Abstract
Objective
: Cognitive deficit and white matter alteration relationships in Parkinson’s disease (PD) were investigated.
Methods
: Comparison of 64 patients with PD (M:F, 34:30; 64.4 ± 10.4 years) classified as cognitively normal (PD-CogNL, n = 24), mild cognitive impairment (PD-MCI, n = 30), and dementia (PD-D, n = 10) with 21 healthy participants (M:F, 10:11; 60.1 ± 13.6 years) was conducted using white matter fractional anisotropy (FA), region-of-interest analysis, and diffusion tensor imaging.
Results
: The PD-D and PD-MCI exhibited higher Unified Parkinson’s Disease Rating Scale motor scores (P < .001; P < .01) and Hoehn-Yahr stages (P < .001; P < .05) and FA reductions in left frontal/right temporal white matter and bilateral anterior cingulated bundles. Largest FA reductions occurred in PD-D left anterior cingulated bundle and corpus callosum splenium. Disease durations of PD-D = 6.8 ± 6.86, PD-MCI = 5.1 ± 2.9, and PD-CogNL = 4.7 ± 3.4 years, suggesting progressive deterioration.
Conclusions
: Cerebral white matter deterioration may underlie progressive cognitive impairment in PD.
Keywords: Parkinson’s disease, cognitive impairment, white matter, diffusion tensor imaging, fractional anisotropy
Introduction
Cognitive dysfunction is a common occurrence in patients with Parkinson’s disease (PD), demonstrating variant intensities ranging from mild cognitive impairment (MCI) to dementia. The MCI has been estimated to occur in 20% to 30% of all patients with PD 1 , and 30% to 40% of patients with PD eventually develop dementia. 2 Notably, MCI occurs in both current and newly diagnosed patients with PD, 3 where it has been shown to be a predictor of cognitive decline that can potentially lead to dementia.1,3,4 In addition to the disability associated with PD-associated dementia (PD-D), the symptoms of dementia double mortality risk and increase caregiver burden. 5 The MCI, and subsequent worsening of cognitive functions, significantly contributes to the morbidity and mortality associated with PD.6,7 Because MCI is a key risk factor for PD-D,8,9 early diagnosis of PD with MCI (PD-MCI) offers an opportunity for therapeutic intervention to prevent further cognitive decline. Therefore, early MCI diagnosis is important to ensure that optimal therapeutic interventions are initiated to slow the progression of cognitive dysfunction, thus delaying or preventing the PD-D onset.
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique utilized in the indirect evaluation of white matter tract integrity. The DTI functions through the use of a pulsed field gradient, which induces a measurable rate of 3-dimensional (3D) water molecule diffusion (Brownian motion). 10 When white matter pathology disrupts axon orientations, fractional anisotropy (FA) of the tissue decreases. Thus, DTI is able to detect the microstructural changes in white matter prior to overt volume loss .11,12 The technique has been increasingly used to measure white matter tissue microstructure in neurological diseases, such as multiple system atrophy, 13 diffuse Lewy body disease, 14 multiple sclerosis, 15 and amyotrophic lateral sclerosis.16,17 However, few studies have applied DTI for the characterization of altered white matter diffusion in patients with PD.
Decreased FA and increased mean diffusivity in white matter of the frontal lobe 18 and superior cerebellar peduncle 19 of patients with PD have been described in a few isolated reports. These findings imply that microstructural degradation is occurring in these areas in a progressive manner, potentially leading to worsening cognitive dysfunction over time in untreated patients. In the early stages of PD, patients most commonly exhibit relatively mild cognitive deficits, manifesting as MCI characterized predominantly by executive dysfunction and visuospatial deficits, impairments likely associated with subcortical cognitive deficits. 20 Similarly, a recent study reported significant FA reductions in the bilateral frontal regions of patients with PD-D compared to normally functioning patients. 18 Although some evidence has been provided in these studies which suggests an association between white matter abnormalities and cognitive impairment in PD, this relationship requires further exploration.
Microstructural abnormalities in white matter of patient with PD were compared to variant cognitive statuses using region-of-interest (ROI) analysis and DTI in order to investigate the relationship between cognitive deficits and white matter alteration in these patients. Furthermore, additional information useful in the elucidation of the pathologic process of cognitive impairment in PD was explored.
Materials and Methods
Patients
A total of 64 idiopathic patients with PD (34 male and 30 female, mean age 64.4 ± 10.4 years) admitted to the Liuhuaqiao Hospital (Guangzhou, China) between June 2010 and October 2011 were included in the current study. Basic demographic and clinical information, including the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Hoehn and Yahr stage, were obtained from all included patients.
