Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder of the central nervous system. Galectin-3 (Gal-3) is characterized by a conserved sequence within the carbohydrate recognition domain. The effect of Gal-3 in AD is presently unknown. In this study, we found significantly increased Gal-3 serum levels in patients with AD compared to control participants (P = .017). There was no significant difference between patients with mild cognitive impairment (MCI) and healthy controls (P = .143) or between patients with AD and MCI (P = .688). The degree of cognitive impairment, as measured by the Mini-Mental Status Examination score, was found to have a significant correlation with the Gal-3 serum levels in all patients and healthy controls. These data suggest that Gal-3 potentially plays a role in the neuropathogenesis of AD. The Gal-3 found in serum could be a potential candidate for a biomarker panel for AD diagnosis.
Keywords: Alzheimer’s disease, β-amyloid, galectin-3, biomarker, serum
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder of the central nervous system (CNS), which correlates with the appearance of neurofibrillary tangles and senile plaques (SPs). 1 The major component of SPs is β-amyloid peptide, which is believed to be the most probable cause of AD. 2 The AD is the most common form of dementia among older people. However, there are currently no diagnostic tests for AD. The AD can only be diagnosed with complete accuracy after death, when microscopic examination of the brain reveals the presence of the characteristic plaques and tangles. 3 The discovery of AD biomarkers, defined as biological indicators of disease presence, activity, and/or progression, will have a major impact on the efficiency of therapeutic clinical trials for this universally neurodegenerative disease.
Galectin-3 (Gal-3) is a member of the galectin family, which is characterized by a conserved sequence within the carbohydrate recognition domain (CRD) that has an affinity for β-galactoside residues. 4,5 The C-terminal half (ie, the CRD), composed of approximately 130 amino acids that form a globular structure, accommodates the entire carbohydrate-binding site and is thus responsible for the lectin activity of Gal-3. The N-terminal half contains 110 to 130 amino acids and is highly conserved among the Gal-3 molecules that have been isolated from other various species. 6,7 Early studies have shown that Gal-3 could bind to human umbilical vein endothelial cells and begin their migration in vitro. The Gal-3 has also been shown to induce new blood vessel formation in the Matrigel and tumor angiogenesis in vivo. 8–10 Increased levels of soluble and/or cellular Gal-3 have been associated with autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and Behçet’s disease. 11 The presence of Gal-3 correlates with the disease activities. 11 Importantly, real-time polymerase chain reaction and Western blot results have revealed that the expression of Gal-3 protein and messenger RNA was induced in scrapie-affected brains in the process of neurodegeneration in prion diseases. Moreover, increased levels of Gal-3 have been considered a potential biomarker for amyotrophic lateral sclerosis (ALS). 12 The expression patterns of Gal-3 in AD are, however, still unknown. This situation led us to focus on the role of Gal-3 in the pathogenesis of AD. As an initial step, we compared the Gal-3 levels in the serum of patients with AD, mild cognitive impairment (MCI), and controls to evaluate its association with the clinical features of each disease.
Materials and Methods
Patients
Serum samples, frozen at −80°C until assayed, were obtained from 41 consecutive patients with AD, 32 patients with MCI, and 46 healthy elderly controls. Patients and healthy elderly controls were recruited from the Department of Neurology in Yuhuangding Hospital and Qilu Hospital of Shandong University. The study was approved by the ethics committee of Yuhuangding Hospital and Shandong University. Written informed consent was obtained from each participant. Demographic parameters and baseline characteristics were investigated and listed in Table 1. Standard Mini-Mental Status Examination (MMSE) method was used to assess the clinical severity of cognitive impairment as described previously. 13 All enrolled patients with AD fulfilled the criteria of International Classification of Diseases, Tenth Revision, Diagnostic and Statistical Manual of Mental Disorders (Third Edition), and National Institute of Neurological and Communicative Disorders and Stroke Alzheimer’s Disease and Related Disorders Association. 14 Patients with MCI fulfilled the criteria as described previously. 15
Table 1.
Patients’ Demographic and Clinical Details.
| Characteristics | All (n = 119) | AD (n = 41) | MCI (n = 32) | Control (n = 46) | P value (AD vs controls) | P value (AD vs MCI) |
|---|---|---|---|---|---|---|
| Age, years | 69.9 ± 9.4 | 71.2 ± 8.1 | 70.6 ± 10.2 | 69.8 ± 10.9 | .053a | .062 |
| Sex (no. [%]) | .346b | .620 | ||||
| Females | 61 | 19 | 17 | 25 | ||
| Males | 58 | 22 | 15 | 21 | ||
| Mini-Mental State Examination score | 24.3 ± 5.8 | 19.1 ± 4.6 | 27.2 ± 1.8 | 29.5 ± 0.6 | <.001 | <.001 |
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment.
aMann-Whitney U test.
bChi-square test.
