Abstract
The aim of this study was to determine whether an egocentric topographical working memory (WM) deficit is present in the early stages of Alzheimer's disease (AD) with respect to other forms of visuospatial WM. Further, we would investigate whether this deficit could be present in patients having AD without topographical disorientation (TD) signs in everyday life assessed through an informal interview to caregivers. Seven patients with AD and 20 healthy participants performed the Walking Corsi Test and the Corsi Block-Tapping Test. The former test requires memorizing a sequence of places by following a path and the latter is a well-known visuospatial memory task. Patients with AD also performed a verbal WM test to exclude the presence of general WM impairments. Preliminary results suggest that egocentric topographical WM is selectively impaired, with respect to visuospatial and verbal WM, even without TD suggesting an important role of this memory in the early stages of AD.
Keywords: working memory, Corsi Block-Tapping Test, Alzheimer’s disease, topographical disorientation, environmental human navigation
Introduction
Recently, interest in topographical memory has increased along with interest in the mechanisms involved in human navigation of space. During navigation of the environment, we acquire spatial information about location, distance, and direction which we store in memory for future retrieval when we try to reach a familiar place or give directions about its location to others. Recent evidence shows that different types of visuospatial working memory (WM) for reaching and navigational space are selectively impaired in brain-damaged patients and patients with temporal lobe epilepsy. 1,2 Results of these studies confirm that the route memory system is associated with navigational disorders and is segregated from the memory system for reaching space. Among navigational disorders, topographical disorientation (TD) is one of the earliest symptoms of Alzheimer’s disease (AD 3 ). In a clinical taxonomy, Aguirre and D’Esposito 4 identified and described 4 types of TD: landmark agnosia when patients are unable to recognize familiar places; egocentric disorientation when they are unable to represent the environment from a first-person perspective; heading disorientation when they do not process directional information from landmarks; and anterograde disorientation when they are unable to learn new environmental representations. The TD has a significant impact on the lives of the patients with AD and their caregivers and is present as one of the earliest symptoms 5,6 in approximately 40% to 50% of patients with AD; it is still unclear, however, which navigational skill is impaired. Burgess et al 7 reported the case of a patient in an early stage of AD who showed a peculiar type of TD. She showed spared recognition of landmarks, objects, and scenes, and her performance on general egocentric representation tasks was preserved. In contrast, her allocentric representation of the environment was severely deficient. Subsequently, Bird et al 8 found deficits in topographical WM in patients with AD and in patients with amnesic mild cognitive impairment, suggesting that a core cognitive deficit associated with the earliest stages of AD is the inability to form and retain allocentric representations of large-scale environments. According to these authors, this deficit could underlie TD in AD. The existence of selective WM deficits related to the type of information processed is intriguing and requires investigation. Selective WM deficits could set alarm bells ringing in the earliest stages of AD, when symptoms are not severe enough to fulfill the AD diagnostic criteria. Although one of the early cognitive deficits in AD is episodic memory impairment, there is increasing evidence that WM is also impaired in the earliest stages of AD (for a review see Huntley et al. 9 ). The existence of different types of WM might also explain the inconsistency in determining the extent of WM deficits in early AD. Indeed, Bird et al 8 found a topographical WM deficit restricted to allocentric environmental representation. We believe, however, that a distinction should be made between the egocentric and the allocentric aspects of topographical WM. This deficit could impede the retention and the manipulation of egocentric navigational information for short periods and, thus, affect navigational skill. Evidence from patients with right brain damage having spatial hemineglect indicates that the presence of a topographical WM impairment affects patients’ performance on environmental tasks, especially their ability to use path integration to reach the location of a previously learned hidden target. 10 Discovering whether this aspect of topographical WM is also affected in early AD would help clarify why some cognitive deficits vary from one patient with AD to another. For example, although TD is very common in advanced stages of AD, it can also be one of the first symptoms. 11 -13 These differences in the presence/absence of TD might be due to impairments in different aspects (allocentric and egocentric) of topographical WM. Indeed, navigational WM seems to be a crucial cognitive process underlying TD phenomena. For example, in developmental TD, 14 navigational WM impairment causes different types of navigational impairment. As in AD, the posterior parietal cortex is one of the cerebral regions that is altered, 15 and as this structure is known to be involved in egocentric processing, 16 we hypothesized that some patients with AD could have egocentric topographical WM damage. To investigate this hypothesis, we set out to determine whether patients with AD have an egocentric topographical WM impairment in the very early stages of the illness. Specifically, we investigated whether they show signs of TD in everyday life through an informal interview with caregivers. The second aim was to compare 2 different types of WM, that is, the one measured in navigational space (i.e. egocentric topographical WM) and the other in reaching space to determine whether selective WM deficits are present in patients with AD. For the purposes of this study, we adopted the Walking Corsi Test (WalCT 17,18 ), which requires memorizing a sequence of places by following a path previously shown by the examiner and observed in a first-person perspective, and the Corsi Block-Tapping Test (CBT 19 ), which is a well-known visuospatial memory task administered in reaching space. Patients with AD also performed a verbal WM test (Digit Span [DS]) to rule out the presence of general WM impairments.