Patients who exhibited (1) previous or current treatment with deep brain stimulation, (2) MRI evidence of atypical parkinsonism and other disorders of the brain or central nervous system, and (3) presence of other chronic diseases such as uncontrolled hypertension or chronic kidney disease were excluded. Patients who scored >20 on the Hamilton Depression Scale were also excluded due to the known effects of depression on neuropsychological test results. 21
A total of 21 age- and sex-matched healthy participants were also included (control group). All patients of the control group demonstrated negative anamnesis for neurologic and psychiatric disorders. Additionally, no control patients reported a family history of parkinsonism, exhibited MRI abnormalities, or demonstrated any current or previous condition that might impair cognition such as head injury or substance abuse. All control patients scored >28 on the Mini-Mental State Examination (MMSE).
The study was approved by the institutional review board of the Liuhuaqiao Hospital. Written informed consent was obtained from all the patients prior to inclusion in the study.
Study Design
A cross-sectional study was conducted using 3 patient groups constructed based on cognitive status indicated by neuropsychological test battery results (Table 1). Cognitively normal patients with PD (PD-CogNL) reported no functional deficits and were confirmed to be cognitively intact. Specifically, these patients did not meet criteria for either MCI or dementia. Patients with PD-MCI were selected according to the modified criteria of Petersen et al. 27 These patients exhibited subjective cognitive complaints, demonstrated a deficit of at least 1.5 standard deviations (SDs) below normative data mean scores in at least 1 of the 4 cognitive domains assessed, and presented with no functional decline due to cognitive deficits. Patients with PD PD-D were selected using the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition; American Psychiatric Association, 1994) criteria. 28
Table 1.
Neuropsychological Test Battery.
| Test | Cognitive Domain |
|---|---|
| Digits forward and backward (WAIS-RC)22,23 | Attention |
| Block design test (WAIS-RC) 23 | Visuospatial |
| Similarities (from WAIS-RC24,23) and CDT 25 | Executive function/planning skills |
| Verbal Paired Associates Visual Reproduction and Logical Memory (WMS) 26 | Memory (immediate, visual working, and delayed memory) |
Abbreviations: CDT, Clock Drawing Test; WAIS-CR, Chinese revision of the Wechsler Adult Intelligence Scale; WMS, Chinese revision of the Wechsler Memory Scale.
Diagnosis
The diagnosis of possible or probable PD was confirmed by a single experienced movement disorder specialist using previously established criteria. 29
The MRI Acquisition Protocol
All MRI scans were acquired on a single 3.0-T clinical scanner (GE Signa HDxt America, GE Healthcare, Waukesha, Wisconsin) equipped with an 8-channel head coil. Restraining foam pads provided by the manufacturer were used to minimize head motion. Axial high-resolution T1-weighted MR images were acquired using a fast-spin echo sequence with parameters 288 × 224 acquisition, 240 mm field of view, 320 × 256 voxels, 27.3 milliseconds TE, and 1794 milliseconds TR. The DT images were obtained using single-shot echo-planar acquisition from 16 gradient directions with parameters 130 × 128 acquisition matrix with 704 slices, 240 mm field of view, 3.4 × 3.4 × 3 mm3 voxels, 87.9 milliseconds TE, 12 seconds TR, and b factor of 1000 s/mm2, without cardiac gating.
The ROI Analysis
All images were postprocessed on an AW4.4 workstation using FuncTool (GE Healthcare) image analysis software. The FA values were obtained from various white matter regions on the DTI scan using ROI analysis,30,31,24 as shown in Figure 1. The ROI settings and FA value measurements were performed by an experienced neuroradiologist blinded to the patient groups. The ROI FA values were compared among the 3 experimental groups and the control group.
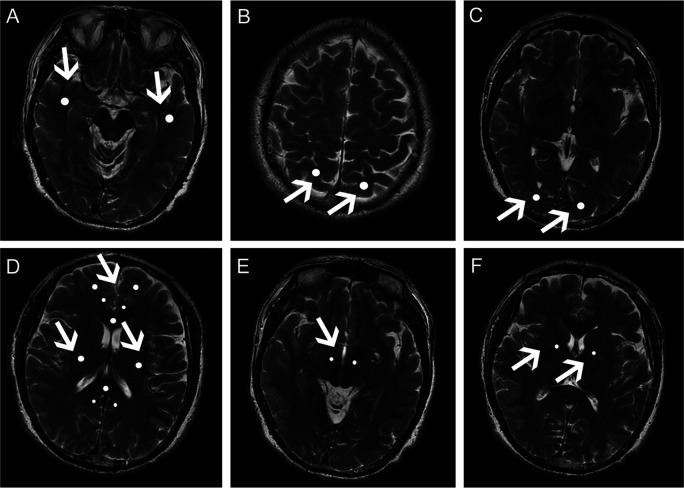
Figure 1.
Regions of interest (ROI) in white matter of patients with Parkinson’s disease. Apparent ROIs (the light regions indicated by white arrows) are shown in the (A) temporal white matter; (B) parietal white matter; (C) occipital white matter; (D) frontal white matter and anterior/posterior cingulate bundles, genu and splenium of the corpus callosum, and superior longitudinal fasciculus; (E) corticospinal tract in midbrain; (F) corticospinal tract in internal capsule of patients with Parkinson’s disease.
