Abstract
Capgras delusion is characterized by the misidentification of people and by the delusional belief that the misidentified persons have been replaced by impostors, generally perceived as persecutors. Since little is known regarding the neural correlates of Capgras syndrome, the cerebral metabolic pattern of a patient with probable Alzheimer’s disease (AD) and Capgras syndrome was compared with those of 24-healthy elderly participants and 26 patients with AD without delusional syndrome. Comparing the healthy group with the AD group, the patient with AD had significant hypometabolism in frontal and posterior midline structures. In the light of current neural models of face perception, our patients with Capgras syndrome may be related to impaired recognition of a familiar face, subserved by the posterior cingulate/precuneus cortex, and impaired reflection about personally relevant knowledge related to a face, subserved by the dorsomedial prefrontal cortex.
Keywords: capgras, delusion of misidentification, face recognition, brain glucose metabolism, neuroimaging
Introduction
Capgras syndrome is one of the most fascinating syndromes in neurology. It was initially identified in 1923 by French psychiatrist J. M. J. Capgras and his assistant, J. Reboul-Lachaux, in their work on the clinical case of “Mrs M.” 1 Clinically, Capgras syndrome is characterized by misidentification of one or more people and by the delusional belief that the misidentified person (generally a close relative of the patient) has been replaced by a double, an impostor with a significant resemblance to the original, who is generally perceived as a persecutor. 2 This belief can be recurrent, transient, or sustained. 3
The patient most often remains mentally quite lucid in other aspects of his or her social life. 4 He or she usually uses small or delusional differences in the behavior, clothing, or physical appearance of the relative and the alleged impostor to justify the delusional conviction. 5 –8 In rare cases, Capgras syndrome may involve objects 9,10 or animals 11,12 but always things for which the patient has a strong affective bond. 5
The misidentified target has no existence by itself and is only defined as the absence of the loved person. In fact, it is never seen at the same time as the loved one, as the perception of one excludes the perception of the other. 5 The patient is usually aggressive toward the “impostor.” 5,13 Moreover, Capgras syndrome concerns one or more misidentified persons, 14 –16 and indeed, the number of misidentified persons tends to increase as time goes by, 17 possibly progressing to include all the patient’s acquaintances. Furthermore, the misidentified targets themselves are frequently replaced by other “impostors” in a continuous, daily delusional process, sometimes thousands of times. Capgras delusion must be distinguished from prosopagnosia, an impairment of familiar face recognition frequently associated with right hemispheric damage. 18
Capgras delusion generally occurs as part of a psychiatric disorder, 7,13,19 most often during paranoid schizophrenia, but more than one-third of the cases occur subsequent to intoxications, brain injuries, dementing illnesses, or various organic conditions. 13 It was long considered to be a psychic nonorganic disorder, but the observation of Capgras syndrome further to organic disorders made the initial psychodynamic hypothesis relatively difficult to sustain. 13,20 Thanks to the advances in neuropsychology and neuroimaging over the last 20 years, Capgras syndrome has been recognized as a complex clinical entity, rather than a symptom of some other illness. 5 Thus, for many authors, Capgras delusion became an interesting paradigm for unravelling face recognition mechanisms. In fact, it was at the origin of several face processing models. Unfortunately, given that the delusion is very rare, these models have mostly been generated by discussions of single case reports and have rarely been tested against a control population. 21 Critically, the face recognition impairments in prosopagnosia and Capgras syndrome have been compared. 8,22 Along these lines, an explanatory hypothesis was advanced, expanding Bauer’s and Tranel and Damasio’s work on covert recognition in prosopagnosia. 23,24 According to this hypothesis, Capgras delusion could be the mirror image of prosopagnosia. 21,25,26 The argument is as follows: face recognition depends on 2 pathways. The ventral pathway, from the visual cortex to the temporal lobes via the inferior longitudinal fasciculus, is the main route for overt conscious face recognition. The dorsal pathway, from the visual cortex to the limbic system via the inferior parietal lobule, is involved in registering the emotional significance of a face. In prosopagnosia, the ventral route is disrupted while the dorsal one remains functional, whereas in Capgras delusion, the dorsal route is disconnected but the ventral one remains intact, generating inappropriate or insufficient affective response to a face. In other words, the delusion may consist of misattributing changes in the patient’s internal world to changes in the external world. 15 This model was strengthened by the demonstration, in a study registering electrodermal conductance, of reduced autonomic responses to familiar faces in patients with Capgras syndrome. 21
