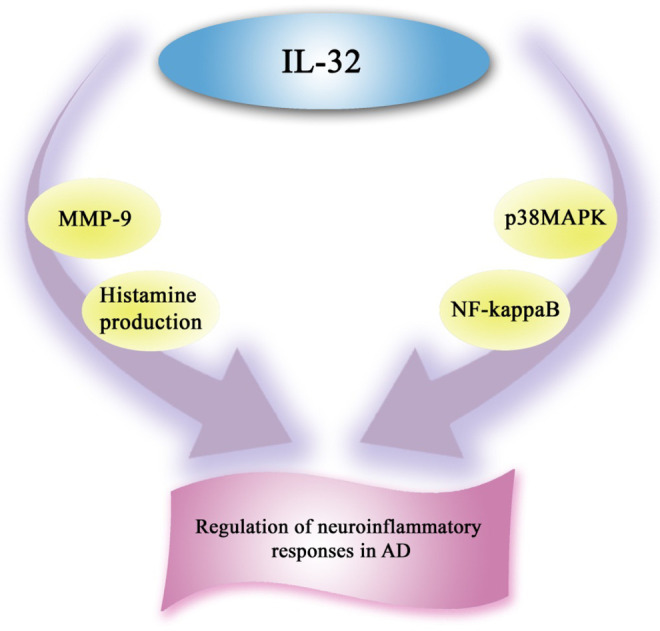
Interleukin 32 is a proinflammatory cytokine that activates both the p38 mitogen-activated protein kinase (p38MAPK) and nuclear factor κB (NF-κB) pathways. Interleukin 32 could provoke the production of histamine and matrix metalloprotein 9 (MMP-9). Thus, it regulates neuroinflammatory responses in Alzheimer's disease.
Histamine
Histamine is produced from l-histidine and plays a role as a neurotransmitter in the mammalian brain. Two important catabolism pathways are described for histamine: (1) diamine oxidase in the peripheral tissue and (2) histamine methyl transferase in the brain. Histaminergic fibers can control the production of nonhistaminergic neurotransmitters through histamine heteroreceptors that are found on the nonhistaminergic nerve endings. 32 Histamine is a major allergic mediator and has 2 roles: one, as a neurotransmitter in the nervous system and another, as a signaling molecule in the skin, the gut, and the immune system. 17,33 Histamine-secreting neurons are at the posterior hypothalamus in the tuberomammillary nucleus of the brain. 34 The monoamine histamine is involved in many biological processes such as neurotransmission in the CNS and peripheral nervous system (PNS). Furthermore, it has a role in inflammatory reactions, including gastric acid secretion and smooth muscle contraction. 35 Brain histamine is involved in many physiological processes including temperature regulation, emesis, water intake, and avoidance behavior. Moreover, it can regulate antidiuretic hormone and release both luteinizing and prolactin hormones. 36 Histamine is produced by several cells such as basophils, mast cells, neurons, lymphocytes, and gastric enterochromaffin-like cells. The pleiotropic effects of histamine are mediated by 4 GPCR subtypes H1R to H4R. 15,37 The H1R, H2R, and H3R can bind to glutamate N-methyl-d-aspartate receptors and have many functions in the brain, such as excitability and plasticity. 33 The release of histamine is regulated by glutamatergic neurons and nitric oxide of neuronal origin. Cholinergic transmission is controlled by neighboring histaminergic neurons through H1R, H2R, and H3R. 38 The histaminergic and hypocretin/orexin (hcrt) neurotransmitter systems have a major role in alertness and wakefulness in rodents. 39 Furthermore, the histaminergic system has a crucial role in memory and learning. Recently, the deficient histaminergic transmission in the brain in vascular dementia has been reported. 40 Both H1R and H4R play a role in allergic airway inflammation. It has been indicated that histamine has crucial role in IL-4-driven eosinophilic inflammation. 17 In addition, there has been a decline in eosinophilic inflammation and an eosinophil recruitment factor (CCL24) expression in the H2R knockout mice. 17 Two histamine-gated chloride (HisCl α1 and α2) channels have been identified in invertebrates. The HisCl channels belong to the Cys-loop receptor superfamily of ligand-gated ion channels that correlate with the mammalian γ-aminobutyric acid A (GABA A) and glycine receptor. Moreover, they have high homology within the ligand binding and ion channel domains. 41 Histamine has been found in the nervous tissue of animals, such as the snail Helix aspersa, the crab Carcinus maenas, the goldfish Carassius auratus, the frog Rana pipiens, and the mouse Mus musculus. 42 Histamine is a major regulator of energy intake and expenditure. It has been shown that H1R and H2R signaling can control both glucose and lipid metabolism and development of hyperlipidemia-induced nonalcoholic steatohepatitis. 43 On the other hand, histamine participates in host defense against infections. Furthermore, it may play a role in bacteria-host symbiosis, dysbiosis, and pathogenicity. 44
Histamine H1 Receptors
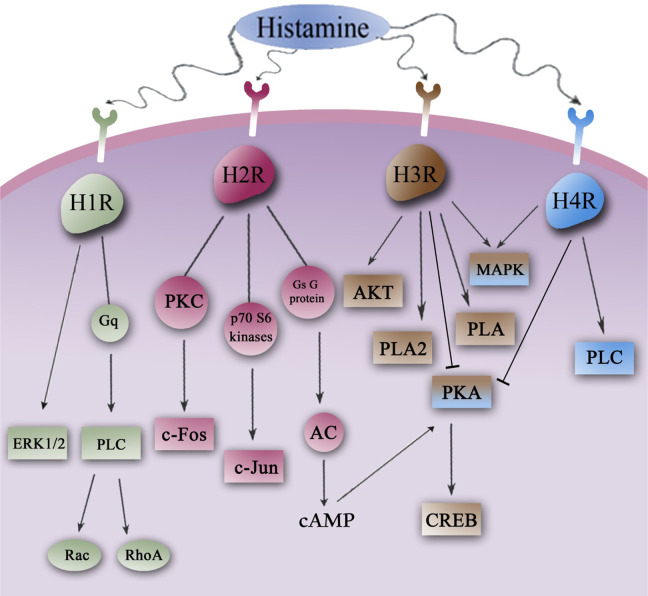