Temporal white matter ROIs (50 mm2) were sampled posterolateral to the lateral fissure on most caudal slice, the site where the lateral fissure was apparent. Frontal white matter (50 mm2), anterior/posterior cingulate bundle (20 mm2), corpus callosum genu and splenium (50 mm2), and superior longitudinal fasciculus (50 mm2) ROIs were sampled on the slice that included a full-volume lateral ventricle. The ROIs in parietal white matter (50 mm2) were selected from the region posterior to the central sulcus on the most caudal slice, where it was visible. Occipital white matter (50 mm2) was within the optic radiations on the most caudal slice, where the occipital horn of the lateral ventricle was visible. Two additional ROIs (20 mm2 each) were selected from the corticospinal tract at the levels where the red nucleus and internal capsule were apparent.
Statistical Analysis
All data were statistically analyzed using SPSS version 13.0 (SPSS Inc, Chicago, Illinois) software. Statistical analysis of demographic and clinical data was performed using analysis of variance (ANOVA) with a post hoc Tukey honest significant difference test for continuous variables. Kruskal-Wallis tests with post hoc Mann-Whitney U tests were applied to analyze noncontinuous variables, and chi-square tests were employed for categorical data.
In the primary analysis, FA values were compared among the 4 groups using 1-way ANOVA. Fisher least significant difference t test was used for post hoc analysis. Logistic regression analysis was used to adjust the influence of significantly different factors in the primary analysis. Spearman correlation test was used to examine associations between FA values and disease status. Two-sided values of P <.05 were considered statistically significant.
Results
No significant differences in demographic and clinical characteristics, including age, gender, disease duration, or education years, were observed among the 3 patient groups with PD (Table 2). However, disease duration increased with increasing severity of cognitive dysfunction with durations of 4.7 ± 3.4 years in the PD-CogNL group, 5.1 ± 2.9 years in the PD-MCI group, and 6.8±6.86 years in the PD-D group (PD-CogNL < PD-MCI < PD-D). Both the UPDRS motor score and Hoehn-Yahr stage were significantly higher in the PD-D group (UPDRS: P < .001; Hoehn-Yahr: P < .001) and PD-MCI group (UPDRS: P < .01; Hoehn-Yahr: P < .05) than those observed in patients with no cognitive dysfunction (PD-CogNL group), as shown in Table 2.
Table 2.
Demographic and Clinical Data of Patients With PD With Different Cognitive Status and Controls.a
| Control (n = 21) | PD-CogNL (n = 24) | PD-MCI (n = 30) | PD-D (n = 10) | P Value | |
|---|---|---|---|---|---|
| Male, no of patients | 10 | 14 | 15 | 5 | NS |
| Mean ± SD | |||||
| Age, years | 60.1 ± 13.6 | 62.1 ± 8.6 | 65.1 ± 11.8 | 69.0 ± 9.7 | NS |
| Education, years | 8.57 ± 4.68 | 10.39 ± 4.9 | 8.0 ± 3.0 | 7.6 ± 4.3 | NS |
| Disease duration, years | − | 4.7 ± 3.4 | 5.1 ± 2.9 | 6.8 ± 6.86 | NS |
| UPDRS motor score | − | 30.2 ± 12.2 | 42.4 ± 14.0b | 56.0 ± 15.3b,c | <.001 |
| Hoehn-Yahr stage | − | 2.2 ± 0.8 | 2.8 ± 0.7b | 3.7 ± 0.9b,c | <.001 |
| Madopar equivalent dose, mg | − | 299.5 ± 122.5 | 477.1 ± 82.9b,c | 581.3 ± 137.6b,c | <.001 |
Abbreviations: PD-CogNL, Parkinson’s disease with normal cognitive function; PD-MCI, Parkinson’s disease with mild cognitive impairment; PD-D, Parkinson’s disease with dementia; UPDRS, Unified Parkinson’s Disease Rating Scale; NS, nonsignificant; SD, standard deviation.
a Statistical comparisons were made using 1-way ANOVA, Fisher least significant difference t test was used for post hoc analysis. P > .05 was considered nonsignificant.
b P < .05, compared to PD-CogNL.
c P < .05, compared to PD-MCI.
Compared to patients of the control group, patients with PD-D and PD-MCI showed significant FA reduction in the left frontal and right temporal white matter as well as in the bilateral anterior cingulate bundles. Patients with PD-CogNL showed significant FA reductions in left occipital white matter and the left anterior cingulate bundle compared to patients of the control group. Median FA values for each of the white matter regions in all the 3 experimental groups and the control group are shown in detail in Table 3.
Table 3.