At the cerebral level, Capgras delusion is more frequently associated with right than left hemispheric lesions, 12,27,28 but the majority of patients with Capgras syndrome show bilateral cerebral dysfunction, 29,30 involving many brain regions, notably the frontal 27,29 and temporal cortex. 29,31 Computed tomography (CT) 32 scans and electroencephalographic studies have demonstrated that Capgras syndrome is associated with global brain atrophy in dementia, 33 in combination with more severe right than left hemisphere involvement. 27 In cases with Alzheimer’s disease (AD), in particular, degeneration of the right frontal lobe and a relative preservation of the left frontal lobe 13 have been described, along with parietal damage. 34 A single photon emission computed tomography (CT) 35 and a positron emission tomography (PET) study 11 have shown multiple nonspecific cortical hypoactivities. To our knowledge, no functional magnetic resonance imaging (MRI) study has yet been conducted on a patient with Capgras syndrome. Finally, observations of transient episodes of Capgras delusion 13,36 suggest that a structural lesion may not always be required to develop the syndrome. 9
Recent advances in neural models of face recognition provide interesting avenues to explain the genesis of delusional misidentification syndromes and Capgras delusion. Recently, a neural multisystems model for familiar face recognition has been developed, 37 expanding Haxby’s model of face recognition. 38 In this model, familiar face recognition depends on the interactions between a core system, which analyzes the visual appearance of a face, and an extended system, which extracts further personal and emotional information related to the face. The core system includes the inferior occipital gyrus, the lateral fusiform gyrus, and the superior temporal sulcus and encodes the face’s perceptual characteristics. 39 The extended system consists of 2 parts, the first of which is responsible for encoding and retrieving person knowledge related to a familiar face. It includes the anterior paracingulate cortex, the posterior temporoparietal junction, the anterior temporal cortex, and the precuneus/posterior cingulate cortex (PCC). The other part of the extended system comprises a set of areas involved in the representation of emotions and in emotional responses to familiar faces, including the amygdala, insula, and striatum reward system. 40 In relation to the involvement of the paracingulate, other authors have emphasized the role of the medial prefrontal cortex (MPFC) in face recognition, especially in the retrieval or representation of personal knowledge of faces independent of their perceptual familiarity. 41 Indeed, parts of the MPFC are involved in self-referential processing 42 –46 and in forming impressions of others. 42,47 In such an integrated system, Capgras delusion might be related to (partially) impaired transfer of the visual appearance of a face to regions involved in the retrieval of the personal knowledge of people and/or emotional information that makes relatives’ faces seem familiar. In addition to impaired recognition of a close relative (misidentification), a patient with Capgras syndrome would not be able to modify his or her judgement by forming corrected impression of the relative based on personally relevant cues (leading to delusive impression of imposture).
In this article, we present the case of “F,” a patient having probable AD who presented transient but recurrent Capgras syndrome. Our aim was to explore the functional cerebral correlates of Capgras delusion by means of fluorodeoxyglucose PET (FDG-PET) and to interpret the patient’s profile of metabolic impairment in the light of the leading model of Capgras syndrome 21,26 and of current models of face recognition. 37,38
Materials and Methods
Case Report
At the time of presentation, “F,” a right-handed woman, was 75 years old. Her medical history was unremarkable. Her main symptom was face misidentification. She was convinced that her husband had been replaced by an impostor or by her father who died. She used small differences in her husband’s clothing or physical appearance to warrant her delusional conviction, arguing for instance that the impostor had taken her husband’s watch or that they had the same mole. She also spent time searching for her vanished husband. The clinical phenomenon occurred transiently, especially when “F” had just woken up or when her husband came back from some errand. Only her husband’s face was misidentified. She also presented some nonspecific memory complaints. In addition, she was quite anosognosic, minimizing her behavioral disturbances, and developed some paranoid beliefs. However, “F” remained able to identify her husband and her father in old photographs, and she recognized the differences between them. Intellectually, she understood that her father was dead, but this fact had no influence on her delusional conviction. The relationship between the patient and her father appeared unremarkable. A diagnostic neuropsychological evaluation highlighted an episodic memory deficit affecting auditory-verbal learning, with a score below the fifth percentile of a control population at a free and cued recall test. Visuospatial skills were well preserved. The “F” displayed poor lexical access, altered by perseverations during semantic fluency tasks. She performed poorly on some executive tasks (Stroop test, part B of the trail making test and Luria’s graphic series). She scored 27 of 30 on the “Mini-Mental State Examination” and 128 of 144 on Mattis dementia rating scale. 48 Impairment in some daily activities was reported by the husband. Except for her husband, “F”’s ability to recognize faces was not altered. This was observed, for example, for famous faces. These nonspecific findings seem to be common in misidentification syndromes. 49 The neurological examination was normal. Her visual fields were full to confrontation, and she had no visual deficit. A CT scan of the brain did not show any obvious structural brain abnormality. A clinical MRI of the brain without contrast showed diffuse cerebral atrophy, without vascular lesions and a mild hippocampal atrophy. According to the clinical presentation and the neuropsychological and neuroradiological findings, 50 a diagnosis of Capgras syndrome in a context of probable AD 51,52 was made.