An intronless gene that is located on chromosome 3p25 encodes 56-kd H1R with 487 amino acids. The H1Rs have been shown to bind to the Gαq/11 family of G proteins that induce Ca2+ mobilization in a pertussis-toxin-insensitive fashion. 45 The H1Rs regulate several physiological functions including wakefulness, emotion, learning and memory, and stress. 46 The H1R is expressed by various kinds of cell types, such as neurons, vascular smooth muscle cells, neutrophils, dendritic cells (DCs), and Tand B cells. 9 It is also proved that the histaminergic system can modulate wakefulness and orexin/hypocretin neuron development through H1R in zebra fish. 39 Antihistamines are described as first- or second-generation drugs, due to its chemical structure and adverse effects. The adverse effects of antihistamines on the CNS rely on their ability to cross the BBB and link to the central H1Rs. 47 Dimebolin (latrepirdine), a compound with several drug targets, is considered in clinical trials for the treatment of AD, and it has been reported that dimebon could both slow the disease process and increase aspects of cognition. It has been also indicated that dimebon increases components of working memory in monkeys. 48 Histamine can generate several symptoms of allergic disease in the nose, skin, and lower airways by the Gq/11-coupled H1R. 49 Histamine has both activating and inhibitory effects: its activating effect is induced by stimulating of H1Rrs that are located on the membrane of myocytes, but its inhibitory effect is caused by stimulation of H2Rs located on the membrane of myocytes and produces nitric oxide by the endothelial cells of lymph node sinus. 50 It has been shown that 2 inverse agonists, carebastine and mepyramine, decline inositol phosphate accumulation and H1R gene expression. Thus, inverse agonists can relieve allergy symptoms by both preventing upregulation of H1R gene expression and inhibiting basal histamine signaling through their inverse agonistic activity. 51 Histamine controls cell proliferation via the activation of the H1R, and H1R binds to phospholipase C (PLC) through Gq (Table 1). The H1R antagonists such as mepyramine, regulator of G-protein-signaling 2, and the PLC inhibitor U73122 suppress histamine response. Thus, Rac and RhoA activation is controlled by H1R via Gq binding to PLC stimulation (Figure 2). The H1R-induced extracellular signal-regulated kinase (ERK1/2) activation was suppressed by U73122. Therefore, ERK1/2 activation relies on PLC but not on RhoA or Rac. Recent studies show that the functional binding of the H1R to Gq-PLC causes the activation of RhoA and Rac small guanine triphosphatases (GTPases; Figure 2). Thus, some roles for Rho GTPases in the control of cell proliferation by histamine have been expected. 52 Moreover, it has been indicated that H1R stimulation may lead to the production of arachidonic acid by phospholipase A2 (PLA2) enzymatic action (Table 1). 53
Table 1.
Properties of HRs.
| CNS Expression | Peripheral Expression | General Function | Binding Affinity to Histamine (pKi) | Signaling Pathway | |
|---|---|---|---|---|---|
| H1R | Thalamus, hippocampus, cortex, amygdala, basal forebrain | Ileum, smooth muscle, heart | Wakefulness, inflammatory responses, decreasing blood pressure | 4.2 | PLC |
| H2R | Basal ganglia, hippocampus, amygdala, pyramidal cells, raphe nuclei, substantia nigra | Lung, heart, intestine, smooth muscle | Regulation of gastric acid secretion, decreasing blood pressure, relaxation of airway and vascular smooth muscle, excitation, fluid balance, regulation of hormonal secretion | 4.3 | Activation of PKC |
| H3R | CNS, cerebral cortex, basal ganglia and hypothalamus | Lung, cardiovascular system, Intestine | Regulation of histamine release and generation | 8.0 | Inhibition of PKA, activation of PLA2, MAPK |
| H4R | Cerebellum, hippocampus | Hematopoietic cells | Modulation of immune system | 7.8 | Inhibition of PKA, activation of PLC, MAPK |
Abbreviations: HRs, histamine receptors, H1R, histamine H1 receptor, H2R, histamine H2 receptor, H3R, histamine H3 receptor, H4R, histamine H4 receptor, PLC, phospholipase C, PKA, protein kinase A, PLA2, phospholipase A2, MAPK, mitogen-activated protein kinase; CNS, central nervous system; PKC, protein kinase C.
Figure 2.
Signaling pathways in histamine receptors. Histamine can mediate its functions via its 4 G-protein-coupled H1R to H4R. Rac and RhoA activation is mediated by H1R via Gq binding to PLC stimulation. Moreover, H2R controls both c-Fos and c-Jun expression by distinct signaling pathways such as protein kinase C (PKC) and p70 S6 kinases, respectively. The H3R could activate AKT, PLA2, PLA, and MAPK pathways, and H4R stimulates MAPK and PLC pathways. H1R indicates histamine H1 receptor; MAPK, mitogen-activated protein kinase; PLA indicates phospholipase A; PLC, phospholipase C.
