Abstract
To investigate the value of hydrogen proton magnet resonance spectroscopy (1H-MRS) in the differential diagnosis of multiple-domain amnestic mild cognitive impairment (M-aMCI) and vascular cognitive impairment with no dementia (VCIND); 1H-MRS was performed in patients with M-aMCI and VCIND. The level was determined for N-acetylaspartate (NAA), glutamate (Glu), inositol (mI), choline (Cho), and creatine (Cr). Compared with the normal control group, the NAA–Cr ratio in all regions studied was significantly lower in the M-aMCI and VCIND groups. The Glu–Cr ratio in the posterior cingulate gyrus of the M-aMCI group was significantly lower than in the VCIND. The mI–Cr ratio in the frontal white matter of the VCIND was significantly higher than in the M-aMCI group. In the white matter adjacent to the lateral ventricles, the Cho–Cr ratio was significantly higher in the VCIND than the M-aMCI. Our results suggested 1H-MRS is an effective method in the differential diagnosis of M-aMCI and VCIND.
Keywords: multi-domain amnestic mild cognitive impairment, vascular cognitive impairment without dementia, Alzheimer’s disease, magnet resonance spectroscopy
Introduction
Amnestic mild cognitive impairment (aMCI) is a transitional stage between normal aging and Alzheimer’s disease (AD), with a risk for AD conversion of up to 10% per year, whereas the risk for AD conversion from normal aging was approximately 1% to 2% per year. 1 Amnestic mild cognitive impairment includes the following 2 subtypes: single-domain aMCI (S-aMCI) and multiple-domain aMCI (M-aMCI). Single-domain aMCI mainly has memory impairment, whereas M-aMCI has memory impairment and functional impairments in language, execution, attention, or other cognitive domains 2 and a higher AD conversion rate than S-aMCI. 3 Vascular cognitive impairment with no dementia (VCIND) is a prodromal stage of vascular dementia (VD). Wentzel et al 4 showed that approximately 50% of patients with VCIND converted to VD within 5 years. Previous studies have concluded that VCIND mainly affects executive functional disorder, but recent research has confirmed that VCIND is often accompanied by multiregional cognitive impairments in memory, attention, and visuospatial ability. 5
Compared with patients experiencing impairment in a single cognitive function, clinical manifestations of patients with M-aMCI or VCIND vary more broadly and have a worse prognosis. 6 Therefore, early and definitive diagnosis may effectively guide the clinical therapy and the prognosis of patients having M-aMCI or VCIND. However, solely relying on clinical manifestations, neuropsychological testing, and routine imaging cannot effectively differentiate between M-aMCI and VCIND. This study used hydrogen proton magnet resonance spectroscopy (1H-MRS) to determine the features of metabolic change in patients with M-aMCI and VCIND in different brain regions using imaging to provide a better understanding between M-aMCI and VCIND and a clinical basis for early diagnosis of either disease.
Materials and Methods
Research Participants
Thirty-eight patients with M-aMCI and 44 patients with VCIND from the Suzhou Hospital affiliated to Nanjing Medical University were randomly selected from July 2011 to June 2014, and 30 age-matched healthy elderly population served as normal controls in this study (see Table 1 for age, gender, and education level). All tested participants underwent epidemiological investigation, neuropsychological testing, and conventional radiographic examination to exclude patients with severe depression, schizophrenia, and other mental disorders. All tested participants were right-handed and signed a written informed consent form that had been approved by the ethics committee of Nanjing Medical University.
Table 1.
Characteristics of Patients in M-aMCI, VCIND and NC Groups.
| M-aMCI | VCIND | NC | Test Value | P | |
|---|---|---|---|---|---|
| Gender (M/F) | 38 (22/16) | 44 (27/17) | 30 (17/13) | 5.273 | .05 |
| Age, years | 68.32 ± 6.13 | 70.46 ± 6.77 | 70.74 ± 6.58 | 4.152 | .05 |
| Education, years | 9.6 ± 2.3 | 8.7 ± 3.1 | 9.1 ± 3.3 | 1.663 | .05 |
Abbreviations: M-aMCI, multiple-domain amnestic mild cognitive impairment; NC, normal cognitive; VCIND, vascular cognitive impairment with no dementia.
Diagnostic Standard
Diagnosis of M-aMCI was revised primarily based on the criteria of Petersen et al. 7 (1) Patients complained about memory loss, which was corroborated by their family members. (2) In addition to memory dysfunction, objective evidence existed for cognitive impairment in one or more of the following functions: language, executive function, and calculation (consistent with a Mini-Mental State Evaluation [MMSE] score of 24-27; a Clinical Dementia Rating [CDR] score of 0.5; and a Montreal Cognitive Assessment [MoCA] score of 23-26). However, the patients did not meet the diagnostic criteria of dementia according to the Diagnostic and Statistical Manual of Mental Disorder IV. (3) The daily activities were not affected (ie, the activity of daily living [ADL] score below 22).