Healthy Elderly Control Group
A total of 24 FDG-PET images of the right-handed healthy elderly controls were included, selected from our database (see Table 1). Each participant was more than 60 years old. Participant in this group had no cognitive or psychiatric problems, were free of medication that could affect cognitive functioning, reported being in good health, and did not have any relevant medical history. No structural brain imaging or psychometric testing was done for this group. The Ethics Committee of the University Hospital approved the study. Informed consent was obtained from all the participants.
Table 1.
Demographic Characteristics and Clinical Data of the Participants.a
| Variable | Patients with AD | EC | Patients with Capgras syndrome |
|---|---|---|---|
| n | 26 | 24 | 1 |
| Age, years | 77.73 (6.94) | 71.85 (6.89) | 75 |
| Education, years | 10.27 (3.14) | 12.80 (2.91) | 12 |
| Gender | |||
| Male | 7 | 9 | |
| Female | 19 | 15 | |
| Mattis DRS | 123.30 (7.45) | 139.21 (5.42) | 128 |
Abbreviations: AD, Alzheimer’s disease; EC, eigenvector centrality; DRS, dementia rating scale.
aNumbers are means with standard deviations.
Alzheimer Group
A total of 26 FDG-PET images of right-handed patients with AD were included, selected from our database. The patients with AD had been diagnosed in the memory clinic of the University Hospital, Liege. Each patient was more than 60 years old. Diagnosis was made after a clinical interview with the patient and his or her relative, a clinical examination, neuropsychological assessment, and structural brain imaging. All patients met the diagnostic criteria for mild AD 51,52 (see Table 1). A characteristic PET pattern (that is a visual observation of posterior associative cortices hypometabolism, unilateral or bilateral, with or without involvement of other cerebral associative cortices) was used as a biomarker. No psychoactive symptoms or delirious beliefs were reported among this group. The University Hospital’s Ethics Committee approved the study. Informed consent was obtained from all the patients. Both the AD and healthy elderly groups have similar age, gender, handedness, education, and medical history (see Table 1).
Neuroimaging Data Acquisition
The PET scanner images were acquired on a CTI ECAT HR+ scanner (Siemens, Knoxville, Tennessee; 3D mode; 63 slices; 15.2-cm axial field of view; 5.6-mm transaxial resolution, and 2.4-mm slice interval). Images of brain tracer distribution (scan duration 20 minutes) were obtained during quiet wakefulness with eyes closed, 30 minutes after an intravenous injection of 2-[18F]fluoro-2-deoxy-d-glucose (123 to 290 MBq). Images were reconstructed using the filtered backprojection algorithm including correction for measured attenuation and scatter using standard manufacturer software (Siemens/Cps Ecat Pet software version 7.2.2).
The MRI data were acquired on a 3-T Siemens scanner (Siemens, Allegra, Erlangen, Germany). Head movements were minimized by restraining the patient’s head with a cushion. A structural MRI image was acquired to compare regional atrophy between the groups. It consisted in a high-resolution T1-weighted image (3D MP-RAGE sequence) with the following parameters: repetition time = 7.92 ms, echo time = 2.4 ms, inversion time = 910 ms, flip angle = 15°, field of view = 256 × 224 × 176 mm3, 1 mm isotropic spatial resolution.
It is interesting to note that the patient still had delusional symptoms when she was scanned.
Data Analysis
Statistical analyses were performed using the Statistical Parametric Mapping SPM8 software (Wellcome Department of Cognitive Neurology, London, United Kingdom), implemented in MATLAB (Mathworks Inc, Sherborn, Massachusetts). To make sure that the impaired regional metabolism observed in our patient was not explained by cerebral atrophy, we conducted a voxel-based morphometry (VBM) study using SPM8 and its Diffeomorphic Anatomical Registration toolbox (DARTEL) to extract and compare regional gray matter density.
Each PET image was registered to its corresponding MRI image. Then we applied DARTEL, an algorithm for accurate diffeomorphic image registration, to create a set of group-specific templates. The MRI images of the brain were segmented, normalized, and modulated using these templates. The output images were still in the average brain space. Additional warping from the Montreal Neurological Institute (MNI) space was given to brain images. In order to avoid differences due to the normalization process or the use of different referential templates, PET data were normalized using the flow-field parameters generated by DARTEL. Gray matter probability images and normalized PET images were then smoothed with a 12-mm Gaussian kernel (full width at half maximum).