Method
Participants
The study included 7 patients (5 women), recruited at San Camillo-Forlanini Hospital in Rome, who had a diagnosis of early stage AD, and 20 healthy control participants (10 women, defined briefly as C). Mean age was 76.8 (3.6 standard deviation [SD]) years for patients with AD and 77.8 (2.2 SD) years for C; basic education was 5.4 (2.5 SD) years on average for AD (2.6 SD) and 6.1 (2.5 s.d) years for C. Groups did not differ significantly with respect to these variables (age: t 1,25 = −0.43, P = not significant [ns]; education: t 1,25 = −0.67, P = ns). All participants with AD did not show any motor deficit as revealed from neurological examination.
All C participants were given the Mini-Mental State Examination (MMSE 20 ) to exclude the presence of AD; they obtained a score higher than 23 (mean score = 27.44; SD = 2.08; cutoff ≤ 23; Magni et al 20 ). No C participants had a family history of dementia.
Concerning the AD group, prior to recruitment, an expert neurologist made the clinical diagnosis of AD according to the ICD-10 criteria. Patients also fulfilled the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA, Alzheimer's Criteria 21 ) for probable or possible AD. All patients with AD were submitted to the Mental Deterioration Battery, 22 (MDB; see Table 1) and to the MMSE. 20 To be included in the sample, patients had to score less than 23 on the MMSE 20 and perform under the cutoff in 2 or more tests of the MDB. 22 In an informal interview with the patients’ relatives, it emerged that 2 of the 7 patients with AD were prone to getting lost at the beginning of the illness.
Table 1.
Means Score (SD) and Cutoff of Participants With AD.
| Mean Scores of Patients With AD | ADL | IADL | IR | DR | PM | WF | IVM | CD | CDL | PC |
|---|---|---|---|---|---|---|---|---|---|---|
| Means (SD) | 5.8 (0.45) | 3.4 (1.14) | 23.62 (5.61) | 3.7 (1.77) | 20.9 (6.58) | 16.3 (12.03) | 15.86 (3.67) | 8.65 (2.64) | 55.3 (13.13) | 16.6 (16.26) |
| Cutoff | – | – | 28.53 | 4.69 | 18.96 | 17.35 | 13.85 | 7.18 | 61.85 | 8.72 |
Abbreviations: ADL, Activity Daily Life; IADL, Instrumental Activity Daily Life; IR, Rey’s 15 Words Auditory Learning Test-Immediate Recall; DR, Rey’s 15 Words Auditory Learning Test-Delayed Recall; PM, Raven’s 47 Progressive Coloured Matrices; WF, Word fluency; IVM, Immediate Visual Memory; CD, Free-hand copying of drawings; CDL, Copying Drawings with Landmarks; PC, Phrase Construction; AD, Alzheimer’s disease; SD, standard deviation.
Demographic data are reported in Table 2.
Table 2.
Personal Data of Participants With AD.
| Case | Group | Age | Sex | Education, yrs | MRI |
|---|---|---|---|---|---|
| PA | AD | 75 | F | 3 | Negative |
| DA | AD | 72 | F | 5 | Negative |
| CR | AD | 74 | F | 5 | Enlarged ventricles, corticosubcortical atrophy, predominantly in the right hemisphere |
| FG | AD | 79 | M | 5 | Enlarged ventricles and subcortical atrophy |
| BI | AD | 78 | F | 5 | Moderate cortical atrophy |
| PC | AD | 83 | M | 11 | Negative |
| CC | AD | 77 | F | 4 | Left posterior parietal hypodensity |
Abbreviations: AD, Alzheimer’s disease; MRI, magnetic resonance imaging.