Diagnoses of VCIND were revised according to the VCIND criteria 8 proposed by National Institute of Neurological Disorders and Stroke-Canadian Stroke Network (NINDS-CSN). (1) Patients had impairment of executive function, which was confirmed by their family members. (2) Patients also had other cognitive impairments (eg, memory loss). (3) However, the cognitive impairments did not meet the diagnostic criteria for NINDS. The MoCA score was 23 to 26, and the CDR score was 0.5. (4) The daily activities were not affected, with an ADL score below 22. (5) Cerebrovascular disease was considered to be a risk factor for or a cause of cognitive impairment and confirmed by corresponding focal neurological signs or imaging data, with a Hachinski Ischemic Score (HIS) of above 7.
Tested participants in the normal cognitive (NC) function group had no clinical cognitive dysfunction and an uneventful neurological history, as well as had normal scores of MMSE, CDR, and MoCA.
Neuropsychological Evaluation
All tested participants underwent neuropsychological assessments by senior neurologist to examine their cognitive function using MMSE, ADL, CDR, MoCA, and HIS.
Hydrogen Proton Magnet Resonance Spectroscopy
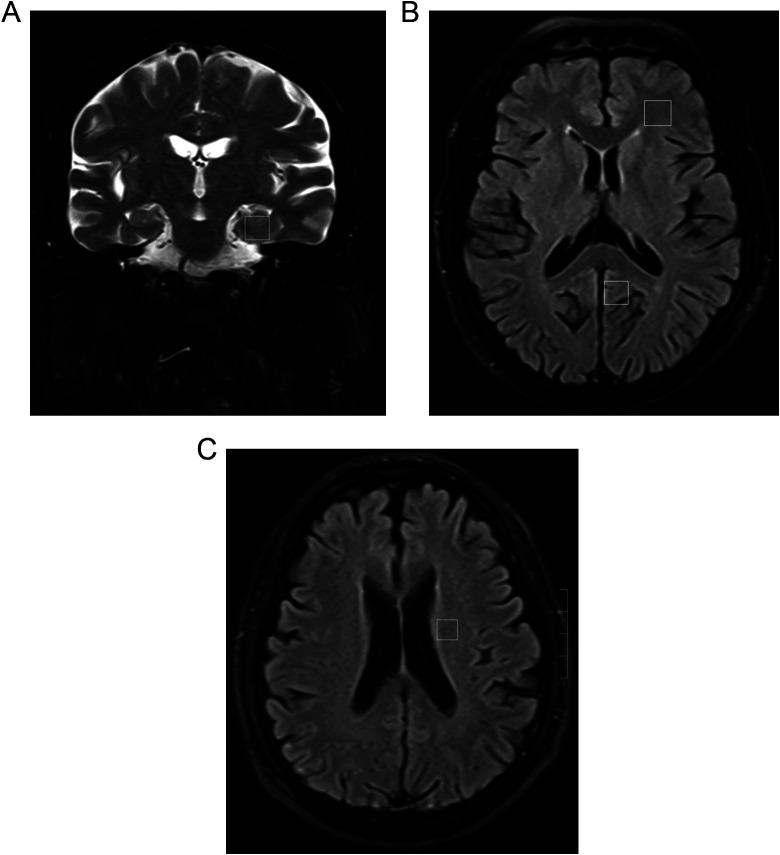
All tested participants were examined using a Siemens MAGNETOM Aera 3.0T magnetic resonance imaging (MRI) scanner, and image capture was performed using a 24-channel standard quadrature head coil. All tested participants first underwent conventional scanning sequences of T1WI, T2WI, and fluid attenuation inversion recovery, followed by using point-resolved spatially localized spectroscopy for 3-dimensional single-voxel 1H-MRS, with scanning parameters: repetition time/echo time = 2200/45 ms, rotation angle of 90°, field of vision 180 × 240 × 60 mm, incentive number of 200, and each voxel size of 0.8 × 0.8 × 1.0 cm. The regions of interest in the left hippocampus, the posterior cingulate gyrus, the frontal white matter, and the white matter adjacent to the lateral ventricles were selected (Figure 1) and oriented in 3 directions (ie, cross-sectional, coronal, and sagittal sections) to avoid impact from the skull, blood vessels, fat tissue, or cerebrospinal fluid, with the setting of automatic shimming, water suppression, and full width at half-maximum less than 15 Hz. After scanning was completed, raw data were transferred to workstations to obtain the spectrum of original phase diagram via a Fourier transformation. The baseline calibration and the phase correction were automatically completed by the MRI software. The area under the peaks for N-acetylaspartate (NAA), glutamate (Glu), inositol (mI), choline (Cho), and creatine (Cr) were measured to calculate the ratio differences of NAA–Cr, Glu–Cr, mI–Cr, and Cho–Cr.