The FA Values Comparison Among Patients With PD With Different Cognitive Status (Mean ± SD).a
| Control(n = 21) | PD-CogNL (n = 24) | PD-MCI (n = 30) | PD-D (n = 10) | ANOVA, P Value | Logistic Regression Analysis | ||
|---|---|---|---|---|---|---|---|
| PD-MCI/PD-CogNL | PDD/PD-CogNL | ||||||
| Frontal white matter | |||||||
| Left | 0.519 ± 0.096 | 0.485 ± 0.040 | 0.463 ± 0.074b | 0.446 ± 0.047c | .017 | NA | NA |
| Right | 0.486 ± 0.076 | 0.468 ± 0.115 | 0.465 ± 0.069 | 0.466 ± 0.478 | NS | NA | NA |
| Parietal white matter | |||||||
| Left | 0.467 ± 0.095 | 0.449 ± 0.094 | 0.454 ± 0.068 | 0.442 ± 0.036 | NS | NA | NA |
| Right | 0.463 ± 0.060 | 0.433 ± 0.039 | 0.450 ± 0.051 | 0.424 ± 0.066 | NS | NA | NA |
| Temporal white matter | |||||||
| Left | 0.503 ± 0.061 | 0.474 ± 0.039 | 0.467 ± 0.059 | 0.476 ± 0.049 | NS | NA | NA |
| Right | 0.507 ± 0.059 | 0.483 ± 0.050 | 0.451 ± 0.052 b,d | 0.457 ± 0.052c | .003 | NS | NA |
| Occipital white matter | |||||||
| Left | 0.433 ± 0.560 | 0.486 ± 0.087e | 0.459 ± 0.074 | 0.414 ± 0.081f | .034 | NA | NS |
| Right | 0.482 ± 0.043 | 0.469 ± 0.083 | 0.448 ± 0.065 | 0.449 ± 0.066 | NS | NA | NA |
| Anterior cingulate bundle | |||||||
| Left | 0.532 ± 0.096 | 0.470 ± 0.071e | 0.454 ± 0.079d | 0.384 ± 0.071 c,f,g | .000 | NA | 0.049 |
| Right | 0.438 ± 0.713 | 0.425 ± 0.066 | 0.398 ± 0.064a | 0.378 ± 0.046b | .039 | NA | NA |
| Posterior cingulate bundle | |||||||
| Left | 0.440 ± 0.046 | 0.456 ± 0.063 | 0.427 ± 0.048 | 0.437 ± 0.056 | NS | NA | NA |
| Right | 0.489 ± 0.070 | 0.469 ± 0.104 | 0.462 ± 0.075 | 0.409 ± 0.069 | NS | NA | NA |
| Splenium of the corpus callosum | 0.798 ± 0.051 | 0.760 ± 0.069 | 0.765 ± 0.075 | 0.687 ± 0.121c,f,g | .004 | NA | NS |
| Genu of the corpus callosum | 0.716 ± 0.063 | 0.727 ± 0.086 | 0.667 ± 0.098 | 0.696 ± 0.066 | NS | NA | NA |
| Corticospinal tract in midbrain | |||||||
| Left | 0.715 ± 0.069 | 0.714 ± 0.043 | 0.697 ± 0.041 | 0.709 ± 0.064 | NS | NA | NA |
| Right | 0.706 ± 0.071 | 0.712 ± 0.049 | 0.729 ± 0.064 | 0.722 ± 0.062 | NS | NA | NA |
| Corticospinal tract in internal capsule | |||||||
| Left | 0.631 ± 0.044 | 0.930 ± 0.047 | 0.632 ± 0.061 | 0.637 ± 0.054 | NS | NA | NA |
| Right | 0.627 ± 0.066 | 0.940 ± 0.015 | 0.629 ± 0.064 | 0.656 ± 0.056 | NS | NA | NA |
| Superior longitudinal fasciculus | |||||||
| Left | 0.620 ± 0.065 | 0.629 ± 0.059 | 0.625 ± 0.070 | 0.643 ± 0.041 | NS | NA | NA |
| Right | 0.644 ± 0.045 | 0.637 ± 0.066 | 0.622 ± 0.074 | 0.649 ± 0.050 | NS | NA | NA |
Abbreviations: NA, not applicable; NS, nonsignificant; FA, fractional anisotropy; PD-CogNL, Parkinson’s disease with normal cognitive function; PD-MCI, Parkinson’s disease with mild cognitive impairment; PD-D, Parkinson’s disease with dementia; ANOVA, analysis of variance; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
a Post hoc logistic regression analysis was undertaken to account for baseline differences in UPDRS/Hoehn-Yahr scores. P >.05 was considered nonsignificant.
b Significant FA reductions in PD-MCI compared to controls.
c Significant FA reductions in PD-D compared to controls.
d significant FA reductions in PD-MCI compared to PD-CogNL.
e Significant fractional anisotropy reductions in PD-CogNL compared to controls.
f Significant FA reductions in PD-D compared to PD-CogNL.
g significant FA reductions in PD-D compared to PD-MCI.
In general, patients with PD-D showed significant FA reductions in the left anterior cingulate bundle and corpus callosum splenium compared to the other 3 groups. The FA reduction in the left anterior cingulate bundle remained significant even after performing logistic regression analysis to adjust for the influence of UPDRS motor score and Hoehn-Yahr stage. The FA values in the left occipital white matter of patients with PD-D were significantly lower than in patients with PD-CogNL. However, no significant FA difference was demonstrated between other areas among the 4 groups.