Measurement of Gal-3 Levels in Serum
Serum Gal-3 levels were measured by the use of specific enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota). Briefly, 100 μL of the serum was added to polystyrene 96-well plates coated with anti-Gal-3 antibodies and incubated at room temperature (RT) for 2 hours. After being washed 3 times, the wells were incubated at RT for 2 hours with horseradish peroxidase-conjugated secondary antibody. Next, the wells were washed again with the addition of tetramethylbenzidine and incubated at RT for 30 minutes. Finally, the reaction was terminated using H2SO4, and the absorbance was measured at 450 nm. The standard curve was used to calculate serum Gal-3 levels.
Data Analysis
All of the statistical analyses were carried out using the statistical analysis software package SPSS 19 (SPSS, Munich, Germany). A univariate analysis of variance (ANOVA) was calculated to compare serum Gal-3 levels between patients with AD and the control group. The data are presented as mean ± standard deviation (SD). P < .05 was considered as a statistical significance. A bivariate correlation analysis (Pearson correlation) between age, MMSE scores, and serum Gal-3 levels was conducted. Differences between 2 groups were assessed using the 2-tailed t test in case of normal distribution. The Mann-Whitney U test was used to assess differences between 2 groups in case of nonnormal distribution. Differences in gender between the 2 groups were assessed using the chi-square test.
Results
In order to include the age difference as a factor between controls and patients with AD, age was used as a covariate for the ANOVA. We found a difference in serum levels between the 3 different groups (P = .005). Pairwise comparisons with an independent t test revealed a significant difference in the Gal-3 serum levels between patients with AD and controls (AD vs healthy controls [mean ± SD] 6.42 ± 2.51 vs 5.27 ± 1.91 ng/mL; P = .017; Figure 1). However, there was no significant difference between patients with MCI and healthy controls (P = .143) nor between patients with AD and MCI (P = .688). There was a significant positive correlation between the MMSE score (as a measure for cognitive status) and the Gal-3 serum levels seen in all patients and healthy controls (r = .341; P < .001; Figure 2).
Figure 1.

The Gal-3 serum levels (ng/mL) in patients with Alzheimer’s disease (AD), patients with mild cognitive impairment (MCI), and healthy elderly controls. Patients with AD showed significantly higher Gal-3 serum levels compared with healthy controls (P = .017). There was no significant difference between patients with MCI and healthy controls (P = .143) nor between patients with AD and MCI (P = .688). Gal-3 indicates galectin-3.
Figure 2.
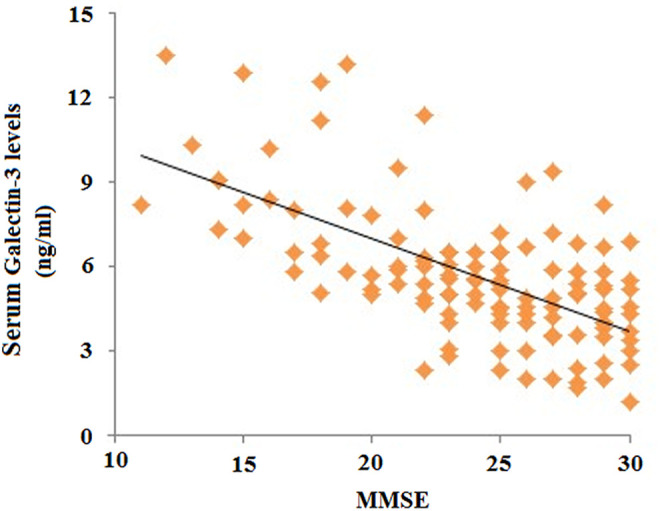
In all patients and healthy controls (n = 119), there is a significant correlation between the Mini-Mental Status Examination (MMSE) score (as a measure for cognitive status) and galectin (Gal-3) serum levels (r = .341; P < .001).