Figure 1.
Localized proton magnet resonance spectroscopy (MRS) of patient: (A) hippocampus (HIP); (B) white matter of the frontal lobe (FLWM) and posterior cingulate gyrus (PCG); (C) white matter adjacent to the lateral ventricles (WMALV). Region of interest (ROI) sizes were approximately 0.8 × 0.8 × 1.0 cm.
Statistical Analysis
SPSS for Windows 17.0 software was used for statistical analysis of measured data. Data were presented using mean ± standard deviation ( ). Qualitative data were analyzed using χ2 test, whereas quantitative data were measured using 1-way analysis of variance. Pairwise comparisons between groups were measured using Fisher least significant difference test. P < .05 was considered as statistically significant difference.
Results
Comparison of clinical features in M-aMCI, VCIND, and NC groups (Table 1).
The age, education level, and gender were homogeneously distributed in the test participants of the 3 groups, with no significant difference between groups (P > .05).
Comparing the neuropsychological test results in M-aMCI, VCIND, and NC groups (Table 2).
Table 2.
The Neuropsychological Feature of the M-aMCI, VCIND, and NC Groups.
| M-aMCI | VCIND | NC | F | P | |
|---|---|---|---|---|---|
| MMSE | 25.49 ± 1.08a | 25.13 ± 1.51a | 29.54 ± 2.01 | 58.841 | <.05 |
| MoCA | 24.28 ± 1.23a | 24.75 ± 1.17a | 28.94 ± 3.52 | 38.695 | <.05 |
| HIS | 2.92 ± 0.39 | 9.32 ± 0.73a,b | 3.03 ± 0.25 | 249.383 | <.05 |
Abbreviations: HIS, Hachinski Ischemic Score; M-aMCI, multiple-domain amnestic mild cognitive impairment; MMSE, Mini-Mental State Evaluation; MoCA, Montreal Cognitive Assessment; NC, normal cognitive; VCIND, vascular cognitive impairment with no dementia.
aP < .05 versus NC group.
bP < .05 versus M-aMCI group.
Univariate analysis of variance showed statistically significant differences in MMSE, MoCA, and HIS scores among the tested participants of the 3 groups (P < .05), whereas the ADL scores were not significantly different between the 3 groups (P > .05). The MMSE and MoCA scores in the M-aMCI and VCIND groups were significantly lower than in the NC group (P < .05 and P < .05, respectively). No statistically significant differences in the MMSE and MoCA scores were found between the M-aMCI and VCIND groups (P > .05). The HIS score in the VCIND group was significantly higher than in the M-aMCI and NC groups (P < .05), whereas no significant difference in the HIS score was found between the M-aMCI and NC groups (P > .05).
Table 3.
The ratio of NAA–Cr From M-aMCI, VCIND, and NC Groups.
| Group | HIP | PCG | FLWM | PAWM |
|---|---|---|---|---|
| NC | 1.19 ± 0.21 | 1.22 ± 0.25 | 1.21 ± 0.18 | 1.21 ± 0.24 |
| M-aMCI | 1.12 ± 0.08a | 1.09 ± 0.09a | 1.08 ± 0.12a | 1.09 ± 0.11a |
| VCIND | 1.10 ± 0.11a | 1.12 ± 0.15a | 1.11 ± 0.10a | 1.08 ± 0.13a |
| F | 8.194 | 7.772 | 11.236 | 9.815 |
| P | .05 | .05 | .05 | .05 |
Abbreviations: Cr, creatine; FLWM, white matter of the frontal lobe; HIP, hippocampus; M-aMCI, multiple-domain amnestic mild cognitive impairment; NAA, N-acetylaspartate; NC, normal cognitive; PAWN, white matter adjacent to the lateral ventricles; PCG, posterior cingulate gyrus; VCIND, vascular cognitive impairment with no dementia.
aP < .05 versus NC.
Table 4.