Histamine H2 Receptor
The gene that encodes the H2R is located on chromosome 5. The H2Rs may bind to adenylate cyclase (AC) and phosphoinositide second messenger systems by guanine triphosphate (GTP)-dependent pathways. Moreover, the human receptor modulates c-Fos and c-Jun expression by distinct signaling pathways such as protein kinase C (PKC) and p70 S6 kinases, respectively (Figure 2). 45 The H2R is observed in many tissues and cells, such as the brain, smooth muscle cells, gastric parietal cells, and T and B cells. Furthermore, the histamine absence can downregulate H2R in a tissue-specific manner. 54 There are no muscular H2Rrs in the tracheal muscle of dogs and guinea pigs. 55 Gastric acid production is induced by 3 GPCR ligands: (1) histamine, (2) acetylcholine, and (3) gastrin. 56 Recent data show that suppressive effects of histamine on methamphetamine-induced behavioral sensitization could be regulated via H1R and H2R in the CNS such as neostriatum. 57 Histamine regulates the expression of P-selectin via both the H2R and calcium, but this expression is decreased by cyclic adenosine monophosphate (cAMP). The histamine-induced expression promotes polymorphonuclear leukocytes (PMN) coupling to the human brain microvessel endothelial cells. Thus, it is expected that P-selectin has a role in the recruitment of acute inflammatory cells to the CNS. 58 On the other sides, H2R binds to Gs G-protein and this binding can activate adenylyl cyclase and increase cAMP. In turn, cAMP activates protein kinase A (PKA) and phosphorylates several proteins (Table 1). As a consequence, both cAMP response element binding (CREB)-activation and the Ca2+-dependent potassium conductance inhibition can occur (Figure 2). 59 -61 It has been proved that agonist stimulation of H2R can activate both AC and PLC, inhibit the receptor and stimulate ERK1/2. 62
Histamine H3 Receptor
At first, H3R was identified in 1983 by Arrang et al as a presynaptic autoreceptor. 63 The H3R, a member of GPCRs family, modulates neurotransmitter release negatively in CNS and PNS. 64 The H3R is encoded by a gene which is located on chromosome 20. It binds to the Gi class of G-proteins and negatively controls the intracellular messenger cAMP. 65 The H3R is expressed in some brain regions including the CNS, cerebral cortex, basal ganglia, and hypothalamus. These brain regions play a major role in cognition, sleep, and homeostatic modulation. 66 Several intracellular proteins have been described to couple to carboxy (C)-termini of GPCRs and mediate their intracellular trafficking and signal transduction pathways. 64 In type I cells of rats, it has been shown that histamine can attenuate calcium-signaling events that are induced by the muscarinic acetylcholine receptor agonist acetyl-β-methylcholine through an H3R-controlled mechanism. Thus, the excitatory presynaptic functions of acetylcholine are downregulated by histamine. 67 Thioperamide, which can cause a strong activation of histamine release and convert histamine to N-tele-methylhistamine, competitively suppresses the conversion of N-tele-methylhistamine to N-tele-methylimidazoleacetic acid in the brains of human and monkey due to monoamine oxidase B existence. 68 It has been proved that some neurotransmitter systems, such as acetylcholine, dopamine, serotonin, and glutamate are involved in specific aspects of cognition. The H3R antagonists can stimulate the release of histamine, norepinephrine, dopamine, and acetylcholine. Thus, H3R antagonists can be thought as an efficient drug target for cognitive disorders. 66 The H3Rs’ constitutive activity in rat brain cortex suppresses the AC/PKA pathway (Figure 2). It has been shown that the agonist-activated H3 autoreceptors can also suppress histamine production that is regulated by calcium/calmodulin- and cAMP-dependent protein kinases. 69 The postsynaptic H3Rs are observed in striatum at GABAergic cell bodies of the medium spiny neurons that are located on the striatonigral neurons of both direct and indirect movement pathway. 70 Activation of H3Rs suppresses the production of neuropeptides and neurogenic–vasogenic inflammation that cause headache. The V-allele genotypes in the H3R gene play several roles such as increasing the number of inactive receptors, inhibiting the negative feedback mechanism on the H3R, and stimulating histamine release, which relates to migraine attacks in susceptible cases. 71 Chloride intracellular channel 4 (CLIC4), a member of CLIC protein family that is expressed in multicellular organisms, has anion channel activities in planar lipid bilayer systems. It has been indicated that CLIC4 has an influence on H3R cell surface expression via coupling to the C-terminal cytoplasmic domain of the receptor. 64 The H3R antagonists are identified as a therapeutic target for treatment of AD and several serious disorders such as obesity, myocardial ischemia, migraine, and inflammatory diseases. 72,73 Yohimbine suppresses the effect of brimonidine on histamine release. Moreover, the H3R agonist R-(alpha)-methylhistamine can decline histamine production, as well as thioperamide inhibits the effect of R-(alpha)-methylhistamine, but when they were used singly, yohimbine and thioperamide could stimulate histamine release. It has been proved that both prejunctional alpha (2) receptors and histamine H3R may mediate and cooperate to control the sympathetic histamine production. 74 Thioperamide is a H4R/H3R antagonist that can prevent mast cell and eosinophil migration. 75 H3receptor density in cortical tissue of spontaneously hypertensive rats promotes with age where the number of the expressed amplicons of the detected H3R declines. Although the number of expressed amplicons of the H3R declines, the expression of the larger amplicon (∼500 bp) increases. 76 The H3R affects various signaling pathways such as activation of phospholipase A(2), AkT, and the mitogen-activated kinase, G(i/o)-dependent inhibition of adenylyl cyclase as well as the inhibition of both Na+/H+ exchanger and K+-induced Ca2+ mobilization (Figure 2). 73 Recent studies show that H3Rs mediate noradrenaline release in rat olfactory bulb through a presynaptic action. 77
The H4R