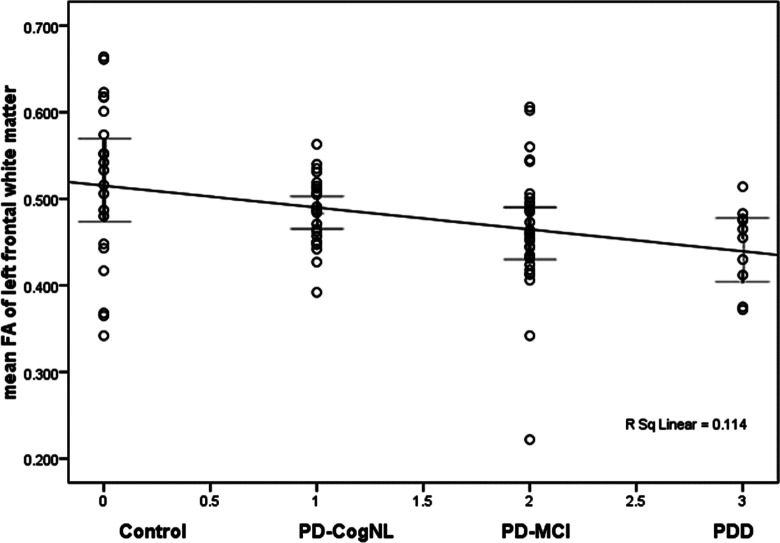
Spearman correlation analyses of FA values and cognitive status of patient with PD revealed significant negative correlations between PD cognitive status and FA values of some white matter areas, including the left frontal (−0.334; P = .002), right temporal (−0.378; P = .002), left occipital white matter (−0.220; P = .043), left anterior cingulate bundles (−0.413; P = .000), right anterior cingulate bundles (−.299; P = .005), and corpus callosum splenium (−0.266; P = .014). Table 4 shows the correlation analysis of the 2 variables; the FA values of the different parts and the cognitive status indicators grouped according to cognitive function status with the assigned variables 1 (PD-cogNL), 2 (PD-MCI), and 3 (PD-D). Figures 2 and 3 demonstrate that the data for each ROI group can be represented as a plot with few outliers, indicating the degree of separation between the groups. Figure 4 provides the complete FA values data for patients with PD with different cognitive status according to ROI, demonstrating the degree of separation between all the groups.
Table 4.
Spearman Correlation Analysis of FA Value and Cognitive Status of Patient with PD.
| White Matter | Correlation Coefficient | P Value |
|---|---|---|
| Left frontal | −0.334 | .002 |
| Right temporal | −0.378 | .002 |
| Left occipital | −0.220 | .043 |
| Left anterior cingulate bundle | −0.413 | .000 |
| Right anterior cingulate bundle | −0.299 | .005 |
| Splenium of the corpus callosum | −0.266 | .014 |
Abbreviations: FA, fractional anisotropy; PD, Parkinson’s disease.
Figure 2.
Fractional anisotropy (FA) values of left frontal white matter among patients with Parkinson’s disease (PD) with different cognitive statuses.Notably few outliers were observed in the regions-of-interest (ROI) regions.
Figure 3.
Fractional anisotropy (FA) values of right frontal white matter among patients with Parkinson’s disease (PD) with different cognitive statuses. Notably few outliers were observed in the regions-of-interest (ROI) regions.
Figure 4.
Fractional anisotropy (FA) values among patients with Parkinson’s disease (PD) with different cognitive statuses.The degree of separation in each regions-of-interest (ROI) group is clearly shown in the line graphs corresponding to each ROI. Notably, few outliers were observed in each region.
Discussion
The white matter of patients with PD was shown to undergo progressive deterioration linked to cognitive dysfunction of variant severity. The current study further demonstrated that MIC may progress into more severe cognitive dysfunction with increasing disease duration, potentially resulting in PD-D. Because PD-D represents a significant reduction in patient quality of life and places additional strain on care providers, these findings indicate that early intervention for patients with PD-MIC may be employed to beneficially prevent or delay PD-D onset in future.
White matter comprises many interconnecting tracts between variant regions of the brain that play critical roles in higher functions. These functions may be disrupted by pathologies involving white matter abnormalities, such as PD-D and dementia associated with Alzheimer’s disease (AD).32,33 Unlike AD, however, no neuropathological studies have been reported to date that investigate the relationship between white matter deterioration and cognitive status in patients with PD. Notably, the impaired cognitive domains of PD-D have been shown to be distinct from those observed in patients with AD, who predominantly exhibit memory and language impairments. Conversely, patients with PD often exhibit visuospatial, attention, memory, and executive function impairments that occur throughout the disease course, even in early stages.34–37 Though some symptoms are similar, these findings suggest that subcortical cognitive impairment is dominant in PD-D, unlike AD.