The study was designed in accordance with the ethical principles of human experimentation stated in the Declaration of Helsinki and was approved by the local ethics committee. All participants’ relatives provided written informed consent after the procedures had been fully explained to them, and each of the patients with AD gave verbal assent.
Instruments and Procedure
Working Memory in Reaching Space: The CBT
Spatial WM in reaching space was tested using the CBT. 19 This test consists of 9 wooden blocks (4.5 × 4.5 cm) fixed on a board (30 × 25 cm) in a scattered array. On the experimenter’s side, the blocks are numbered for easy identification.
The examiner tapped a sequence of blocks at the rate of 1 block per 2 seconds, and the patient had to reproduce the same sequence in the same order. Sequences of increasing length (i.e. number of blocks starting from a 2-block sequence) were presented until the patient failed to reproduce 2 out of 3 trials of a given length.
The patients were tested individually in a quiet hospital room with artificial lighting. They were seated facing the examiner on a height-adjustable office chair in front of the CBT board.
Working Memory in Navigational Space: The WalCT
A larger version of the CBT (3 × 2.5 m; scale 1:10 of the CBT) was set up in an empty room. In this test, the patient had to actually walk and reach different locations. The WalCT 18 consists of 9 black squares placed on a light gray carpet in the same positions as the standard CBT (see Figure 1). Although the same experimental conditions were adopted in the WalCT and the CBT, the starting position was different; that is, in the WalCT, but not in the CBT, both the examiner and the participants started from the same point. The examiner illustrated the sequences of increasing length (starting from a 2-square sequence) by walking on the carpet and stopping on each square for 2 seconds. Then, the participant had to repeat the same sequence as the examiner by walking and stopping on the squares included in the sequence.
Figure 1.

Examiner performing the Walking Corsi Test (WalCT). The apparatus used for administering the WalCT. The scale was 10:1 of the Corsi Block-Tapping Test (CBT) and measured 2.5 × 3 m; black squares were 30 × 30 cm.
Verbal Working Memory: The Digit Span
DS is a subtest of the Wechsler Intelligence scales. 23,24 Auditory digit span is assessed using number sequences; 2 test items are presented at each sequence length. The number of correct items is recorded as a digit score and a DS is calculated as the longest sequence of digits recalled correctly. The administration order of the CBT, WalCT, and DS was counterbalanced across the participants in each group.
Results
To verify the presence of differences between patients with AD and C for WM skills in both reaching (CBT) and navigational (WalCT) space, we performed unpaired t tests for each experimental task.
We found no difference between patients with AD and C in the span assessed in reaching space (CBT: AD = 3.71, SD = 0.95; C = 3.85, SD = 0.67; t 1,25 = −0.41, P = ns; Cohen’s d = 0.16; effect size [r] = .08) but found that patients with AD had a significantly shorter span than C in navigational space (WalCT: AD = 3, SD = 0.82; C = 3.8, SD = 0.83; t 1,27 = −2.19, P < .05; Cohen’s d = 0.88; effect size [r] = .4; Figure 2).
Figure 2.