Human H4R is encoded by a gene that is located on chromosome 18. The H4R is a 390-amino-acid receptor that has 37% to 43% homology to H3R. Both H3R and H4R are bound to Gi/o and activate several signaling pathways. 45 The H4Rs are found in various cell types of the gut, such as immune cells, neurons, paracrine, and endocrine cells. 78 In the gastrointestinal tract, H4R controls gastric acid production, gastric mucosal defense, inflammation, immunity, and carcinogenesis. 78 Furthermore, it may be expressed in adult colorectal cancer. 78 Recent data demonstrate that H4Rs can be expressed on mature basophils and other effector cells of allergic reactions, like eosinophils. 79 Moreover, the H4R is expressed on both CD4(+) T cells and human CD4(+) Th2-polarized T cells. Both CD4(+) T-cell and histamine can be found in psoriatic plaques and in acute skin lesions of atopic dermatitis. Histamine is produced in psoriasis and atopic dermatitis diseases and can promote the immunomodulatory potency of skin-infiltrating Th17 cells. 80 The H4R is also expressed on plasmacytoid dendritic cells (pDCs) in psoriasis and affects cytokine synthesis and migration of pDCs. Thus, the H4R alone or together with the H2R can be identified as a therapeutic approach for psoriasis. 81 Although the histamine and phorbol 12 myristate 13 acetate have no effects on H4R expression, lipopolysaccharides and indomethacin increase H4R messenger RNA expression, and 20 μmol/L dexamethasone can promote H4R protein levels. 82 On the other hand, the dog H4R shows a 61% to 71% homology to the receptors of other species and has a 71% homology with the human receptor. The dog H4R affinity for histamine is 18 nmol/L. So that, the dog affinity for histamine is 3-fold lower than that of human. The agonists of the human H4R can be identified as the agonists of the dog receptor but with different effects. 83 The H4Rs are expressed on neurons in the rat lumbar dorsal root ganglia and in the lumbar spinal cord. Thus, the presence of H4R in human and rodent CNS indicates that H4R can play a role in itch and pain. 84 It has been shown that histamine can activate signal transduction pathways, such as G(i)-protein-dependent actin reorganization, increase intracellular calcium, and stimulate migratory responses in γδ T lymphocytes through the H4R. But it can decrease γδ T-cell modulated cytotoxicity via H2Rs and G(s)-protein-coupled signaling. It has been thought that histamine-activated γδ T cells can regulate immunological surveillance of tumor tissue. 85 Histamine induces the hyperphosphorylation of STAT6. The H1R antagonist, pyrilamine, may inhibit the phosphorylation of STAT6 by histamine, whereas H2R antagonist, ranitidine, and H3R/H4R antagonist, thioperamide, have no effect on the histamine-regulated hyperphosphorylation of STAT6. 86
Alzheimer and Histamine
Histamine functions are regulated by H1R, H2R, and H3R. The decline in histamine receptor binding may have a role in the cognitive deficits of patients with AD. 87 The H3R can suppress histamine release in brain. Thus, H3R inverse agonists increase histaminergic neuron activity. The histaminergic system has a major role in cognition and H3R inverse agonists are identified as therapeutics target for cognitive deficits of AD. A significant loss of histaminergic neurons has been reported in patients with Alzheimer. 88 Moreover, the histamine metabolite levels in the CSF of patients with AD indicate a decline in their global activity. 88 The H3 antagonists that enhance the release of brain histamine, acetylcholine, and neurotransmitters are responsible for mediating cognitive processes. Thus, this ability can affect attention and vigilance. Furthermore, histamine regulates both short-term and long-term memory. 89 Recent studies have indicated a strong neurofibrillary degeneration of the tuberomamillary nucleus that is the origin of histamine neurons in AD. Histaminergic neurons activity increases cognition and memory. Thus, their degeneration may have a role in the cognitive decline of AD. The brain histaminergic system involves mast cells and microglia that synthesize histamine. However, the histaminergic system level of activity in patients with Alzheimer is not clear. 90 The histaminergic system is a crucial modulatory system that controls both basic homeostatic and higher functions such as arousal, cognition, and circadian. It has been proved that histamine can regulate learning in different types of behavioral tasks, but the exact part of regulation and its mechanisms remains unknown. 91 It has been reported that the cognitive-increasing effects of H3R antagonists through multiple cognitive domains in a several preclinical cognition models can be a new therapeutic approach for AD treatment. 92 The H3R integrity has been reported in different stages of AD. Thus, this can be a good reason to use H3 antagonists as a therapeutic strategy in AD treatment. 93 Histamine can decrease neurotoxicity which is caused by βA(1-42), and this can be regulated by H2R and H3R. 94 Among siblings at high risk of AD, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) could delay onset and decline the risk of AD 95 and among NSAIDs, aspirin has been proved to decrease the occurrence of AD. Moreover, an inverse association of AD and use of H2-receptor agonist have been observed. 96 The homozygous H1R knockout (H1R-KO) mice show severe memory deficits in episodic-like memory and procedural memory. It should be noted that some changes in acetylcholine esterase activity in the hippocampus and also dopamine (DA) turnover in the cerebellum in AD have been reported. 97 New experiments in mice with a null mutation of gene coding histamine H1R or H2R indicate that both H1R and H2R play a major role in learning and memory and the frontal cortex, amygdala and hippocampus are involved in these processes. 98 It has been proved that both H1R and H2R are excitatory, but H3 is only an inhibitory auto- and heteroreceptor. The interactions of histaminergic and other aminergic and peptidergic systems can regulate major homeostatic functions, such as sleep–wake regulation, synaptic plasticity, learning, and memory. 99 Furthermore, recent data show that both IL-17 and TNF-α can intensify the effect of histamine via the H1-receptor. 100 It has been reported that ebastine, a second-generation H1 antihistamine, has no effect on cognitive impairment or subjective sleepiness. Moreover, the CNS impairment can be correlated with plasma (+)-chlorpheniramine, H1R antagonist that also works as a serotonin–norepinephrine reuptake inhibitor concentration. 101 6-[(3-Cyclobutyl-2,3,4,5-tetrahydro-1H-3-benzazepin-7-yl)oxy]-N-methyl-3 pyridinecarboxamide hydrochloride (GSK189254), a H3R antagonist with high affinity for both human and rat H3R, has been shown to have a therapeutic effect on dementia in AD and other cognitive diseases. 