A few previous publications have, however, examined the neuropathological relationship between white matter deterioration and cognitive status in patients with PD using MRI methodologies. For example, patients with PD-D were found to have significantly higher levels of white matter abnormalities than patients with PD without dementia using MRI data.38–40 Notably, structural MRI results are usually normal in patients with PD, even in those with a long disease course. Although some researchers have attempted the use of single-photon emission computed tomography and positron-emission tomography due to their high level of diagnostic sensitivity and specificity, the associated ionization has limited their use in this area of study. 41 Overall, radiographic findings cumulatively suggest that white matter alterations may contribute to PD-D, though the precise effect of white matter alteration on cognitive dysfunction will require further study using a combination of techniques.42,43
Both DTI and derived FA measurements have been successfully applied to quantitatively measure white matter microstructural alterations by analyzing in vivo water diffusion. 44 The FA values are predominantly determined by the axonal membrane and cytoskeletal components, such as neurofilaments and microtubules, with lower values indicative of white matter abnormality. 45 In patients with PD, DTI studies have reported reduced FA in the frontal lobes, premotor areas, and the cingulum46,47 as well as the corpus callosum and the superior longitudinal fasciculus. 20 Furthermore, ROIs along the line between the substantia nigra and the lower part of the putamen/caudate complex have also been shown to exhibit low FA values. 46 These findings are suggestive of widespread microstructural damage to white matter in the early stages of PD.
To date, few studies have applied DTI to investigate the changes in patients with PD.21,27,48,49 Of these, only the study of Hattori et al 49 specifically explored the relationship between white matter alteration and cognitive status in PD, including PD-MCI and PD-D. Unfortunately, this study did not assess the detailed neuropsychological status of patients, such as the executive, amnestic, visuospatial, attention, and language deficits. It also did not define patients with PD-MCI based on the widely accepted criterion of 1.5 SDs below the mean normal score. 27 Conversely, the current study defined PD-MCI based on a neuropsychological test battery, with DTI findings based on specific ROIs. This simple and practical technique can be implemented in any clinical radiological setting. This is well suited to investigate the relationship between white matter abnormalities and cognitive status in patients with PD-MCI and PD-D. Furthermore, data reported by Hattori et al were acquired by MRI scanning using a 1.5-T clinical scanner, which provides less accuracy than the more contemporary 3.0-T clinical scanner employed in the current study.
Cognitive impairments associated with frontal lobe dysfunction commonly occur throughout the course of PD, and may even occur in the earliest PD stages in rare cases.15,50 Deterioration of the white matter in the frontal cortex can impair attention set shifting.51,52 This frontostriatal circuit deficits have been recently considered the basis for cognitive impairments in PD. 53 As PD progresses, further dysfunctions associate with the dorsolateral prefrontal cortex may occur, impairing the functions of set shifting, working memory, response generation, set maintenance, memory retrieval, and conditioned associate learning. Similarly, deterioration of the orbitofrontal cortex may result in impairments in perseverance, impulse control, stimulus-driven behavior, decision making, and disinhibition, often resulting in clinical depression. 54
A previous DTI study 27 evaluated various cognitive functions and compared FA values in patients with PD with and without executive dysfunction. The results showed that conventional tasks associated with the frontal lobe were affected by impairments of both the frontal and parietal lobes. Another study 13 reported a significant decrease in FA values in the bilateral frontal areas of patients with PD-D. In the current study, reduced FA values were observed in the left frontal white matter of patients with PD-D and PD-MCI compared to healthy control patients, consistent with previous findings.13,27 It is important, however, to note that this difference was not statistically significant following correction for UPDRS and Hoehn-Yahr scores.
The anterior cingulate cortex has been reported to play a role in response initiation, conflict monitoring, motivation, and apathy. 54 Kamagata et al 55 reported metabolic abnormalities in the posterior cingulate regions of patients with PD, and Karagulle et al 47 reported FA alterations in the rostral and anterior cingulate regions in patients with PD. Similarly, the current study demonstrated that FA values in the bilateral anterior cingulate bundles were significantly reduced in patients with PD-D and PD-MCI, even after accounting for UPDRS motor score and Hoehn-Yahr stage. Thus, the posterior cingulate bundles may play an important role in PD-D development and severity. These findings are supported by the report by Kamagata et al 55 , which showed a significant correlation between tensor tractography FA values and MMSE scores in patients with PD-D.