The PET images of the patients were compared to those of the control groups and patients with AD using proportional scaling by cerebral global mean values to control for individual variation in global FDG uptake. Age and sex were introduced as covariates. Given the specificities of our study and the a priori hypothesis concerning the systems involved, P < .001 uncorrected was used as the threshold of significance.
Note that all the stereotaxic coordinates given below will therefore refer to the MNI space.
Results
Structural MRI
To make sure that the differences in the cerebral metabolism were not due to cerebral atrophy, “F”’s structural MRI image was compared with the structural MRI images from the healthy control group and from the AD group, using VBM in SPM8 and a statistical threshold P < .001 uncorrected for multiple comparisons. This comparison did not show any significant differences in regional brain atrophy. The comparison between structural images from the AD group and the healthy elder control group, performed at a “liberal” statistical threshold of P < .01, showed expected large clusters of atrophy in precuneus cortex, PCC, and temporoparietal regions. Finally, the comparison, at P < .01 between “F” structural image and structural images from the control group, showed an atrophy in the PCC and precuneus cortex.
Resting FDG-PET
Brain metabolic images of the 3 groups were introduced in a single design matrix. The comparison between “F” and the healthy elderly control groups revealed a hypometabolic cluster in “F”’s left dorsal and posterior MPFC (dorsal posterior MPFC, −15, 21, and 63) and left upper precuneus/PCC (−9, −72, and 33; see Figure 1 and Table 2). The comparison between the AD group and the healthy elderly controls yielded a typical AD pattern of hypometabolism involving the left PCC/precuneus (−3, −57, and 27), left inferior temporal regions (−54, −27, and −30), and parts of the posterior parietal cortex (L: −48, −63, and 33; R: 57, −54, and 39; see Figure 1 and Table 3). The comparison between “F” and the AD group showed that “F” presented hypometabolism of midline structures involving the left dorsal posterior MPFC (−12, 21 and 63) and upper left precuneus/PCC (−10, −75, and 33; see Figure 1 and Table 4). Noteworthy, a bilateral hypometabolism was observed when we decreased the significance level of our analysis to a “liberal” threshold of P < .01.
Figure 1.
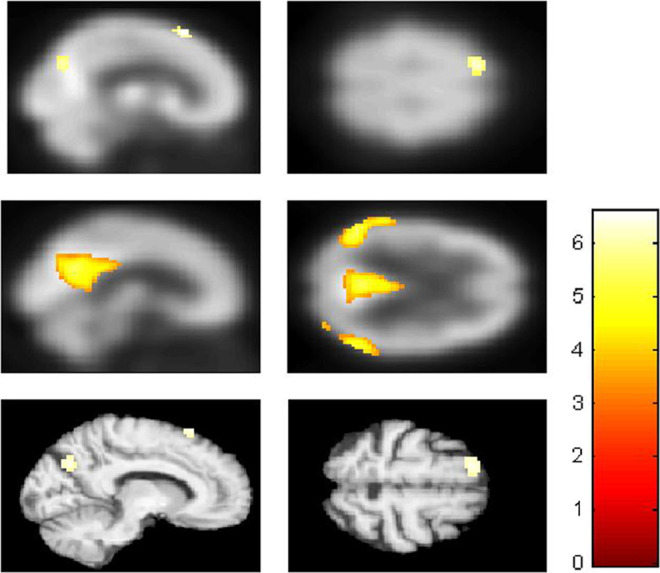
Discrepancy in the resting state brain metabolism between “F,” Alzheimer’s disease (AD) group, and healthy elderly control group. A, Displayed at P < .001 uncorrected on the mean positron emission tomography (PET) of all participants. Results of the comparison, “F” < controls: hypometabolic cluster in the dorsal and posterior medial prefrontal cortex (MPFC) and the upper precuneus/posterior cingulate. B, Displayed at P < .001 uncorrected on the mean PET of all the participants. Results of the comparison, AD group < controls: hypometabolism in the posterior cingulate cortex/precuneus, inferior temporal regions, and parts of the posterior parietal lobe. C, Displayed at P < .001 uncorrected on the patient’s normalized structural magnetic resonance image. Results of the comparison, “F” < AD group: hypometabolism involving the dorsal posterior MPFC and upper precuneus/posterior cingulate cortex.
Table 2.
Metabolic Differences Between Brain Regions of Capgras Patient “F” and the Healthy Elderly Control Group, Coordinates, P, and Z Values.
| Brain region | MNI coordinates | Z score | Peak level | |||
|---|---|---|---|---|---|---|
| x | y | z | P FWEcorr | Voxels | ||
| L dMPFC | −15 | 21 | 63 | 3.73 | .162a | 51 |
| L PCC/precuneus | −9 | −72 | 33 | 3.47 | .320a | 41 |
Abbreviations: L, left hemisphere; dMPFC, dorsal medial prefrontal cortex with z coordinate > 10 mm; PCC, posterior cingulate cortex with z coordinate > 10 mm; MNI, Montreal Neurological Institute; P FWEcorr, familywise error correction.
aFor a P value uncorrected < .001.