102 The interactions between histamine and H3Rs can decline the cholinergic tone in both the frontal cortex and the hippocampus, and this could be important in learning and memory. Both H3 and 5-HT3 receptors are targets for exogenous compounds that are functioning as antagonists. Thus, both thioperamide (H3R antagonist) and ondansetron (5-HT3 antagonist) can cure the deficits that are caused by cholinergic hypofunction. 103 It has been reported that H3-antagonist-mediated signaling does not depend on ACh-stimulated α7 nAChR activation. Furthermore, H3-antagonist (ABT-239) activates biochemical signaling that increases both cognitive performance and attenuation of tau hyperphosphorylation. Thus, the concept that H3 antagonists can improve AD symptoms and have a therapeutic role in AD has been confirmed. 104 Histamine receptors regulate the functional activities of DC subsets. Histamine-induced reduction of CD1a (+) DCs as well as enhanced production of both IL-6 and IL-10, upregulation of chemokines, expression of C5aR1 via the CD1a (−) DC subset, and increased migration of activated DC subsets that is stimulated by the secretion of MMP-9 and MMP-12 enzymes have been detected in a H2R-specific manner, which is identified by the antagonist of H2R. 105 Store-operated calcium entry (SOCE) has been thought as the major Ca2+ influx pathway of DCs. DCs primed with histamine can induce Th2 immune response through various types of histamine receptors. Histamine stimulates DCs to produce Ca2+ from internal store. Histamine increases the level of IL-10 but reduces the level of IL-12p70 produced by DCs. Pretreatment of SOC blockers and H1R, and H4R antagonists with DCs can suppress the Th2 polarization of T helper cells caused by histamine in mixed lymphocyte responses. Recent studies indicate that SOCE has a major role in histamine-induced maturation and Th(2) response of DCs via both H1R and H4R. 106 It has been demonstrated that A beta (1-42)-specific Th1-type T-cell memory is present in young people who are secreting large amounts of IFN-γ and IL-2. With increasing age, a decline in the synthesis of both IFN-γ and IL-2 has been reported and also a significant rise in CD4(+) T-cell-derived regulatory IL-10 secretion has been detected. However, the patients with AD can synthesize IL-10 in the absence of any effector cytokine. 107 The IL-32 is a proinflammatory cytokine that can activate both the p38 mitogen-activated protein kinase (p38MAPK) and nuclear factor κB pathways. The IL-32 can provoke histamine production in human-derived cord blood mast cells (HDCBMCs; Figure 1). Thus, it has been proved that IL-32 can be species specific, and it can function in mature human mast cells (HDCBMCs) more than in transformed mast cells (LAD 2 cells). 108 It has been shown that IL-32 can have a role in the regulation of neuroinflammatory responses in many neurological disease include AD. 109
Conclusion
The AD is a neurodegenerative disease that is characterized by 2 factors: (1) cognition impairment and (2) accumulation of βA plaques. Histamine is identified as a neurotransmitter in the nervous system, and it has been proved that the histaminergic system has a major role in cognition. Furthermore, its main role in memory and learning has been reported. Recent studies indicate that receptor function in AD may be compromised due to disrupted postreceptor signal transduction that is controlled by the G-protein that modulates phosphoinositide hydrolysis and AC pathways. Moreover, H3Rs constitutive activity in rat brain cortex could suppress the AC/PKA pathway. The H3R antagonists are suggested to be a therapeutic approach for cognitive deficits of AD. Moreover, histamine can decrease neurotoxicity that is caused by βA(1-42) and this can be controlled by H2R and H3R. It has been shown that histamine activates PKC-δ, ERK1/2, p38 kinase, and c-Jun N-terminal kinase prior to early growth response factor 1 (Egr-1) induction. The H2Rs bind to both AC and phosphoinositide second messenger systems by GTP-dependent pathways. Furthermore, the human receptor mediates c-Fos and c-Jun expression by distinct signaling pathways including PKC and p70 S6 kinases, respectively. The PKC is responsible for synaptic remodeling, induction of protein synthesis, and several other processes that are crucial in learning and memory. Thus, activation of neuronal PKC might be important for all phases of learning, such as acquisition, consolidation, and reconsolidation. Thus, we expect that histamine antagonists against H3R and regulation of H2R could be effective for AD therapy. Collectively, there is no information about the effect of H4R on AD. Thus, study about the role of H4R in cognition and βA plaques might be a new target for future experiments. Several studies have been carried out about histamine and H1R to H4R and these data indicate that histamine could be a promising compound for AD therapy.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Speciale L, Calabrese E, Saresella M, et al. Lymphocyte subset patterns and cytokine production in Alzheimer’s disease patients. Neurobiol Aging. 2007;28(8):1163–1169. [DOI] [PubMed] [Google Scholar]
- 2. Jaeger LB, Dohgu S, Sultana R, et al. Lipopolysaccharide alters the blood–brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23(4):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu TY, Chen CP, Jinn TR. Alzheimer's disease: aging, insomnia and epigenetics. Taiwan J Obstet Gynecol. 2010;49(4):468–472. [DOI] [PubMed] [Google Scholar]
- 4. Wu TY, Chen CP, Jinn TR. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50(2):131–135. [DOI] [PubMed] [Google Scholar]
- 5. Small DH. Network dysfunction in Alzheimer’s disease: does synaptic scaling drive disease progression? Trends Mol Med. 2008;14(3):103–108. [DOI] [PubMed] [Google Scholar]
- 6. Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24(2-3):167–176. [DOI] [PubMed] [Google Scholar]
- 7. Saresella M, Calabrese E, Marventano I, et al. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav Immun. 2011;25(3):539–547. [DOI] [PubMed] [Google Scholar]
- 8. Richartz-Salzburger E, Batra A, Stransky E, et al. Altered lymphocyte distribution in Alzheimer’s disease. J Psychiatr Res. 2007;41(1-2):174–178. [DOI] [PubMed] [Google Scholar]
- 9. O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128(6):1153–1162. [DOI] [PubMed] [Google Scholar]