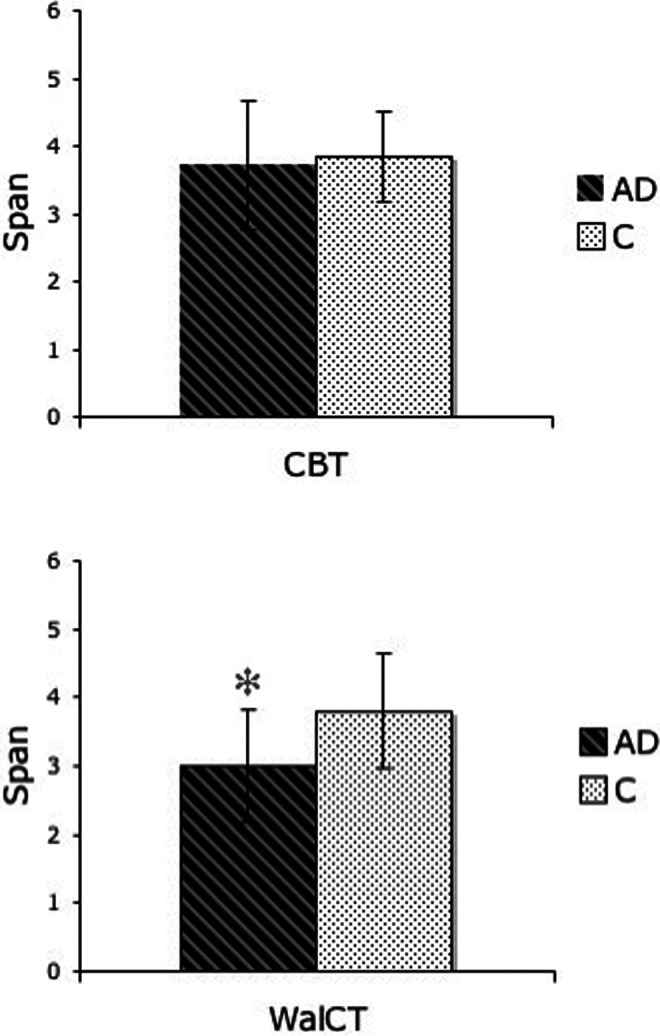
Graphs showing patients with Alzheimer's disease (AD) and C mean and standard deviation (SD) performance on the Corsi Block-Tapping Test (CBT; above) and the WalCT (below). * indicates significance between the group differences.
Concerning verbal WM skills, none of the patients with AD performed pathologically on the DS subtest (see Table 3).
Table 3.
Reports of Patients’ Scores: Mean (No. of Cubes/Squares/Digits) Correctly Recalled in Short-Term Memory and (SD) for Each Working Memory Task Performed by the Groups.a
| Group | MMSE | Reaching Space Working Memory | Egocentric Topographical Working Memory | Verbal Working Memory |
|---|---|---|---|---|
| CBT (No. of Cubes) | WalCT (No. of Squares) | Digit Span (No. of Digits) | ||
| Patients with AD (no. = 7) | 22.23 (0.78) | 3.71 (0.95) | 3.00 (0.82) | 5.43 (0.98) |
| C participants (no. = 20) | 27.44 (2.08) | 3.85 (0.67) | 3.8 (0.83) | 5.39 (0.86) |
Abbreviations: AD, Alzheimer’s disease; CBT, Corsi Block-Tapping Test; MMSE, Mini-Mental State Examination; SD, standard deviation; WalCT, Walking Corsi Test.
a C participants performed only the WalCT and CBT. Mean and SD of MMSE score are reported in the second column.
Discussion
The increase in life expectancy and the prevalence of age-related cognitive disorders, such as AD, have led to great interest in identifying the predictors of AD. Considering the great burden of AD on national health care systems (i.e. in terms of cost and therapy), research aimed at improving the early diagnosis of this pathology is mandatory. In this study, we used an informal interview with caregivers to investigate whether egocentric topographical WM deficits were present in patients having early-stage AD with or without TD. The performance of patients with AD on the egocentric topographical WM test was compared with their reaching space WM and verbal WM performance on well-known tests that are widely used in clinical practice (i.e. CBT and DS). We found that egocentric topographical WM was selectively impaired with respect to reaching space and verbal WM. Therefore, the presence of this kind of memory may be an indicator of early-stage AD. This deficit also seems to be present in the absence of TD, which is considered an early symptom of AD following precocious reduction in hippocampal volume. 25,26 Indeed, in our sample, 4 out of 7 patients with AD showed signs of TD; specifically, 3 out of 4 had deficits in both familiar and unfamiliar environments and 1 out of 4 got lost only in unfamiliar environments. Of the remaining patients with AD without TD, 2 out of 3 showed an egocentric topographical WM performance less than 1 SD of the C mean. As Bird et al 8 pointed out, navigational ability is a multifaceted competence; therefore, it is necessary to investigate the underlying cognitive processes. Clarification of which of these processes are impaired first in AD would facilitate the development of compensatory aids and the identification of patients with mild cognitive impairment (MCI) most at risk of progressing to AD during the prodromal stages of the disease. Bird et al 8 found a specific impairment of allocentric topographical WM in patients with amnestic MCI and in patients with early-stage AD but not in patients with frontotemporal lobar degeneration. This indicates that, unlike AD in which TD may be one of the first manifestations, frontotemporal lobar degeneration is not characterized by the presence of TD symptoms. Pengas et al 27 investigated the neural basis of the topographical memory impairment in AD. They found correlations that converged on a region encompassing the retrosplenial/isthmus/posterior cingulate cortex and possibly the hippocampal tail. They also found a predominantly right hemispheric network that reflected elements of the topographical network highlighted in past functional magnetic resonance imaging (fMRI) studies of human route learning in healthy volunteers. More recently, an fMRI study by Nemmi et al 28 found activation of the retrosplenial cortex during performance of the WalCT. These authors investigated the regions involved and found activations in the right dorsolateral prefrontal cortex, calcarine sulcus, and lingual gyrus. Specifically, the region in which the calcarine sulcus joins the parieto-occipital sulcus is part of the restrosplenial complex, 29,30 a region that has been found active in several navigational tasks. 31,32 Furthermore, according to Byrne et al, 33 this area also transforms egocentric representations into an allocentric frame and vice versa.