Table 3.
Metabolic Differences Between Brain Regions of the AD Group and the Healthy Elderly Control Group, Coordinates, P and Z Values.a
| Brain region | MNI coordinates | Z score | Peak level | |||
|---|---|---|---|---|---|---|
| x | y | z | P FWEcorr | Voxels | ||
| R/L PCC/precuneus | −3 | −57 | 27 | 4.66 | .006 | 110 |
| L Posterior parietal | −48 | −63 | 33 | 4.75 | .005 | 264 |
| L Inferior temporal | −54 | −27 | −30 | 5.47 | .001 | 98 |
| R Posterior parietal | 57 | −54 | 39 | 5.17 | .009 | 106 |
Abbreviations: R, right hemisphere; L, left hemisphere; PCC, posterior cingulate cortex with z coordinate > 10 mm; MNI, Montreal Neurological Institute; P FWEcorr, familywise error correction.
aFor a P value uncorrected < .001.
Table 4.
Metabolic Differences Between Brain Regions of Capgras Patient “F” and the AD Group, Coordinates, P and Z Values.
| Brain region | MNI coordinates | Z score | Peak level | |||
|---|---|---|---|---|---|---|
| x | y | z | P FWEcorr | Voxels | ||
| L dMPFC | −12 | 21 | 63 | 3.05 | .69a | 19 |
| L PCC/precuneus | −10 | −75 | 33 | 3.09 | .65a | 21 |
Abbreviations: L, Left hemisphere; dMPFC, dorsal medial prefrontal cortex with z coordinate > 10 mm; PCC, posterior cingulate cortex with z coordinate > 10 mm; MNI, Montreal Neurological Institute; P FWEcorr, familywise error correction.
aFor a P value uncorrected < .001.
Beyond the statistical analysis, the visual observation of “F” FDG-PET image showed a discrete hypometabolism in the inferior temporal lobe suggesting a dysfunction in the visual ventral pathway.
Discussion
According to the 2-pathway face recognition model, 21,25,26 Capgras delusion results from a dysfunction of the dorsal route that generates insufficient affective response to recognize a somehow familiar face as personally relevant. The delusion could result from the patient’s attributing changes in his or her internal world to a change in the external world. 15 To our knowledge, this is the only Capgras delusion model that is experimentally supported by an electrodermal conductance study. 21 In the current study, we tested whether this model can explain the profile of resting-state hypometabolism demonstrated by a patient with probable AD presenting with Capgras delusion. Contradictory to the model-based hypothesis, the results did not show significant hypometabolism in the dorsal path assumed to support covert/emotional face recognition, namely, in the visual cortex, inferior parietal lobule, and emotional nodes of the limbic system.
Actually, when compared to the control and AD groups, and taking into account age and sex as confounding variables, the PET image of patients with Capgras syndrome was mainly characterized by hypometabolism of midline structures in the dorsal posterior MPFC and PCC/precuneus regions. Our data thus suggest that an impaired activity in the theoretical dorsal route is not mandatory to generate Capgras delusion. However, this does not invalidate the 2-pathway model that has been experimentally supported; 4,21 rather, we tentatively suggest that affective representations and person knowledge related to face recognition may pass through different neurological pathways from those considered originally.
Our results are consistent with the previous reports of frontal 13 and parietal 34 involvement in patients with AD with Capgras syndrome and with the recent model for familiar face recognition. 37 In this model, familiar face recognition depends on the interactions between a core system that analyzes the visual appearance of a face and an extended system that extracts further information related to this face. Some authors have emphasized the role of the MPFC in the retrieval or representation of personal knowledge about faces. 41 The posterior dorsal MPFC is also involved in forming impressions of others, 47 in self- and other-referential processing 44 –46 and in perspective taking. 43 All those processes may be involved in flexibly reflecting on personal information related to a familiar face. A number of studies have also demonstrated activation of the PCC/precuneus during perception of familiar faces 39 or voices. 53 More generally, both the MPFC and the PCC/precuneus form part of the so-called social brain. 54
In keeping with the impaired reflection on delusional beliefs characteristic of Capgras syndrome, impaired MPFC activity 55 –57 and PCC activity 58 have also been found to be related to anosognosia in neurodegenerative diseases, a clinical condition in which patients do not recognize their erroneous functioning.