- 10. Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23(5):255–263. [DOI] [PubMed] [Google Scholar]
- 11. Del Rio R, Noubade R, Saligrama N, et al. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol. 2012;188(2):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wulff BS, Hastrup S, Rimvall K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol. 2002;453(1):33–41. [DOI] [PubMed] [Google Scholar]
- 13. Fernańndez-Novoa L, Cacabelos R. Histamine function in brain disorders. Behav Brain Res. 2001;124(2):213–233. [DOI] [PubMed] [Google Scholar]
- 14. Zarrindast MR, Eidi M, Eidi A, Oryan S. Effects of histamine and opioid systems on memory retention of passive avoidance learning in rats. Eur J Pharmacol. 2002;452(2):193–197. [DOI] [PubMed] [Google Scholar]
- 15. Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533(1-3):69–76. [DOI] [PubMed] [Google Scholar]
- 16. Crane K, Shih DT. Development of a homogeneous binding assay for histamine receptors. Anal Biochem. 2004;335(1):42–49. [DOI] [PubMed] [Google Scholar]
- 17. Swartzendruber JA, Byrne AJ, Bryce PJ. Cutting edge: histamine is required for IL-4-driven eosinophilic allergic responses. J Immunol. 2012;188(2):536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiligada E, Kyriakidis K, Chazot PL, Passani MB. Histamine pharmacology and new CNS drug targets. CNS Neurosci Ther. 2011;17(6):620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin N, Covington M, Bian D, et al. INCB38579, a novel and potent histamine H (4) receptor small molecule antagonist with anti-inflammatory pain and anti-pruritic functions. Eur J Pharmacol. 2012;675(1-3):47–56. [DOI] [PubMed] [Google Scholar]
- 20. Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Bolam JP. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci. 2011;31(43):15340–15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benetti F, Silveira CK, Silva WC, Cammarota M, Izquierdo I. Histamine reverses a memory deficit induced in rats by early postnatal maternal deprivation. Neurobiol Learn Mem. 2012;97(1):54–58. [DOI] [PubMed] [Google Scholar]
- 22. Galimberti D, Scarpini E. Inflammation and oxidative damage in Alzheimer's disease: friend or foe? Front Biosci (Schol Ed). 2011;3:252–266. [DOI] [PubMed] [Google Scholar]
- 23. Fehér A, Juhász A, Rimanóczy A, Kálmán J, Janka Z. Association study of interferon-γ, cytosolic phospholipase A2, and cyclooxy-genase-2 gene polymorphisms in Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):983–987. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Y, Hou H, Rezai-Zadeh K, et al. CD45 deficiency drives amyloid-β peptide oligomers and neuronal loss in Alzheimer's disease mice. J Neurosci. 2011;31(4):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGeer EG, McGeer PL. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19(1):355–361. [DOI] [PubMed] [Google Scholar]
- 26. Saresella M, Calabrese E, Marventano I, et al. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer's disease. Brain Behav Immun. 2011;25(3):539–547. [DOI] [PubMed] [Google Scholar]
- 27. Galimberti D, Venturelli E, Fenoglio C, et al. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer's disease and frontotemporal lobar degeneration. J Neurol. 2008;255(4):539–544. [DOI] [PubMed] [Google Scholar]
- 28. Rentzos M, Zoga M, Paraskevas GP, et al. IL-15 is elevated in cerebrospinal fluid of patients with Alzheimer's disease and frontotemporal dementia. J Geriatr Psychiatry Neurol. 2006;19(2):114–117. [DOI] [PubMed] [Google Scholar]
- 29. Cho KS, Park SH, Joo SH, Kim SH, Shin CY. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochem Biophys Res Commun. 2010;402(1):48–53. [DOI] [PubMed] [Google Scholar]
- 30. Chapuis J, Hot D, Hansmannel F, et al. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Mol Psychiatry. 2009;14(11):1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bossù P, Ciaramella A, Salani F, et al. Interleukin-18, from neuroinflammation to Alzheimer's disease. Curr Pharm Des. 2010;16(38):4213–4224. [DOI] [PubMed] [Google Scholar]
- 32. Adachi N. Cerebral ischemia and brain histamine. Brain Res Brain Res Rev. 2005;50(2):275–286. [DOI] [PubMed] [Google Scholar]
- 33. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. [DOI] [PubMed] [Google Scholar]
- 34. Alvarez EO. The role of histamine on cognition. Behav Brain Res. 2009;199(2):183–189. [DOI] [PubMed] [Google Scholar]
- 35. Wulff BS, Hastrup S, Rimvall K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol. 2002;453(1):33–41. [DOI] [PubMed] [Google Scholar]
- 36. Mazurkiewicz-kwilecki IM, Prell GD. Brain histamine: plasma corticosterone spontaneous locomotor activity and temperature. Pharmacol Biochem Behav. 1980;12(4):549–553. [DOI] [PubMed] [Google Scholar]
- 37. Walter M, Stark H. Histamine receptor subtypes: a century of rational drug design. Front Biosci (Schol Ed). 2012;4:461–488. [DOI] [PubMed] [Google Scholar]
- 38. Philippu A, Prast H. Importance of histamine in modulatory processes, locomotion and memory. Behav Brain Res. 2001;124(2):151–159. [DOI] [PubMed] [Google Scholar]
- 39. Sundvik M, Kudo H, Toivonen P, Rozov S, Chen YC, Panula P. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011;25(12):4338–4347. [DOI] [PubMed] [Google Scholar]
- 40. Stasiak A, Mussur M, Unzeta M, Lazewska D, Kiec-Kononowicz K, Fogel WA. The central histamine level in rat model of vascular dementia. J Physiol Pharmacol. 2011;62(5):549–558. [PubMed] [Google Scholar]
- 41. Fleck MW, Thomson JL, Hough LB. Histamine-gated ion channels in mammals? Biochem Pharmacol. 2012;83(9):1127–1135. [DOI] [PubMed] [Google Scholar]
- 42. Woodruff GN, Oniwinde AB, Kerkut GA. Histamine in tissues of the snail, crab, goldfish, frog and mouse. Comp Biochem Physiol. 1969;31(4):599–603. [DOI] [PubMed] [Google Scholar]
- 43. Wang KY, Tanimoto A, Yamada S, et al. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am J Pathol. 2010;177(2):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kyriakidis DA, Theodorou MC, Tiligada E. Histamine in two component system-mediated bacterial signaling. Front Biosci. 2012;17:1108–1119. [DOI] [PubMed] [Google Scholar]
- 45. Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23(5):255–263. [DOI] [PubMed] [Google Scholar]
- 46. Yanai K, Tashiro M. The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies. Pharmacol Ther. 2007;113(1):1–15. [DOI] [PubMed] [Google Scholar]
- 47. Montoro J, Mullol J, Dávila I, et al. Bilastine and the central nervous system. J Investig Allergol Clin Immunol. 2011;21(suppl 3):9–15. [PubMed] [Google Scholar]