The Ratio of Glu–Cr From M-aMCI, VCIND, and NC Groups.
| Group | HIP | PCG | FLWM | PAWM |
|---|---|---|---|---|
| NC | 0.41 ± 0.05 | 0.40 ± 0.05 | 0.39 ± 0.03 | 0.39 ± 0.04 |
| M-aMCI | 0.39 ± 0.05 | 0.31 ± 0.02a,b | 0.40 ± 0.03 | 0.39 ± 0.04 |
| VCIND | 0.38 ± 0.04 | 0.42 ± 0.05 | 0.41 ± 0.04 | 0.41 ± 0.03 |
| F | 0.512 | 7.418 | 0.843 | 0.626 |
| P | .05 | .05 | .05 | .05 |
Abbreviations: Cr, creatine; FLWM, white matter of the frontal lobe; Glu, glutamate; HIP, hippocampus; M-aMCI, multiple-domain amnestic mild cognitive impairment; NC, normal cognitive; PAWN, white matter adjacent to the lateral ventricles; PCG, posterior cingulate gyrus; VCIND, vascular cognitive impairment with no dementia.
aP < .05 versus NC.
bP < .05 versus VCIND.
Table 5.
The Ratio of mI–Cr from M-aMCI, VCIND, and NC Groups.
| Group | HIP | PCG | FLWM | PAWM |
|---|---|---|---|---|
| NC | 0.46 ± 0.05 | 0.42 ± 0.05 | 0.43 ± 0.06 | 0.45 ± 0.04 |
| M-aMCI | 0.69 ± 0.09a,b | 0.71 ± 0.08 | 0.40 ± 0.04 | 0.39 ± 0.04 |
| VCIND | 0.49 ± 0.04 | 0.44 ± 0.05 | 0.61 ± 0.07a,c | 0.42 ± 0.03 |
| F | 2.672 | 5.491 | 4.463 | 0.774 |
| P | .05 | .05 | .05 | .05 |
Abbreviations: Cr, creatine; FLWM, white matter of the frontal lobe; HIP, hippocampus; M-aMCI, multiple-domain amnestic mild cognitive impairment; mI, inositol; NC, normal cognitive; PAWN, white matter adjacent to the lateral ventricles; PCG, posterior cingulate gyrus; VCIND, vascular cognitive impairment with no dementia.
aP < .05 versus NC.
bP < .05 versus VCIND.
cP < .05 versus M-aMCI.
Table 6.
The Ratio of Cho–Cr From M-aMCI, VCIND, and NC Groups.
| Group | HIP | PCG | FLWM | PAWM |
|---|---|---|---|---|
| NC | 0.92 ± 0.05 | 0.94 ± 0.05 | 1.06 ± 0.09 | 1.02 ± 0.05 |
| M-aMCI | 0.95 ± 0.06 | 0.92 ± 0.06 | 1.05 ± 0.06 | 0.99 ± 0.04 |
| VCIND | 0.99 ± 0.04 | 0.94 ± 0.05 | 1.03 ± 0.06 | 1.12 ± 0.11a,b |
| F | 0.651 | 0.349 | 0.720 | 7.813 |
| P | .05 | .05 | .05 | .05 |
Abbreviations: Cho, choline; Cr, creatine; FLWM, white matter of the frontal lobe; HIP, hippocampus; M-aMCI, multiple-domain amnestic mild cognitive impairment; NC, normal cognitive; PAWN, white matter adjacent to the lateral ventricles; PCG, posterior cingulate gyrus; VCIND, vascular cognitive impairment with no dementia.
aP < .05 versus NC.
bP < .05 versus M-aMCI.
The NAA–Cr ratios in the left hippocampus, the posterior cingulate gyrus, the frontal white matter, and the white matter adjacent to the lateral ventricles in the M-aMCI and VCIND groups were significantly lower than in the NC group (P < .05 and P < .05, respectively). No significant difference in NAA–Cr was found between the M-aMCI and VCIND groups in any of the brain regions studied (P > .05).
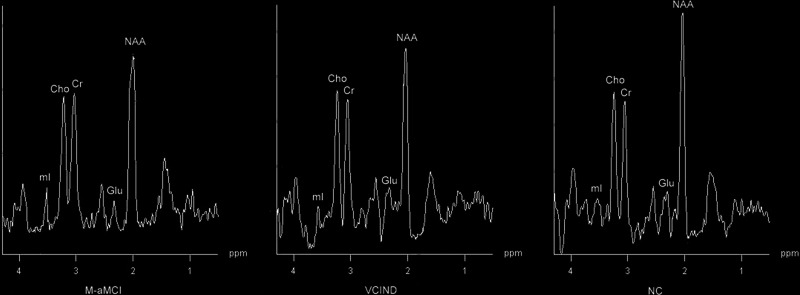
Results of 1H-MRS showed that the Glu–Cr ratio in the left lateral posterior cingulate gyrus of the M-aMCI group was significantly lower than in the VCIND and NC groups (P < .05 and P < .05, respectively), and no significant difference in Glu–Cr was found between the VCIND and NC groups (P > .05; Figure 2). In the left hippocampus, frontal white matter, and white matter adjacent to the lateral ventricles, no significant difference in Glu–Cr was found between the 3 groups (P > .05).