Our findings suggest that precuneus/PCC and dorsal MPFC (dMPFC) dysfunction may play a role in Capgras syndrome. Considering the putative role of these regions in face recognition according to Gobbini et al’s model, an interpretation could be that Capgras delusion results from a defect in subsystems dedicated to retrieval of face-related person knowledge and/or representation and to reflection about familiar face-related social information, even if the structural recognition of faces remains unaltered. Following this, the delusion would arise when the patient cannot properly monitor familiar face-related person knowledge or social representations and misattributes an internal self-dysfunction to an external world change. Anecdotally, the clinical description of the Capgras delusion of voices could also be explained by the PCC/precuneus dysfunction, as this region is also activated when hearing familiar voices. 53
It has been emphasized that the dMPFC mediates cognitive rather than affective aspects of self-referential processing, 59 –61 and in a recent review, van der Meer suggested that activation of the dMPFC is not specific to self-representation and is also apparent when thinking about others. 62 In keeping with our interpretations, the precuneus and the dMPFC have recently been claimed to belong to a cerebral network that subserves thinking about the traits and mental states, and intentions of others 42,63 have also demonstrated that the dMPFC and PCC are activated when evaluating the personalities of both close and distant other people. In addition, the dMPFC is more involved in judgements of dissimilar others than of similar others. 47 According to this literature, in Capgras syndrome, the delusion itself could result from the dMPFC dysfunction, corresponding to impairment of an integrative cognitive subsystem participating in both self-reference and judgement about others and their mental states and intentions.
It is fair to say that these hypometabolic regions are not specific for visual delusions in Capgras syndrome, and that there are probably other metabolic disturbances or disconnections in the system which cannot be demonstrated on a single patient statistic analysis (that is in the core “face” visual system or between core and extended systems). Along these lines, one could hypothesize that any lesion or dysfunction of a person-knowledge or emotional-related subsystem taking part in the extended system for face identification or in the paths connecting the core to the extended system could possibly lead to Capgras delusion. This could explain the wide variety of lesion/dysfunction patterns observed in Capgras delusion imaging. 11 –13,27 –31,34,35
According to our data, the “2-pathway” face recognition model of Capgras delusion, 24,26,64 although experimentally based and predictive, must be more detailed in terms of its neural correlates.
According to Ellis, 65 studies of Capgras syndrome and delusional misidentification syndromes by functional imaging could be an irreplaceable means of exploring face recognition and the social brain network and integrating clinical findings with our knowledge of neuroanatomy and neurophysiology much more precisely. Further investigations are necessary to more finely characterize the cerebral lesions resulting in Capgras delusion and to specify the exact role of each component of the networks involved in the genesis of the delusion. To reach this objective, additional functional imaging studies exploring groups of patients with Capgras syndrome performing complex visual tasks involving faces are also required. The comparison of functional differences between patients with dementia and patients with schizophrenia having Capgras syndrome during these tasks could be especially interesting. The main difficulty that currently remains is the relative scarcity of Capgras syndrome and other delusional misidentification syndromes among the general population and the difficulty of diagnosing them.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HJ is a FRS-FNRS research fellow, DF is funded by ARC 06/11-340. CB is a researcher in a Belgian InterUniversity Attraction Pole (IUAP6/29 and IUAP7), FC is a Senior Research Associate, MAB is a Logistic Collaborator, and PM is the research director at the National Fund for Scientific Research (FNRS).
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the FRS-FNRS.