The presence of topographical WM defects in patients with AD which affect the processing of allocentric and egocentric information suggests that an in-depth analysis of topographical memory is crucial when making precocious diagnoses even when clinical symptoms are absent. Indeed, in a study investigating the role of confounding factors (such as age, sex, educational achievements, and depressive symptoms), Pengas et al 34 found that topographical memory (unlike other memory complaints 35 ) is insensitive to depression. The evidence that topographical memory is less sensitive to the presence of depression, but seems to be one of the first symptoms in AD, could help clinicians make early differential diagnoses.
According to Mapstone et al, 36 the navigational deficits observed in AD are more related to perceptual impairment of visual motion in optic flow than to memory impairment. Although we did not fully investigate perceptual competence, our sample was above the cutoff in both abstract visuospatial reasoning and free-hand copying of drawings. The patients with AD we tested failed only in Copying Drawings with Landmarks. Our results suggest that the presence/absence of memory deficits may depend on the type of memory assessed. It is well known that the existence of a decay is due to aging in spatial WM. 37 -40 However, healthy controls did not show a significant difference in performing CBT and WalCT, even if the span in the CBT was slightly greater than that in the WalCT. This nonsignificant difference could be explained by the rate of stimuli presentation differentiating the 2 tests. Indeed, the WalCT has a longer interstimulus interval than the CBT, and there are reports in the literature that the interstimulus interval could have effect on performance in the elderly population. 41,42 Our previous studies strongly suggest independence of the 2 types of memory system, that is, that route memory has a smaller WM store. In our AD sample, both verbal WM and spatial WM seemed to be preserved in reaching space but not navigationl space, showing that WM deficits are present in a specific domain. Further, involvement of the retrosplenial cortex in performing the WalCT 25 and its impairment in patients with AD27 could account for the selective deficit observed in this topographical WM task with respect to the other WM tasks. Dissociations in WM are not completely new in AD. For example, Grossi et al 43 found 2 patients with AD who presented contrasting patterns of impairment. One patient scored normally on the CBT and deficiently on the visual matrix task (VMT 44 ); the second patient presented the opposite pattern. These results suggest that although the CBT and the VMT seem to test the same spatial memory processing, they may actually assess different functions. More specifically, VMT and CBT differ in stimulus presentation, that is, spatial simultaneous versus spatial sequential. In the present study, the different performance of our patients with AD on the CBT and the WalCT excludes that WM deficits in AD are linked just to stimulus presentation (i.e. simultaneous vs. sequential) and strongly suggests the need to more thoroughly investigate specific visuospatial domains, in particular the navigational domain.
In conclusion, the present study shows the importance of considering egocentric information when assessing WM of patients with AD in navigational space. Our study is preliminary and suggests the importance of investigating this ability in a study with a larger sample, a more detailed neuropsychological evaluation and more refined statistical techniques.
If a more exhaustive study confirms the present results, the WalCT could help clinicians identify individuals in the prodromal phase of AD, called the “predementia stage of AD” in Dubois’ 45 diagnostic criteria for AD. Recognition of the early symptomatic phase of AD, before progression to the typical phase of AD, is crucial to ensure that patients receive the proper rehabilitation and preventive therapies.