Figure 2.
Spectra from the left lateral posterior cingulate gyrus: glutamate (Glu) level of the multiple-domain amnestic mild cognitive impairment (M-aMCI) group was significantly lower than in the vascular cognitive impairment with no dementia (VCIND) and normal cognitive (NC) groups.
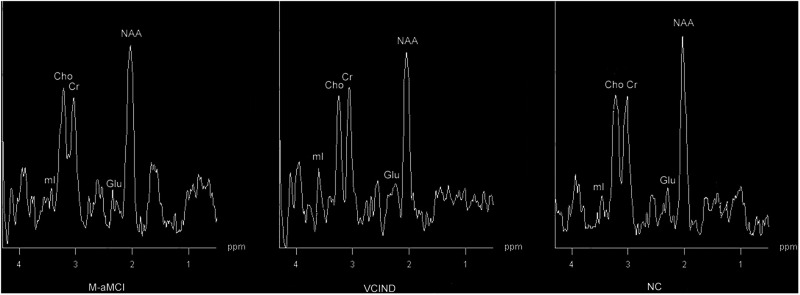
The mI–Cr ratios in the left hippocampus and posterior cingulate gyrus in the M-aMCI group were significantly higher than those in the VCIND and NC groups (P < .05). No significant difference in the mI–Cr ratio was found between the VCIND and NC groups (P > .05). In the region of the left lateral posterior cingulate gyrus, the mI–Cr ratio in the VCIND group was significantly higher than in the M-aMCI and NC groups (P < .05; Figure 3). In the white matter adjacent to the lateral ventricles, no significant difference in the mI–Cr ratio was found between the 3 groups (P > .05).
Figure 3.
Spectra from the left lateral frontal white matter: inositol (mI) level of the vascular cognitive impairment with no dementia (VCIND) group was higher than those in the multiple-domain amnestic mild cognitive impairment (M-aMCI) and normal cognitive (NC) groups.
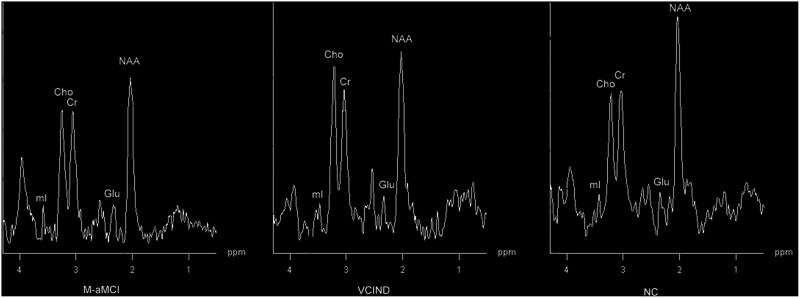
No significant difference in Cho–Cr ratio in the left hippocampus, cingulate gyrus, and frontal white matter was found between the M-aMCI and VCIND groups (P > .05); in the white matter adjacent to the lateral ventricle, the ratio was significantly higher in the VCIND group than in the M-aMCI and NC groups (P < .05; Figure 4).
Figure 4.
Spectra from the white matter adjacent to the left lateral ventricles: choline (Cho) level of the vascular cognitive impairment with no dementia (VCIND) group was significantly higher than those in the M-aMCI and NC groups.
Discussion
Pathological features of M-aMCI are amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) in the hippocampus and entorhinal area, whereas the histopathological feature of VCIND is subcortical ischemic vascular disease with small vessel disease. Differences in the pathological basis between M-aMCI and VCIND lead to the development of different types of dementia. 9 Initially, aMCI was considered to show memory impairment only and no other cognitive dysfunctions. 10 However, later studies showed that many patients showed impairment in memory, executive function, and information-processing capacity compared with normal elderly participants, that is, cognitive dysfunction was seen in multiple brain regions. 11,12 Similarly, although VCIND shows prominent impairment of executive function, it shares common features with M-aMCI that include memory loss and other areas of cognitive impairment. 6 Even with imaging, some VCIND caused by undetected cerebrovascular disease (eg, low perfusion and incomplete infarction) may appear to be negative on MRI examination. Patients with M-aMCI often have cerebrovascular disease (eg, lacunar infarction and leukoaraiosis) as a comorbidity. Thus, although neuropsychological assessment and conventional neuroimaging are important strategies for the diagnosis of different types of cognitive dysfunction, in practice, M-aMCI and VCIND are difficult to distinguish clinically.