In addition to white matter alterations in the frontal lobe and bilateral anterior cingulate bundles, the current study produced evidence of significant alterations in the right temporal white matter in both patients with PD-D and patients with PD-MCI. Notably, white matter hyperintensities in cholinergic pathways have been shown to be significantly higher in PD-D compared to PD-MCI. These altered MRI findings correlated with the degree of decline in frontal executive function and attention observed in these patients. 56 These findings are further supported by the report by Weintraub et al 57 , who suggested that hippocampal atrophy may be a useful biomarker of initial cognitive decline in PD. 57
The current findings largely confirm and extend those of previous studies, demonstrating that decreased FA values in white matter occur in PD as cognitive function worsens.13,27,46,47,58 Consistent with the results of previous DTI studies, the FA values in the corticospinal tract were not significantly altered in any patient group compared to controls. Groups that employed transcranial magnetic stimulation59,60 also reported a relatively unaffected corticospinal tract. 61 In the current study, FA values in some white matter regions were negatively correlated with cognitive status in patients with PD. These findings further support the hypothesis that worsening cognitive status correlates with decreasing FA values, indicative of altered axonal structure with or without synaptic dysfunction and impaired axonal transport. Therefore, the current findings support the recent hypothesis that white matter abnormalities may play an important role in PD-associated cognitive deficits, likely due to cortical–subcortical disconnection.
The PD-D is found only rarely in the general population, making large cohort sizes difficult to achieve. Because this relatively small cross-sectional study included only 10 patients with PD-D, compared with 20 to 30 patients in the other groups, a possible statistical impact due to unequal sample sizes exists. This potential error, however, was adequately accounted for by the ANOVA method. It is important to note that the selected ROIs were measured only once, making it impossible to discuss interrater reliability; however, rater bias was minimized by blinding the assessing neuroradiologist to the study groups. Still, prospective studies to measure FA values at fixed time points over longer, multiyear periods will be required to further elucidate the relationship between white matter abnormalities and cognitive status in patients with PD. Studies of longer duration will be particularly necessary in determining the mechanisms of cognitive dysfunction progression in these patients.
The current results demonstrate that PD is associated with significant microstructural alterations to numerous regions of the cerebral white matter. Notably, these alterations showed a positive correlation with cognitive dysfunction in these patients. Therefore, white matter damage may be a significant factor in the underlying mechanism of progressive cognitive impairment throughout the course of PD. Because the full pathologic processes responsible for cognitive dysfunction during PD are varied and multifaceted, further studies will be required to fully determine these mechanisms.
Acknowledgment
We thank all of the participants, the Neurology department staff of Guangzhou Military Region General Hospital, and Li Zhen-sheng and Wang Li-min for help with data collection.
Footnotes
Authors’ Note: The authors Bingmei Deng and Yuhu Zhang contributed equally to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Natural Science Foundation of China (No 30870863, No 30801219), the Natural Science Foundation of Guangdong Province (No 10151008004000030), the Science and Technology Planning Project of Guangdong Province (No 2009B030801251, No 2011B080701087), and the Medical Scientific Research Foundation of Guangdong Province, China (No A2011442, No A2009038).
References
- 1.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(9):1272–1277. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001;56(3):730–736. [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(pt 7):1787–1798. [DOI] [PubMed] [Google Scholar]
- 4.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. [DOI] [PubMed] [Google Scholar]
- 5.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59(11):1708–1713. [DOI] [PubMed] [Google Scholar]
- 6.Burn DJ, McKeith IG. Current treatment of dementia with Lewy bodies and dementia associated with Parkinson's disease. Mov Disord. 2003;(18 suppl 6):S72–S79. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson's disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118–123. [DOI] [PubMed] [Google Scholar]
- 8.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–2324. [DOI] [PubMed] [Google Scholar]
- 9.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. [DOI] [PubMed] [Google Scholar]
- 10.Minati L, We̢glarz WP. Physical foundations, models, and methods of diffusion magnetic resonance imaging of the brain: a review. Concept Magnetic Res A. 2007;30(5):278–307. [Google Scholar]
- 11.Abe O, Aoki S, Hayashi N, et al. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging. 2002;23(3):433–441. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30(6):749–761. [DOI] [PubMed] [Google Scholar]
- 13.Schocke MF, Seppi K, Esterhammer R, et al. Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson's disease. Neuroimage. 2004;21(4):1443–1451. [DOI] [PubMed] [Google Scholar]
- 14.Bozzali M, Falini A, Cercignani M, et al. Brain tissue damage in dementia with Lewy bodies: an in vivo diffusion tensor MRI study. Brain. 2005;128(pt 7):1595–1604. [DOI] [PubMed] [Google Scholar]
- 15.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol. 1999;20(8):1491–1499. [PMC free article] [PubMed] [Google Scholar]
- 16.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132(pt 1):239–249. [DOI] [PubMed] [Google Scholar]