Acknowledgments
The authors would like to thank Dr Elisabetta Celia and Michela Bruschini for their help in recruiting patients with AD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Piccardi L, Berthoz A, Baulac M, et al. Different spatial memory systems are involved in small- and large-scale environments: evidence from patients with temporal lobe epilepsy. Exp Brain Res. 2010;206 (2):171–177. [DOI] [PubMed] [Google Scholar]
- 2. Piccardi L, Iaria G, Bianchini F, Zompanti L, Guariglia C. Dissociated deficits of visuo-spatial memory in near space and navigational space: evidence from brain-damaged patients and healthy older participants. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18 (3):362–384. [DOI] [PubMed] [Google Scholar]
- 3. Reisberg B. Clinical presentation, diagnosis, and symptomatology of age-associated cognitive decline and Alzheimer’s disease. In: Reisberg B, editor. Alzheimer’s Disease. New York: The Free Press; 1983:173–187. [Google Scholar]
- 4. Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122 (pt 9):1613–1628. [DOI] [PubMed] [Google Scholar]
- 5. McShane R, Gedling K, Keene J, Fairburn C, Jacoby R, Hope T. Getting lost in dementia: a longitudinal study of a behavioral symptom. Int Psychogeriatr. 1998;10 (3):253–260. [DOI] [PubMed] [Google Scholar]
- 6. Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19 (3):250–255. [DOI] [PubMed] [Google Scholar]
- 7. Burgess N, Trinkler I, King J, Kennedy A, Cipolotti L. Impaired allocentric spatial memory underlying topographical disorientation. Rev Neurosci. 2006;17 (1-2):239–251. [DOI] [PubMed] [Google Scholar]
- 8. Bird CM, Chan D, Hartley T, Pijnenburg YA, Rossor MN, Burgess N. Topographical short-term memory differentiates Alzheimer’s Disease from frontotemporal lobar degeneration. Hippocampus. 2010;20 (10):1154–1169. [DOI] [PubMed] [Google Scholar]
- 9. Huntley JD, Howard RJ. Working memory in early Alzheimer’s disease: a neuropsychological review. Int J Geriatr Psychiatry. 2010;25 (2):121–132. [DOI] [PubMed] [Google Scholar]
- 10. De Nigris A, Piccardi L, Bianchini F, Palermo L, Incoccia C, Guariglia C. Role of visuo-spatial working memory in path integration disorders in neglect. Cortex. 2013;49 (49):920–930. [DOI] [PubMed] [Google Scholar]
- 11. Henderson VW, Mack W, Williams BW. Spatial disorientation in Alzheimer disease. Arch Neurol. 1989;46 (4):391–394. [DOI] [PubMed] [Google Scholar]
- 12. Cherrier MM, Mendez M, Perryman K. Route learning performance in Alzheimer disease patients. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14 (3):159–168. [PubMed] [Google Scholar]
- 13. Guariglia CC, Nitrini R. Topographical disorientation in Alzheimer’s disease. Arq Neuropsiquiatr. 2009;67 (4):967–972. [DOI] [PubMed] [Google Scholar]
- 14. Bianchini F, Palermo L, Piccardi L. et al. Where am I? A new case of developmental topographical disorientation. J Neuropsychol. 2014;8 (1):107–124. doi: 10.1111/jnp.12007. [DOI] [PubMed] [Google Scholar]
- 15. Seo EH, Lee DY, Lee JM, et al. Whole-brain functional networks in cognitively normal, mild cognitive impairment, and Alzheimer’s disease. PLoS One. 2013;8 (1):e53922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3 (7):553–562. [DOI] [PubMed] [Google Scholar]
- 17. Piccardi L, Iaria G, Ricci M, Bianchini F, Zompanti L, Guariglia C. Walking in the Corsi test: which type of memory do you need? Neurosci Lett. 2008;432 (2):127–131. [DOI] [PubMed] [Google Scholar]
- 18. Piccardi L, Bianchini F, Argento O, et al. The walking corsi test (WalCT): standardization of the topographical memory test in an Italian population. Neurol Sci. 2013;34 (6):971–978. [DOI] [PubMed] [Google Scholar]
- 19. Corsi PM. Human memory and the medial temporal region of the brain. Unpublished doctoral dissertation. Montreal, Quebec: McGill University; 1972. [Google Scholar]
- 20. Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M. Mini-mental state examination: a normative study in Italian elderly population. Eur J Neurol. 1996;3 (3):198–202. [DOI] [PubMed] [Google Scholar]