References
- 1. Capgras J, Reboul-Lachaux J. L’illusion des ‘sosies’ dans un délire systématisé chronique. Bull Soc Clin Med Mentale. 1923;2:6–16. [Google Scholar]
- 2. Josephs KA. Capgras syndrome and its relationship to neurodegenerative disease. Arch Neurol. 2007;64(12):1762–1766. [DOI] [PubMed] [Google Scholar]
- 3. Edelstyn NM, Oyebode F. A review of the phenomenology and cognitive neuropsychological origins of the Capgras syndrome. Int J Geriatr Psychiatry. 1999;14(1):48–59. [PubMed] [Google Scholar]
- 4. Hirstein W, Ramachandran VS. Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc Biol Sci. 1997;264(1380):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henriet K, Haouzir S, Petit M. Capgras' syndrome: a delusional interpretation of a deficit of affective identification of face, Revue of literature and proposition of sequential model. Ann Med Psychol. 2008;(166):147–156. [Google Scholar]
- 6. Frazer SJ, Roberts JM. Three cases of Capgras' syndrome. Br J Psychiatry. 1994;164(4):557–559. [DOI] [PubMed] [Google Scholar]
- 7. Christodoulou GN. Treatment of the “syndrome of doubles”. Acta Psychiatr Belg. 1977;77(2):254–259. [PubMed] [Google Scholar]
- 8. Todd J, Dewhurst K, Wallis G. The syndrome of Capgras. Br J Psychiatry. 1981;139:319–327. [DOI] [PubMed] [Google Scholar]
- 9. Young AW, Hellawell D, Wright S, and Ellis HD. Reduplication of visual stimuli. Behav. Neurol. 1994;(7):137–142. [DOI] [PubMed] [Google Scholar]
- 10. Edelstyn NMJ, Oyebode F, Booker E., Humphreys GW, Facial processing and the delusional misidentification syndromes. Cog. Neuropsychiat. 1998;3(4):299–314. [Google Scholar]
- 11. Paillere-Martinot ML, Dao-Castellana MH, Masure MC, Pillon B, Martinot JL. Delusional misidentification: a clinical, neuropsychological and brain imaging case study. Psychopathology. 1994;27(3-5):200–210. [DOI] [PubMed] [Google Scholar]
- 12. Cutting J. Delusional misidentification and the role of the right hemisphere in the appreciation of identity. Br J Psychiatry Suppl. 1991;(14):70–75. [PubMed] [Google Scholar]
- 13. Förstl H, Almeida OP, Owen AM, Burns A, Howard R. Psychiatric, neurological and medical aspects of misidentification syndromes: a review of 260 cases. Psychol Med. 1991;21(4):905–910. [DOI] [PubMed] [Google Scholar]
- 14. Edelstyn NM, Oyebode F, Barrett K. Delusional misidentification: a neuropsychological case study in dementia associated with Parkinson’s disease. Neurocase. 1998;(4):181–188. [Google Scholar]
- 15. Young AW, Reid I, Wright S, Hellawell DJ. Face-processing impairments and the Capgras delusion. Br J Psychiatry. 1993;162:695–698. [DOI] [PubMed] [Google Scholar]
- 16. Oyebode F, Sargeant R. Delusional misidentification syndromes: a descriptive study. Psychopathology. 1996;29(4):209–214. [DOI] [PubMed] [Google Scholar]
- 17. Cenac-Thaly H, Frelot C, Guinard M, Tricot JC, Lacour M. L’illusion des sosies[in French]. Ann Med Psychol. 1962;120(2):481–494. [PubMed] [Google Scholar]
- 18. Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32(4):331–341. [DOI] [PubMed] [Google Scholar]
- 19. Walter-Ryan WG. Capgras' syndrome and misidentification. Am J Psychiatry. 1986;143(1):126. [DOI] [PubMed] [Google Scholar]
- 20. de Pauw KW. Psychodynamic approaches to the Capgras delusion: a critical historical review. Psychopathology. 1994;27(3-5):154–160. [DOI] [PubMed] [Google Scholar]
- 21. Ellis HD, Young AW, Quayle AH, De Pauw KW. Reduced autonomic responses to faces in Capgras delusion. Proc Biol Sci. 1997;264(1384):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis SW. Brain imaging in a case of Capgras' syndrome. Br J Psychiatry. 1987;150:117–121. [DOI] [PubMed] [Google Scholar]
- 23. Bauer RM. Autonomic recognition of names and faces in prosopagnosia: a neuropsychological application of the Guilty Knowledge Test. Neuropsychologia. 1984;22(4):457–469. [DOI] [PubMed] [Google Scholar]
- 24. Tranel D, Damasio AR. Knowledge without awareness: an autonomic index of facial recognition by prosopagnosics. Science. 1985;228(4706):1453–1454. [DOI] [PubMed] [Google Scholar]
- 25. Ellis HD, Young AW. Accounting for delusional misidentifications. Br J Psychiatry. 1990;157:239–248. [DOI] [PubMed] [Google Scholar]
- 26. Breen N, Caine D, Coltheart M. Models of face recognition and delusional misidentification: a critical review. Cogn Neuropsychol. 2000;17(1):55–71. [DOI] [PubMed] [Google Scholar]
- 27. Förstl H, Besthorn C, Burns A, Geiger-Kabisch C, Levy R, Sattel A. Delusional misidentification in Alzheimer's disease: a summary of clinical and biological aspects. Psychopathology. 1994;27(3-5):194–199. [DOI] [PubMed] [Google Scholar]
- 28. Silva JA, Leong GB, Wine DB. Misidentification delusions, facial misrecognition, and right brain injury. Can J Psychiatry. 1993;38(4):239–241. [DOI] [PubMed] [Google Scholar]
- 29. Joseph AB. Focal central nervous system abnormalities in patients with misidentification syndromes. Bibl Psychiatr. 1986;(164):68–79. [DOI] [PubMed] [Google Scholar]