Hydrogen proton magnet resonance spectroscopy is the only imaging technique to study metabolic changes in living tissue noninvasively with good reproducibility and stability. Changes in metabolite levels precede the structural changes in the brain that define the pathological changes in dementia and are widely used in basic and clinical research in dementia. 13 –16 Currently, 1H-MRS studies are mostly conducted in aMCI but rarely reported in VCIND. In addition, in such studies, the tested participants were mainly diagnosed with s-aMCI or VCIND. 17 –19 Studies on the early differential diagnosis of M-aMCI and VCIND have been rarely reported.
N-acetylaspartate, a neuronal cell-specific marker, has been frequently used as a metabolic indicator in studies of cognitive impairment and dementia. Many studies confirmed a significant reduction in NAA levels in multiple brain regions of patients with MCI, AD, and VD. 20 –25 Zhu et al 26 found that there were differences in NAA–Cr related to brain regions between different types of MCI. Kattapong et al 27 also reported that the NAA–Cr and NAA–Cho ratios in the subcortical white matter of patients with VD were significantly reduced compared with patients with AD. In this study, we detected similar changes in NAA levels between patients with M-aMCI and VCIND. Although the NAA levels in multiple brain regions of patients with M-aMCI and VCIND were significantly lower than those in normal elderly participants, suggesting a reduction in neuronal function or demyelination, no significant regional difference in the NAA–Cr ratio was found between M-aMCI and VCIND, suggesting that NAA may lack specificity when differentiating between M-aMCI and VCIND.
Many experts believe that the levels of the mI metabolites may the first to change in the brains of patients with aMCI or AD. 28,29 In the early stage of aMCI or AD, the mI–Cr ratio increases, and this change is associated with a decline in cognitive function. However, the value of mI as a specific marker for cognitive impairment or dementia remains controversial. In the study of Liu et al, 18 increased mI in the right posterior cingulate gyrus of patients with aMCI was observed. Walecki et al 30 evaluated a group of patients with aMCI who eventually developed AD using 1H-MRS and showed no significant change in the mI–Cr ratio of the medial temporal lobe. Kantarci et al 31 showed that the mI–Cr ratio in the posterior cingulate gyrus of patients with S-aMCI was significantly higher than in the control group, but no change was observed in patients with M-aMCI. In contrast, Waldman et al 32 showed no abnormal change in the mI–Cr ratio in the brain of patients with VD. In this study, we demonstrated that the mI–Cr ratio increased abnormally in the hippocampus and the posterior cingulate gyrus of patients with M-aMCI and in the frontal white matter of patients with VCIND, suggesting that mI may be a marker differentiating the features of M-aMCI and VCIND. Although increases in mI concentration reflect a certain proliferation and activation of glial cells or microglial cells, 33 the pathological mechanism associated with the metabolic change in mI in the brain of patients with M-aMCI and VCIND remains unclear. Our analysis of increased mI levels in the hippocampus and posterior cingulate gyrus of patients with M-aMCI may be associated with the changes in pathological characteristic in these brain regions (eg, Aβ protein or NFT), whereas the increased mI levels in the frontal white matter of patients with VCIND may be associated with the first functional damage in the frontal lobe.
Glutamate is the main excitatory neurotransmitter in the brain and is closely related to neuronal survival and formation of synapses 34 involved in regulating learning, memory, and cognition in the cortex. Glutamate homeostasis relies on the functional integrity of neurons and glial cells 35 ; therefore, the level of change in Glu can reflect functional or pathological changes in neurons and glial cells. However, compared with metabolites such as NAA, mI, and Cho, less attention has been paid to Glu studies of cognitive impairment and dementia. This may be due to the difficulties in detecting Glu in <1.5T MRI. Currently, only a few studies such as that by Rupsingh et al 36 and Fayed et al 37 have shown that the Glu–Cr ratio in the hippocampus of patients with AD was significantly reduced compared with the normal controls, but no significant changes were found in patients with MCI. Hattori et al 38 reported that Glu reduction only occurred in the cortex but not in the white matter of patients with AD. With the clinical application of 3.0T MRI, the detection of metabolic changes in Glu in the brain of patients with M-aMCI and VCIND has become feasible. Our study suggests that the Glu–Cr ratio in patients with M-aMCI is first reduced in the posterior cingulate gyrus, indicating that the change in Glu level in this region may be an effective indicator to distinguish between M-aMCI and VCIND. This result also suggests that the posterior cingulate gyrus may play an important role in the neuropsychological process of learning and memory.