- 21. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34 (7):939–944. [DOI] [PubMed] [Google Scholar]
- 22. Carlesimo GA, Caltagirone C, Gainotti G. The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The group for the standardization of the mental deterioration battery. Eur Neurol. 1996;36 (6):378–384. [DOI] [PubMed] [Google Scholar]
- 23. Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci. 1987;8 (6):539–548. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D. Manual for the Wechsler adult intelligence scale – revised (WAIS–R). San Antonio, TX: Psychol Corporation; 1981. [Google Scholar]
- 25. Braak H, Braak E. Neuropathological stageing of Alzheimer related changes. Acta Neuropathol. 1991;82 (4):239–259. [DOI] [PubMed] [Google Scholar]
- 26. Carli M, Luschi R, Samanin R. Dose-related impairment of spatial learning by intrahippocampal scopolamine: antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav Brain Res. 1997;82 (2):185–194. [DOI] [PubMed] [Google Scholar]
- 27. Pengas G, Williams GB, Acosta-Cabronero J, et al. The relationship of topographical memory performance to regional neurodegeneration in Alzheimer’s disease. Front Aging Neurosci. 2012;4 (17):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nemmi F, Boccia M, Piccardi L, Galati G, Guariglia C. Segregation of neural circuits involved in spatial learning in reaching and navigational space. Neuropsychologia. 2013;51 (8):1561–1570. [DOI] [PubMed] [Google Scholar]
- 29. Epstein RA. Parahippocampal and retrosplenial contribution to human navigation. Trends Cogn Sci. 2008;12 (10):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 2007;27 (23):6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25 (3):890–899. [DOI] [PubMed] [Google Scholar]
- 32. Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. [DOI] [PubMed] [Google Scholar]
- 33. Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114 (2):340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pengas G, Patterson K, Arnold RJ, Bird CM, Burgess N, Nestor PJ. Lost and found. bespoke memory testing for Alzheimer’s disease and semantic dementia. J Alzheimers Dis. 2010;21 (4):1347–1365. [DOI] [PubMed] [Google Scholar]
- 35. Ritter E, Despres O, Monsch AU, Manning L. Topographical recognition memory sensitive to amnestic mild cognitive impairment but not to depression. Int J Geriatr Psychiatry. 2006;21 (4):924–929. [DOI] [PubMed] [Google Scholar]
- 36. Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology. 2003;60 (5):802–808. [DOI] [PubMed] [Google Scholar]
- 37. Andiel C, Liu L. Working memory and older adults: Implications for occupational therapy. Am Occup Ther Assoc. 1995;49 (7):681–686. [DOI] [PubMed] [Google Scholar]
- 38. Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38 (4):603–618. [DOI] [PubMed] [Google Scholar]
- 39. Belleville S, Peretz I, Malefant D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia, 1996;34 (3):195–207. [DOI] [PubMed] [Google Scholar]
- 40. Golomb J, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol. 1993;50 (9):967–973. [DOI] [PubMed] [Google Scholar]
- 41. Neils J, Newman CW, Hill M, Weiler E. The effects of rate, sequencing, and memory on auditory processing in the elderly. J Gerontol. 1991;46 (2):71–75. [DOI] [PubMed] [Google Scholar]
- 42. Skottun BC. On the use of discrimination to assess memory. Percept Psychophys. 2004;66 (7):1202–1205. [DOI] [PubMed] [Google Scholar]
- 43. Grossi D, Becker JT, Smith C, Trojano L. Memory for visuospatial patterns in Alzheimer’s disease. Psychol Med. 1993;23 (1):65–70. [DOI] [PubMed] [Google Scholar]
- 44. Della Sala S, Gray C, Baddeley A, Allamano N, Wilson L. Pattern span: a tool for unwelding visuo-spatial memory. Neuropsychologia. 1999;37 (10):1189–1199. [DOI] [PubMed] [Google Scholar]
- 45. Dubois B, Howard H, Feldman HH, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9 (11):1118–1127. [DOI] [PubMed] [Google Scholar]