- 48. Webster SJ, Wilson CA, Lee CH, Mohler EG, Terry AV, Jr, Buccafusco JJ. The acute effects of dimebolin, a potential Alzheimer's disease treatment, on working memory in rhesus monkeys. Br J Pharmacol. 2011;164(3):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Akdis CA, Simons FE, Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533(1-3):69–76. [DOI] [PubMed] [Google Scholar]
- 50. Pan'kova MN, Lobov GI, Chikhman VN, Solnyshkin SD. . Effects of histamine on contractile activity of lymphatic node capsules. The NO role [in Russian]. Ross Fiziol Zh Im I M Sechenova. 2011;97(6):633–640. [PubMed] [Google Scholar]
- 51. Mizuguchi H, Ono S, Hattori M, Fukui H. Inverse agonistic activity of antihistamines and suppression of histamine H (1) receptor gene expression. J Pharmacol Sci. 2012;118(1):117–121. [DOI] [PubMed] [Google Scholar]
- 52. Notcovich C, Diez F, Tubio MR, et al. Histamine acting on H1 receptor promotes inhibition of proliferation via PLC, RAC, and JNK-dependent pathways. Exp Cell Res. 2010;316(3):401–411. [DOI] [PubMed] [Google Scholar]
- 53. Leurs R, Traiffort E, Arrang JM, Tardivel-Lacombe J, Ruat M, Schwartz JC. Guinea pig histamine H1 receptor. II. Stable expression in Chinese hamster ovary cells reveals the interaction with three major signal transduction pathways. J Neurochem. 1994;62(2):519–527. [DOI] [PubMed] [Google Scholar]
- 54. O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128(6):1153–1162. [DOI] [PubMed] [Google Scholar]
- 55. Jolly S, Desmecht D. Functional identification of epithelial and smooth muscle histamine dependent relaxing mechanisms in the bovine trachea, but not in bronchi. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134(1):91–100. [DOI] [PubMed] [Google Scholar]
- 56. Fukushima Y, Shindo T, Anai M, et al. Structural and functional characterization of gastric mucosa and central nervous system in histamine H2 receptor-null mice. Eur J Pharmacol. 2003;468(1):47–58. [DOI] [PubMed] [Google Scholar]
- 57. Ogawa S, Yanai K, Watanabe T, et al. Histamine responses of large neostriatal interneurons in histamine H1 and H2 receptor knock-out mice. Brain Res Bull. 2009;78(4-5):189–194. [DOI] [PubMed] [Google Scholar]
- 58. Easton AS, Dorovini-Zis K. The kinetics, function, and regulation of P-selectin expressed by human brain microvessel endothelial cells in primary culture. Microvasc Res. 2001;62(3):335–345. [DOI] [PubMed] [Google Scholar]
- 59. Baudry M, Martres MP, Schwartz JC. H1 and H2 receptors in the histamine induced accumulation of cyclic AMP in guinea pig brain slices. Nature. 1975;253(5490):362–364. [DOI] [PubMed] [Google Scholar]
- 60. Hegstrand LR, Kanof PD, Greengard P. Histamine sensitive adenylate cyclase in mammalian brain. Nature. 1976;260(5574):163–165. [DOI] [PubMed] [Google Scholar]
- 61. Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252(5011):1427–1430. [DOI] [PubMed] [Google Scholar]
- 62. Xu AJ, Kuramasu A, Maeda K, et al. Agonist-induced internalization of histamine H2 receptor and activation of extracellular signal-regulated kinases are dynamin-dependent. J Neurochem. 2008;107(1):208–217. [DOI] [PubMed] [Google Scholar]
- 63. Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302(5911):832–837. [DOI] [PubMed] [Google Scholar]
- 64. Maeda K, Haraguchi M, Kuramasu A, et al. CLIC4 interacts with histamine H3 receptor and enhances the receptor cell surface expression. Biochem Biophys Res Commun. 2008;369(2):603–608. [DOI] [PubMed] [Google Scholar]
- 65. Wellendorph P, Goodman MW, Burstein ES, Nash NR, Brann MR, Weiner DM. Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H3 receptor. Neuropharmacology. 2002;42(7):929–940. [DOI] [PubMed] [Google Scholar]
- 66. Gemkow MJ, Davenport AJ, Harich S, Ellenbroek BA, Cesura A, Hallett D. The histamine H3 receptor as a threputic drug target for CNS disorders. Drug Discov Today. 2009;14(9-10):509–515. [DOI] [PubMed] [Google Scholar]
- 67. Thompson CM, Troche K, Jordan HL, Barr BL, Wyatt CN. Evidence for functional, inhibitory, histamine H3 receptors in rat carotid body Type I cells. Neurosci Lett. 2010;471(1):15–19. [DOI] [PubMed] [Google Scholar]
- 68. Sakurai E, Sakurai E, Tanaka Y, Watanabe T, Singh Jossan S, Oreland L. Effects of histamine H3-receptor ligands on brain monoamine oxidase in various mammalian species. Brain Res. 2001;906(1-2):180–183. [DOI] [PubMed] [Google Scholar]
- 69. Moreno-Delgado D, Torrent A, Gómez-Ramıŕez J, de Esch I, Blanco I, Ortiz J. Constitutive activity of H3 autoreceptors modulates histamine synthesis in rat brain through the cAMP/PKA pathway. Neuropharmacology. 2006;51(3):517–523. [DOI] [PubMed] [Google Scholar]
- 70. Nuutinen S, Vanhanen J, Pigni MC, Panula P. Effects of histamine H3 receptor ligands on the rewarding, stimulant and motor-impairing effects of ethanol in DBA/2J mice. Neuropharmacology. 2011;60(7-8):1193–1199. [DOI] [PubMed] [Google Scholar]
- 71. Millan-Guerrero RO, Baltazar-Rodrıguez LM, Cardenas-Rojas MI. A280V polymorphism in the histamine H3 receptor as a risk factor for migraine. Arch Med Res. 2011;42(1):44–47. [DOI] [PubMed] [Google Scholar]
- 72. Sheng Y, Lee JH, Medhurst AD, et al. Preservation of cortical histamine H (3) receptors in ischemic vascular and mixed dementias. J Neurol Sci. 2012;315(1-2):110–114. [DOI] [PubMed] [Google Scholar]
- 73. Bongers G, Bakker RA, Leurs R. Molecular aspects of the histamine H3 receptor. Biochem Pharmacol. 2007;73(8):1195–1204. [DOI] [PubMed] [Google Scholar]
- 74. He G, Ma X, Lu J, et al. Alpha2 adrenoceptors modulate histamine release from sympathetic nerves in the guinea pig vas deferens. Neuropharmacology. 2009;57(5-6):506–510. [DOI] [PubMed] [Google Scholar]
- 75. Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci. 2008;82(5-6):324–330. [DOI] [PubMed] [Google Scholar]
- 76. Shaw JB, Cai Q, Mtshali C, Myles EL, Washington B. Heterogeneity of histamine H3 receptor genomic expression in the cerebral cortex of spontaneously hypertensive rat. Cell Mol Biol (Noisy-le-grand). 2007;53(4):45–50. [PubMed] [Google Scholar]
- 77. Aquino-Miranda G, Osorio-Espinoza A, Escamilla-Sánchez J, et al. Histamine H (3) receptors modulate depolarization-evoked [(3) H]-noradrenaline release from rat olfactory bulb slices. Neuropharmacology. 2012;62(2):1127–1133. [DOI] [PubMed] [Google Scholar]
- 78. Coruzzi G, Adami M, Pozzoli C. Role of histamine H4 receptors in the gastrointestinal tract. Front Biosci (Schol Ed). 2012;4:226–239. [DOI] [PubMed] [Google Scholar]