Choline is involved in the formation and metabolism of cell membranes and myelin. Its role as a marker for cognitive impairment and dementia remains unclear and contradictory. A study by Kantarci et al 17 reported that patients with aMCI who developed AD showed a significantly increased Cho–Cr ratio. Walecki et al 30 reported a decreased Cho–Cr ratio in the temporal lobe of patients with aMCI. In this study, the Cho–Cr ratio in the white matter adjacent to the lateral ventricle in the VCIND group was significantly higher than in the M-aMCI and NC groups, suggesting that patients with VCIND having subcortical ischemic vascular disease have excessive destruction in the myelin of white matter adjacent to the lateral ventricles.
Although the degree of cognitive impairment in M-aMCI and VCIND has not (yet) reached the diagnostic criteria for dementia, studies have confirmed that the pathological changes are similar in dementia and M-aMCI and VCIND. Thus, the concepts of M-aMCI and VCIND were proposed to improve the diagnosis of AD and VD. However, the reasons for regional differences in brain metabolism in patients with M-aMCI and VCIND remain unclear. We speculate that they may be associated with the 2 different patterns of cognitive impairment between M-aMCI and VCIND. The cognitive impairment of VCIND belongs to “the frontal–subcortical” pattern. 6 The frontal white matter and white matter adjacent to the lateral ventricles are key regions for the implementation dysfunction, whereas the cognitive impairment of patients with M-aMCI belongs to “temporal lobe–neocortex” pattern. 39 Hence, the main clinical manifestation is a decrease in memory capacity and the metabolic changes mainly in the hippocampus and cingulate gyrus.
In conclusion, our study showed that one of the most important markers of 1H-MRS, that is, the change in NAA levels, lacked specificity to differentiate between M-aMCI and VCIND. The levels of mI, Glu, and Cho were significantly different in different brain regions, suggesting that 1H-MRS is an effective method to differentiate between M-aMCI and VCIND.
Acknowledgments
The authors thank Dr Jun Wang for contributing to the statistical analysis. They also thank Mr Sunxing Zhang for the English revision.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Petersen RC, Negash S. Mild cognitive impairment: overview. CNS Spectr. 2008;13(1):45–53. [DOI] [PubMed] [Google Scholar]
- 2. Seo SW, Im K, Lee JM, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007;36(2):289–297. [DOI] [PubMed] [Google Scholar]
- 3. Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment—long-term courses of four clinical subtypes. Neurology. 2006;67(12):2176–2185. [DOI] [PubMed] [Google Scholar]
- 4. Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;57(4):714–716. [DOI] [PubMed] [Google Scholar]
- 5. Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Göteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81(5):541–546. [DOI] [PubMed] [Google Scholar]
- 6. Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 2004;19(11):1053–1057. [DOI] [PubMed] [Google Scholar]
- 7. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 8. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. [DOI] [PubMed] [Google Scholar]
- 9. Haroutunian V, Hoffman LB, Beeri MS. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialogues Clin Neurosci. 2009;11(2):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. [DOI] [PubMed] [Google Scholar]
- 11. Di Iulio F, Palmer K, Blundo C, et al. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer’s disease and mild cognitive impairment subtypes. Int Psychogeriatr. 2010;22(4):629–640. [DOI] [PubMed] [Google Scholar]
- 12. Loewenstein DA, Acevedo A, Agron J, et al. Cognitive profiles in Alzheimer’s disease and in mild cognitive impairment of different etiologies. Dement Geriatr Cogn Disord. 2006;21(5-6):309–315. [DOI] [PubMed] [Google Scholar]
- 13. Arana E, Martínez-Granados B, Marti-Bonmati L, et al. Dementias: diagnostic contribution of imaging and proton magnetic resonance spectroscopy. Neurologia. 2007;22(5):267–274. [PubMed] [Google Scholar]
- 14. Martínez-Bisbal MC, Arana E, Martí-Bonmatí L, Martínez-Granados B, Celda B. Cognitive impairment: classification by proton magnetic resonance spectroscopy and the contributions of conventional magnetic resonance imaging. Radiologia. 2006;48(5):301–307. [DOI] [PubMed] [Google Scholar]
- 15. Gao F, Barker PB. Various MRS application tools for Alzheimer disease and mild cognitive impairment. AJNR Am J Neuroradiol. 2014;35(6 suppl):s4–s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang T, Xiao S, Li X, et al. Using proton magnetic resonance spectroscopy to identify mild cognitive impairment. Int Psychogeriatr. 2012;24(1):19–27. [DOI] [PubMed] [Google Scholar]