Discussion
Although the underlying mechanisms in the pathogenesis of AD is still unknown, experimental data from patient sera and cells continue to provide us with clues to the pathogenesis of AD. The Gal-3 is a multifunctional protein implicated in a variety of biological processes including fibrosis, angiogenesis, apoptosis, and immune activation, 16 all of which are associated with the development of AD. 17 As an initial step to address this issue, we investigated serum Gal-3 levels and their associations with the clinical features of AD. The major findings of this report are that the presence of Gal-3 in the serum of patients with AD is significantly increased. Furthermore, a significant positive correlation between the MMSE score and the Gal-3 serum levels were found. To our knowledge, the present data are the first to identify elevated Gal-3 associated with AD. The functional roles of Gal-3 in the CNS have been reported. 18 The induction of Gal-3 has been reported in many pathological processes of brain diseases, including ischemic brain lesions, 19 ALS, 20 and prion disease. 21
The Gal-3 has been reported to be expressed by most cell types. It is present not only in the cytosol and nucleus but also in the extracellular space. Importantly, it has been identified in the CNS and peripheral nervous system in astrocytes, macrophages/microglia, endothelial cells, and Schwann cells. Activation of microglia and endothelial cells was associated with the pathogenesis of AD. 22 Moreover, Gal-3 is involved in the physiology of almost all kinds of immune cells. 23 Thus, we speculated that the upregulation of Gal-3 expression is associated with immune activation and regulation in AD. However, there is limited information on the regulatory mechanisms of Gal-3 expression. 24 Further investigations are needed to elucidate the underlying mechanisms.
Speculation of a possible biological role for Gal-3 in the pathogenesis of AD can be brought about by the several known actions of this protein. For example, Gal-3 influences apoptotic cell death pathways; intracellular Gal-3 is antiapoptotic and extracellular Gal-3 is proapototic. 25,26 Moreover, Gal-3 was reported to play an important role in inflammatory pathways. 27 It was proposed that Gal-3 led to an enhanced inflammatory response by suppressing the production of the anti-inflammatory cytokine interleukin 10 (IL-10). 28 It is interesting to note that IL-10 has been suggested as a neuroprotective cytokine released from microglia during the course of AD progression. 29 Studies also reported that Gal-3 inhibited peripheral nerve regeneration after axotomy 30 and exacerbated CNS damage in EAE. 31 The Gal-3 is an important receptor for advanced glycation end products (RAGEs) in the cell surface. The accumulation of irreversible long-lived proteins, advanced glycation end products (AGEs) and the interaction of AGEs with cellular receptors such as Gal-3 and RAGE are considered to be the key events in the development of long-term complications of chronic diseases, such as diabetes mellitus, AD, uremia, and aging. 32 Moreover, it has been proposed that pharmacologic inhibition of AGE receptor-mediated cell activation with specific antagonists may provide the basis for therapeutic intervention in diseases, where AGE accumulation is a suspected etiological factor for vascular complications of diabetes, macrovascular disease, renal insufficiency, and Alzheimer's disease. 33 So far, 15 galectin subtypes have been described in mammals. They have been numbered following the order of their discovery. 34 Among them, Gal-4 is expressed by hippocampal and cortical neurons, where it is sorted to discrete segments of the axonal membrane in a microtubule- and sulfatide-dependent manner. 35 Moreover, neuronal Gal-4 is considered as a candidate for a soluble regulator of oligodendrocytes differentiation and myelination. 36 However, the effects of Gal-4 in neurodegenerative disease are less investigated. Further research is needed to clarify the effect of Gal-4 in neurodegenerative diseases, including AD.