- 17.Sage CA, Van Hecke W, Peeters R, et al. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: revisited. Hum Brain Mapp. 2009;30(11):3657–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui H, Nishinaka K, Oda M, et al. Depression in Parkinson's disease. Diffusion tensor imaging study. J Neurol. 2007;254(9):1170–1173. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. [DOI] [PubMed] [Google Scholar]
- 20.Farina E, Gattellaro G, Pomati S, et al. Researching a differential impairment of frontal functions and explicit memory in early Parkinson's disease. Eur J Neurol. 2000;7(3):259–267. [DOI] [PubMed] [Google Scholar]
- 21.Uekermann J, Daum I, Peters S, Wiebel B, Przuntek H, Müller T. Depressed mood and executive dysfunction in early Parkinson's disease. Acta Neurol Scand. 2003;107(5):341–348. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler DW. Adult Intelligence Scale. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- 23.YX G. Chinese version of the Wechsler Adult Intelligence Scale (WAIS-RC). Hunan Maping Press; 1992. [Google Scholar]
- 27.Matsui H, Nishinaka K, Oda M, et al. Wisconsin Card Sorting Test in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116(2):108–112. [DOI] [PubMed] [Google Scholar]
- 25.Borod JC, Goodglass H, Edith K. Normative AD on the Boston diagnostic aphasia examination, parietal lobe battery and the Boston naming test. J Clin Neuropsychol. 1980;2(3):209–216. [Google Scholar]
- 26.YX G. Chinese revision of the Wechsler Memory Scale. Hunan Maping Press; 1992. [Google Scholar]
- 27.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 28.Apa D. Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. [DOI] [PubMed] [Google Scholar]
- 30.Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30(6):1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui H, Nishinaka K, Oda M, Niikawa H, Kubori T, Udaka F. Dementia in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116(3):177–181. [DOI] [PubMed] [Google Scholar]
- 32.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60(6):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczynski B, Reed B, Mungas D, Weiner M, Chui HC, Jagust W. Cognitive and anatomic contributions of metabolic decline in Alzheimer disease and cerebrovascular disease. Arch Neurol. 2008;65(5):650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson's disease. Adv Neurol. 1995;65:85–95. [PubMed] [Google Scholar]
- 35.Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76(3):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen AM, Sahakian BJ, Hodges JR, et al. Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology. 1995;9(1):126–140. [Google Scholar]
- 37.Emre M. Dementia associated with Parkinson's disease. Lancet Neurol. 2003;2(4):229–237. [DOI] [PubMed] [Google Scholar]
- 38.Beyer MK, Aarsland D, Greve OJ, Larsen JP. Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord. 2006;21(2):223–229. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Kim JS, Yoo JY, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord. 2010;24(3):227–233. [DOI] [PubMed] [Google Scholar]
- 40.Choi SA, Evidente VG, Caviness JN, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol. 2010;119(1):147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravina B, Eidelberg D, Ahlskog JE, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64(2):208–215. [DOI] [PubMed] [Google Scholar]
- 42.Marshall GA, Shchelchkov E, Kaufer DI, Ivanco LS, Bohnen NI. White matter hyperintensities and cortical acetylcholinesterase activity in Parkinsonian dementia. Acta Neurol Scand. 2006;113(2):87–91. [DOI] [PubMed] [Google Scholar]
- 43.Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O'Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14(10):842–849. [DOI] [PubMed] [Google Scholar]
- 44.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7-8):435–455. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa K, Nakata Y, Yamada K, Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry. 2004;75(3):481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P. Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol. 2008;29(3):501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JE, Park HJ, Park B, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson's disease dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2010;81(3):320–326. [DOI] [PubMed] [Google Scholar]
- 49.Hattori T, Orimo S, Aoki S, et al. Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp. 2012;33(3):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci U S A. 2002;99(11):7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagahama Y, Okina T, Suzuki N, Nabatame H, Matsuda M. The cerebral correlates of different types of perseveration in the Wisconsin Card Sorting Test. J Neurol Neurosurg Psychiatry. 2005;76(2):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–537. [DOI] [PubMed] [Google Scholar]
- 54.Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16(4):193–210. [DOI] [PubMed] [Google Scholar]
- 55.Kamagata K, Motoi Y, Abe O, et al. White matter alteration of the cingulum in Parkinson disease with and without dementia: Evaluation by diffusion tensor tract-specific analysis. AJNR Am J Neuroradiol. 2012;33(5):890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin J, Choi S, Lee JE, Lee HS, Sohn YH, Lee PH. Subcortical white matter hyperintensities within the cholinergic pathways of Parkinson's disease patients according to cognitive status. J Neurol Neurosurg Psychiatry. 2012;83(3):315–321. [DOI] [PubMed] [Google Scholar]
- 57.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol. 2011;68(12):1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camicioli RM, Korzan JR, Foster SL, et al. Posterior cingulate metabolic changes occur in Parkinson's disease patients without dementia. Neurosci Lett. 2004;354(3):177–180. [DOI] [PubMed] [Google Scholar]
- 59.Eusebio A, Azulay JP, Witjas T, Rico A, Attarian S. Assessment of cortico-spinal tract impairment in multiple system atrophy using transcranial magnetic stimulation. Clin Neurophysiol. 2007;118(4):815–823. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson C, Markenroth Bloch K, Brockstedt S, et al. Tracking the neurodegeneration of parkinsonian disorders—a pilot study. Neuroradiology. 2007;49(2):111–119. [DOI] [PubMed] [Google Scholar]
- 61.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]