- 17. Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28(9):1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu YY, Yang ZX, Shen ZW, et al. Magnetic resonance spectroscopy study of amnestic mild cognitive impairment and vascular cognitive impairment with no dementia. Am J Alzheimers Dis Other Demen. 2013;29(5):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Zandvoort MJ, van der Grond J, Kappelle LJ, de Haan EH. Cognitive deficits and changes in neurometabolites after a lacunar infarct. J Neurol. 2005;252(2):183–190. [DOI] [PubMed] [Google Scholar]
- 20. Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384(1-2):23–28. [DOI] [PubMed] [Google Scholar]
- 21. García Santos JM, Gavrila D, Antúnez C, et al. Magnetic resonance spectroscopy performance for detection of dementia, Alzheimer’s disease and mild cognitive impairment in a community-based survey. Dement Geriatr Cogn Disord. 2008;26(1):15–25. [DOI] [PubMed] [Google Scholar]
- 22. Jessen F, Gür O, Block W, et al. A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI. Neurology. 2009;72(20):1735–1740. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe T, Shiino A, Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is useful to differentiate amnesic mild cognitive impairment from Alzheimer’s disease and healthy aging. Dement Geriatr Cogn Disord. 2010;30(1):71–77. [DOI] [PubMed] [Google Scholar]
- 24. Waldman AD, Rai GS. The relationship between cognitive impairment and in vivo metabolite ratios in patients with clinical Alzheimer’s disease and vascular dementia: a proton magnetic resonance spectroscopy study. Neuroradiology. 2003;45(8):507–512. [DOI] [PubMed] [Google Scholar]
- 25. MacKay S, Ezekiel F, Di Sclafani V, et al. Alzheimer disease and subcortical ischemic vascular dementia: evaluation by combining MR imaging segmentation and H-1 MR spectroscopic imaging. Radiology. 1996;198(2):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu X, Cao L, Hu X, et al. Brain metabolism assessed via proton magnetic resonance spectroscopy in patients with amnestic or vascular mild cognitive impairment. Clin Neurol Neurosurg. 2015;130:80–85. [DOI] [PubMed] [Google Scholar]
- 27. Kattapong VJ, Brooks WM, Wesley MH, Kodituwakku PW, Rosenberg GA. Proton magnetic resonance spectroscopy of vascular- and Alzheimer-type dementia. Arch Neurol. 1996;53(7):678–680. [DOI] [PubMed] [Google Scholar]
- 28. Franczak M, Prost RW, Antuono PG, Mark LP, Jones JL, Ulmer JL. Proton magnetic resonance spectroscopy of the hippocampus in patients with mild cognitive impairment: a pilot study. J Comput Assist Tomogr. 2007;31(5):666–670. [DOI] [PubMed] [Google Scholar]
- 29. Catani M, Cherubini A, Howard R, et al. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12(11):2315–2317. [DOI] [PubMed] [Google Scholar]
- 30. Walecki J, Barcikowska M, Ćwikła JB, Gabryelewice T. N-acetylaspartate, choline, myoinositol, glutamine and glutamate (glx) concentration changes in proton MR spectroscopy (1H MRS) in patients with mild cognitive impairment (MCI). Med Sci Monit. 2011;17(12):mt105–mt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kantarci K, Petersen RC, Przybelski SA, et al. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65(12):1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waldman AD, Rai GS, McConnell JR, Chaudry M, Grant D. Clinical brain proton magnetic resonance spectroscopy for management of Alzheimer’s and sub-cortical ischemic vascular dementia in older people. Arch Gerontol Geriatr. 2002;35(2):137–142. [DOI] [PubMed] [Google Scholar]
- 33. Sailasuta N, Harris K, Tran T, Ross B. Minimally invasive biomarker confirms glial activation present in Alzheimer’s disease: a preliminary study. Neuropsychiatr Dis Treat. 2011;7:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alvarez EO, Ruarte MB. Glutamic acid and histamine-sensitive neurons in the ventral hippocampus and the basolateral amygdala of the rat: functional interaction on memory and learning processes. Behav Brain Res. 2004;152(2):209–219. [DOI] [PubMed] [Google Scholar]
- 35. Shen J, Rothman DL. Magnetic resonance spectroscopic approaches to studying neuronal: glial interactions. Biol Psychiatry. 2002;52(7):694–700. [DOI] [PubMed] [Google Scholar]
- 36. Rupsingh R, Borne M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32(5):802–810. [DOI] [PubMed] [Google Scholar]
- 37. Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K. Brain glutamate levels are decreased in Alzheimer’s disease: a magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Demen. 2011;26(6):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hattori N, Abe K, Sakoda S, Sawada T. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s disease. Neuroreport. 2002;13(1):183–186. [DOI] [PubMed] [Google Scholar]
- 39. Garrard P, Perry R, Hodges JR. Disorders of semantic memory. J Neurol Neurosurg Psychiatry. 1997;62(5):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]