- 30. Silva JA, Leong GB, Lesser IM, Boone KB. Bilateral cerebral pathology and the genesis of delusional misidentification. Can J Psychiatry. 1995;40(8):498–499. [DOI] [PubMed] [Google Scholar]
- 31. Signer SF. Localization and lateralization in the delusion of substitution. Capgras symptom and its variants. Psychopathology. 1994;27(3-5):168–176. [DOI] [PubMed] [Google Scholar]
- 32. Gallo DA, Chen JM, Wiseman AL, Schacter DL, Budson AE. Retrieval monitoring and anosognosia in Alzheimer's disease. Neuropsychology. 2007;21(5):559–568. [DOI] [PubMed] [Google Scholar]
- 33. Baldwin RC, Snowden JS, Mann DM. Delusional misidentification in association with cortical Lewy body disease – a case report and overview of possible mechanisms. Int J Geriat Psychiat. 1995;10(10):893–898. [Google Scholar]
- 34. Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol. 1983;40(3):143–146. [DOI] [PubMed] [Google Scholar]
- 35. Lebert F, Pasquier F, Steinling M, Cabaret M, Caparros-Lefebvre D, Petit H. SPECT data in a case of secondary Capgras delusion. Psychopathology. 1994;27(3-5):211–214. [DOI] [PubMed] [Google Scholar]
- 36. Tueth MJ, Cheong JA. Successful treatment with pimozide of Capgras syndrome in an elderly male. J Geriatr Psychiatry Neurol. 1992;5(4):217–219. [DOI] [PubMed] [Google Scholar]
- 37. Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. 2007;45(1):163–173. [DOI] [PubMed] [Google Scholar]
- 38. Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51(1):59–67. [DOI] [PubMed] [Google Scholar]
- 39. Gobbini MI, Haxby JV. Neural response to the visual familiarity of faces. Brain Res Bull. 2006;71(1-3):76–82. [DOI] [PubMed] [Google Scholar]
- 40. Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. [DOI] [PubMed] [Google Scholar]
- 41. Cloutier J, Kelley WM, Heatherton TF. The influence of perceptual and knowledge-based familiarity on the neural substrates of face perception. Soc Neurosci. 2011;6(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yaoi K, Osaka N, Osaka M. Is the self special in the dorsomedial prefrontal cortex? An fMRI study. Soc Neurosci. 2009;4(5):455–463. [DOI] [PubMed] [Google Scholar]
- 43. D'Argembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. [DOI] [PubMed] [Google Scholar]
- 44. Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18(9):1586–1594. [DOI] [PubMed] [Google Scholar]
- 45. Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. [DOI] [PubMed] [Google Scholar]
- 46. Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(pt 8):1808–1814. [DOI] [PubMed] [Google Scholar]
- 47. Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. [DOI] [PubMed] [Google Scholar]
- 48. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 49. Breen N, Caine D, Coltheart M. Mirrored-self misidentification: two cases of focal onset dementia. Neurocase. 2001;7(3):239–254. [DOI] [PubMed] [Google Scholar]
- 50. Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351(1):56–67. [DOI] [PubMed] [Google Scholar]
- 51. Association AP. DSM-IV, Manuel diagnostique et statistique des troubles mentaux1996. Paris, France: Masson. [Google Scholar]
- 52. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 53. Shah NJ, Marshall JC, Zafiris O, et al. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(pt 4):804–815. [DOI] [PubMed] [Google Scholar]
- 54. Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ries ML, Jabbar BM, Schmitz TW, et al. Anosognosia in mild cognitive impairment: relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13(3):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosen HJ, Alcantar O, Rothlind J, et al. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49(4):3358–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zamboni G, Wilcock G. Lack of awareness of symptoms in people with dementia: the structural and functional basis. Int J Geriatr Psychiatry. 2011;26(8):783–792. [DOI] [PubMed] [Google Scholar]
- 58. Mimura M. Memory impairment and awareness of memory deficits in early-stage Alzheimer's disease. Tohoku J Exp Med. 2008;215(2):133–140. [DOI] [PubMed] [Google Scholar]
- 59. D'Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E. Valuing one's self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cereb Cortex. 2012;22(3):659–667. [DOI] [PubMed] [Google Scholar]
- 60. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31(4):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34(6):935–946. [DOI] [PubMed] [Google Scholar]
- 63. Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc Cogn Affect Neurosci. 2013;8(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ellis HD, Lewis MB. Capgras delusion: a window on face recognition. Trends Cogn Sci. 2001;5(4):149–156. [DOI] [PubMed] [Google Scholar]
- 65. Ellis HD. Delusions: a suitable case for imaging? Int J Psychophysiol. 2007;63(2):146–151. [DOI] [PubMed] [Google Scholar]