Elevated serum Gal-3 levels might suggest potential proapoptotic activation, inflammation, and impaired neurodegeneration in patients with AD. More importantly, we have also shown an association between the Gal-3 serum levels and the cognitive status in all patients and healthy controls. Given its secretory nature and detectable level in serum, it is a potential biomarker for AD that is worth further evaluation in a prospective cohort of patients and potentially in future clinical trials.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399(6738 suppl):A23–A31. [DOI] [PubMed] [Google Scholar]
- 2. Cotman CW, Su JH. Mechanisms of neuronal death in Alzheimer's disease. Brain Pathol. 1996;6(4):493–506. [DOI] [PubMed] [Google Scholar]
- 3. Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer's disease. Science. 2006;314(5800):781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agrwal N, Sun Q, Wang SY, Wang JL. Carbohydrate-binding protein 35. I. Properties of the recombinant polypeptide and the individuality of the domains. J Bio Chem. 1993;268(20):14932–14939. [PubMed] [Google Scholar]
- 5. Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616–635. [DOI] [PubMed] [Google Scholar]
- 6. Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. [DOI] [PubMed] [Google Scholar]
- 7. Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64(13):1679–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2009;207(9):1981–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial Growth factor receptor-2 in human endothelial cells. J Biol Chem. 2011;286(34):29913–29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taniguchi T, Asano Y, Akamata K, et al. Serum levels of galectin-3: possible association with fibrosis, aberrant angiogenesis, and immune activation in patients with systemic sclerosis. J Rheumatol. 2012;39(3):539–544. [DOI] [PubMed] [Google Scholar]
- 12. Zhou JY, Afjehi-Sadat L, Asress S, et al. Galectin-3 is a candidate biomarker for amyotrophic lateral sclerosis: discovery by a proteomics approach. J Proteome Res. 2010;9(10):5133–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. ‘Mini mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 15. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 16. Radosavljevic G, Volarevic V, Jovanovic I, et al. The roles of galectin-3 in autoimmunity and tumor progression. Immunol Res. 2012;52(1-2):100–110. [DOI] [PubMed] [Google Scholar]
- 17. Tam JH, Pasternak SH. Amyloid and Alzheimer's disease: inside and out. Can J Neurol Sci. 2012;39(3):286–298. [DOI] [PubMed] [Google Scholar]
- 18. Reichert F, Rotshenker S. Galectin-3/MAC-2 in experimental allergic encephalomyelitis. Exp Neurol. 1999;160(2):508–514. [DOI] [PubMed] [Google Scholar]
- 19. Walther M, Kuklinski S, Pesheva P, et al. Galectin-3 is upregulated in microglial cells in response to ischemic brain lesions, but not to facial nerve axotomy. J Neurosci Res. 2000;61(4):430–435. [DOI] [PubMed] [Google Scholar]
- 20. Lerman BJ, Hoffman EP, Sutherland ML, et al. Deletion of galectin-3 exacerbates microglial activation and accelerates disease progression and demise in a SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Brain Behav. 2012;2(5):563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin JK, Na YJ, Song JH, et al. Galectin-3 expression is correlated with abnormal prion protein accumulation in murine scrapie. Neurosci Lett. 2007;420(2):138–143. [DOI] [PubMed] [Google Scholar]
- 22. Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. [DOI] [PubMed] [Google Scholar]
- 23. Filer A, Bik M, Parsonage GN, et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009;60(6):1604–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim K, Mayer EP, Nachtigal M. Galectin-3 expression in macrophages is signaled by Ras/MAP kinase pathway and up-regulated by modified lipoproteins. Biochim Biophys Acta. 2003;1641(1):13–23. [DOI] [PubMed] [Google Scholar]
- 25. Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277(18):15819–15827. [DOI] [PubMed] [Google Scholar]
- 26. Mok SW, Riemer C, Madela K, et al. Role of galectin-3 in prion infections of the CNS. Biochem. Biophys Res Commun. 2007;359(3):672–678. [DOI] [PubMed] [Google Scholar]
- 27. Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23(4):383–392. [DOI] [PubMed] [Google Scholar]
- 28. Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011;73(2):79–84. [DOI] [PubMed] [Google Scholar]
- 29. Di Bona D, Rizzo C, Bonaventura G, Candore G, Caruso C. Association between interleukin-10 polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2012;29(4):751–759. [DOI] [PubMed] [Google Scholar]
- 30. Narciso MS, Mietto Bde S, Marques SA, et al. Sciatic nerve regeneration is accelerated in galectin-3 knockout mice. Exp Neurol. 2009;217(1):7–15. [DOI] [PubMed] [Google Scholar]
- 31. Jiang HR, Al Rasebi Z, Mensah-Brown E, et al. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182(2):1167–1173. [DOI] [PubMed] [Google Scholar]
- 32. Mercer N, Ahmed H, McCarthy AD, Etcheverry SB, Vasta GR, Cortizo AM. AGE-R3/galectin-3 expression in osteoblast-like cells: regulation by AGEs. Mol Cell Biochem. 2004;266(1-2):17–24. [DOI] [PubMed] [Google Scholar]
- 33. Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand). 1998;44(7):1013–1023. [PubMed] [Google Scholar]
- 34. Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol. 2012;946:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Velasco S, Díez-Revuelta N, Hernández-Iglesias T, et al. Neuronal Galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J Neurochem. 2013;125(1):49–62. [DOI] [PubMed] [Google Scholar]
- 36. Stancic M, Slijepcevic D, Nomden A, et al. Galectin-4, a novel neuronal regulator of myelination. Glia. 2012;60(6):919–935. [DOI] [PubMed] [Google Scholar]