- 79. Gibbs BF, Levi-Schaffer F. H4 receptors in mast cells and basophils: a new therapeutic target for allergy? Front Biosci. 2012;17:430–437. [DOI] [PubMed] [Google Scholar]
- 80. Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human memory th17 cells express a functional histamine h4 receptor. Am J Pathol. 2012;180(1):177–185. [DOI] [PubMed] [Google Scholar]
- 81. Gschwandtner M, Mommert S, Köther B, Werfel T, Gutzmer R. The histamine H4 receptor is highly expressed on plasmacytoid dendritic cells in psoriasis and histamine regulates their cytokine production and migration. J Invest Dermatol. 2011;131(8):1668–1676. [DOI] [PubMed] [Google Scholar]
- 82. Ikawa Y, Shiba K, Ohki E, et al. Comparative study of histamine H4 receptor expression in human dermal fibroblasts. J Toxicol Sci. 2008;33(4):503–508. [DOI] [PubMed] [Google Scholar]
- 83. Jiang W, Lim HD, Zhang M, et al. Cloning and pharmacological characterization of the dog histamine H4 receptor. Eur J Pharmacol. 2008;592(1-3):26–32. [DOI] [PubMed] [Google Scholar]
- 84. Strakhova MI, Nikkel AL, Manelli AM, et al. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009;1250:41–48. [DOI] [PubMed] [Google Scholar]
- 85. Truta-Feles K, Lagadari M, Lehmann K, et al. Histamine modulates γδ-T lymphocyte migration and cytotoxicity, via Gi and Gs protein-coupled signalling pathways. Br J Pharmacol. 2010;161(6):1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kharmatea G, Liua Z, Pattersonb E, Khan MM. Histamine affects STAT6 phosphorylation via its effects on IL-4 secretion: role of H1 receptors in the regulation of IL-4 production. Int Immunopharmacol. 2007;7(3):277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Higuchi M, Yanai K, Okamura N, et al. Histamine H (1) receptors in patients with Alzheimer's disease assessed by positron emission tomography. Neuroscience. 2000;99(4):721–729. [DOI] [PubMed] [Google Scholar]
- 88. Motawaj M, Burban A, Davenas E, et al. The histaminergic system: a target for innovative treatments of cognitive deficit. Therapie. 2010;65(5):415–422. [DOI] [PubMed] [Google Scholar]
- 89. Brioni JD, Esbenshade TA, Garrison TR, Bitner SR, Cowart MD. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer's disease. J Pharmacol Exp Ther. 2011;336(1):38–46. [DOI] [PubMed] [Google Scholar]
- 90. Motawaj M, Peoc'h K, Callebert J, Arrang JM. CSF levels of the histamine metabolite tele-methylhistamine are only slightly decreased in Alzheimer's disease. J Alzheimers Dis. 2010;22(3):861–871. [DOI] [PubMed] [Google Scholar]
- 91. Köhler CA, da Silva WC, Benetti F, Bonini JS. Histaminergic mechanisms for modulation of memory systems. Neural Plast. 2011;2011:328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chazot PL. Therapeutic potential of histamine H3 receptor antagonists in dementias. Drug News Perspect. 2010;23(2):99–103. [DOI] [PubMed] [Google Scholar]
- 93. Medhurst AD, Roberts JC, Lee J, et al. Characterization of histamine H3 receptors in Alzheimer's disease brain and amyloid over-expressing TASTPM mice. Br J Pharmacol. 2009;157(1):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fu QL, Dai HB, Shen Y, Chen Z. Reversing effect of histamine on neurotoxicity induced by beta-amyloid1-42. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007;36(2):146–149. [DOI] [PubMed] [Google Scholar]
- 95. Breitner JC, Welsh KA, Helms MJ, et al. Delayed onset of Alzheimer's disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging. 1995;16(4):523–530. [DOI] [PubMed] [Google Scholar]
- 96. Anthony JC, Breitner JC, Zandi PP, et al. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the cache county study. Neurology. 2000;54(11):2066–2071. [DOI] [PubMed] [Google Scholar]
- 97. Dere E, Zlomuzica A, Viggiano D, et al. Episodic-like and procedural memory impairments in histamine H1 receptor knockout mice coincide with changes in acetylcholine esterase activity in the hippocampus and dopamine turnover in the cerebellum. Neuroscience. 2008;157(3):532–541. [DOI] [PubMed] [Google Scholar]
- 98. Dai H, Kaneko K, Kato H, et al. Selective cognitive dysfunction in mice lacking histamineH1 and H2 receptors. Neurosci Res. 2007;57(2):306–313. [DOI] [PubMed] [Google Scholar]
- 99. Haas HL, Selbach O, Sergeeva OA. Sleep and sleep states: histamine role. Encyclopedia Neurosci. 2009;919–928. [Google Scholar]
- 100. Moniaga CS, Egawa G, Doi H, Miyachi Y, Kabashima K. Histamine modulates the responsiveness of keratinocytes to IL-17 and TNF-α through the H1-receptor. J Dermatol Sci. 2011;61(1):79–81. [DOI] [PubMed] [Google Scholar]
- 101. Tagawa M, Kano M, Okamura N, et al. Differential cognitive effects of ebastine and (+)-chlorpheniramine in healthy subjects: correlation between cognitive impairment and plasma drug concentration. Br J Clin Pharmacol. 2002;53(3):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Medhurst AD, Atkins AR, Beresford IJ, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321(3):1032–1045. [DOI] [PubMed] [Google Scholar]
- 103. Passani MB, Blandina P. Cognitive implications for H3 and 5-HT3 receptor modulation of cortical cholinergic function: a parallel story. Methods Find Exp Clin Pharmacol. 1998;20(8):725–733. [DOI] [PubMed] [Google Scholar]
- 104. Bitner RS, Markosyan S, Nikkel AL, Brioni JD. In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer's disease. Neuropharmacology. 2011;60(2-3):460–466. [DOI] [PubMed] [Google Scholar]
- 105. Simon T, Gogolák P, Kis-Tóth K, Jelinek I, László V, Rajnavölgyi E. Histamine modulates multiple functional activities of monocyte-derived dendritic cell subsets via histamine receptor 2. Int Immunol. 2012;24(2):107–116. [DOI] [PubMed] [Google Scholar]
- 106. Geng S, Gao YD, Yang J, Zou JJ, Guo W. Potential role of store-operated Ca (2+) entry in Th(2) response induced by histamine in human monocyte-derived dendritic cells. Int Immunopharmacol. 2012;12(2):358–367. [DOI] [PubMed] [Google Scholar]
- 107. Loewenbrueck KF, Tigno-Aranjuez JT, Boehm BO, Lehmann PV, Tary-Lehmann M. Th1 responses to beta-amyloid in young humans convert to regulatory IL-10 responses in Down syndrome and Alzheimer's disease. Neurobiol Aging. 2010;31(10):1732–1742. [DOI] [PubMed] [Google Scholar]
- 108. Felaco P, Castellani ML, De Lutiis MA, et al. IL-32: a newly-discovered proinflammatory cytokine. J Biol Regul Homeost Agents. 2009;23(3):141–147. [PubMed] [Google Scholar]
- 109. Cho KS, Park SH, Joo SH, Kim SH, Shin CY. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochem Biophys Res Commun. 2010;402(1):48–53. [DOI] [PubMed] [Google Scholar]