Abstract
The objective of this study was to compare the effect of multisensory stimulation environment (MSSE) and one-to-one activity sessions in the symptomatology of elderly individuals with severe dementia. Thirty-two participants were randomly assigned to the following 3 groups: MSSE, activity, and control group. The MSSE and activity groups participated in two 30-minute weekly sessions over 16 weeks. Pre-, mid-, and posttrial; 8-week follow-up behavior; mood; cognitive status; and dementia severity were registered. Patients in the MSSE group demonstrated a significant improvement in the Neuropsychiatric Inventory and Bedford Alzheimer Nursing Severity Scale scores compared with the activity group. Both MSSE and activity groups showed an improvement during the intervention in the Cohen-Mansfield Agitation Inventory aggressive behavior factor and total score, with no significant differences between groups. The MSSE may have better effects on neuropsychiatric symptoms and dementia severity in comparison with one-to-one activity sessions in patients with severe dementia.
Keywords: multisensory stimulation, severe dementia, elderly, neuropsychiatric symptoms, randomized controlled trial
Introduction
The clinical spectrum of dementia is a continuum where the earlier signs may be barely discernible, and the later signs overt and complex. Those with advanced disease present a wide range of symptoms which include marked cognitive, language, and functional impairment and significant neuropsychiatric symptoms. 1,2
Although nowadays there are available pharmacologic treatments to deal with dementia symptoms, it is recognized that they have modest efficacy and notable risks, especially in people with severe dementia. Hence, nonpharmacologic interventions have been recommended as first-line treatments. 3,4 In the last years, the evidence supporting the efficacy of nonpharmacological approaches has increased, 5,6 however, most of the studies have focused on mild–moderate stages of illness. Given that patients with severe cognitive impairment may not necessarily respond to treatments in the same manner than those with mild to moderate cognitive impairment, it is necessary to explore the stage-specific efficacy of nonpharmacological therapies for patients with severe dementia. 7
One of the interventions that could be suitable for reaching persons with severe or very severe dementia is multisensory stimulation environment (MSSE). Multisensory stimulation environment was developed in the Netherlands in the 1970s and was first introduced for people with learning difficulties. Since the beginning of the 1990s, the MSSE has been used as a nonpharmacological therapy in people with dementia. 8 The MSSE typically occurs in a pleasant and relaxing room known as Snoezelen room. Visual, auditory, tactile, and olfactory stimulation is offered to patients in this room using a variety of lights, fiber-optic cables, water columns, aroma therapy, different music/sounds, tactile objects, and screen projectors. 9,10 The main features of Snoezelen are one-to-one attention and the adoption of a nondirective approach, encouraging patients to engage with sensory stimuli of their choice, 10 being compatible with the person-centered care philosophy. 11,12
Snoezelen aims to stimulate the primary senses without the need for intellectual activity from the patient. Stimuli used are nonsequential and unpatterned, experienced moment by moment without relying on short-term memory to link them to previous events 10 Thus, it could be an especially appropriate intervention for dealing with people in advance stages of dementia, 13 where verbal communication is markedly impaired.
Elderly people with severe dementia living in nursing homes are often sensory deprivated or, on the contrary, they are exposed to an excessive sensory stimulation. The Model of Imbalance in Sensoristasis (MIS) 14,15 suggests that agitated behaviors may be initiated or exacerbated due to the imbalance between sensory-stimulating and sensory-calming activity. The hypothesis of this model is that achieving a balance between the sensory-stimulating and the sensory-calming activities will decrease agitation, ameliorate other behavioral symptoms, and prevent functional decline. Therefore, MSSE constitutes an adequate intervention because it can have both stimulating and calming effects, and it can be used to ameliorate both disengaged and high-arousal needs.
Nowadays, the evidence suggesting that MSSE is more effective than individualized interventions for reducing neuropsychiatric symptoms in patients with severe dementia is very limited. 16 Very recently it has been found 17,18 that MSSE in a Snoezelen room was as effective as individualized activity sessions improving the neuropsychiatric symptoms of people with mild to severe dementia (Global Deterioration Scale GDS 4-7). 19 This broad spectrum of participants’ cognitive status could be blurring the data and explaining the few significant differences found between both interventions. This study is presented as a continuation of the previous one to test the hypothesis that people in advanced stages of dementia (GDS 6-7) may benefit more from MSSE than from more cognitively demanding one-to-one activities. Therefore, the main objective of the current study was to compare the effect of MSSE in a Snoezelen room and one-to-one activity sessions on to the behavior, mood, cognitive status, and dementia severity of institutionalized elderly individuals with dementia.
Methods
Design
A pilot randomized controlled trial was conducted among older participants aged 65 years or older, stratified according to their cognitive status being afterward randomly assigned to one of the 3 groups (MSSE, activity, and control).
Participants
The sample was selected among the residents of a specialized dementia elderly center in A Coruña (Spain). The inclusion criteria were a diagnosis of dementia and the presence of severe or very severe cognitive decline (GDS, 6-7) 19 . Dementia diagnoses was noted on the medical history and provided by a neurologist before placement in the gerontological complex, being corroborated by the elderly center’s medical doctor. GDS was applied by a clinical psychologist with experience in assessing people to determine level of severity: severe (GDS 6) or very severe (GDS 7) cognitive decline. The exclusion criteria were the presence of a sensory disorder that would adversely affect interactions with the multisensory stimulation objects (eg, severe vision and hearing impairment) and be bedridden.
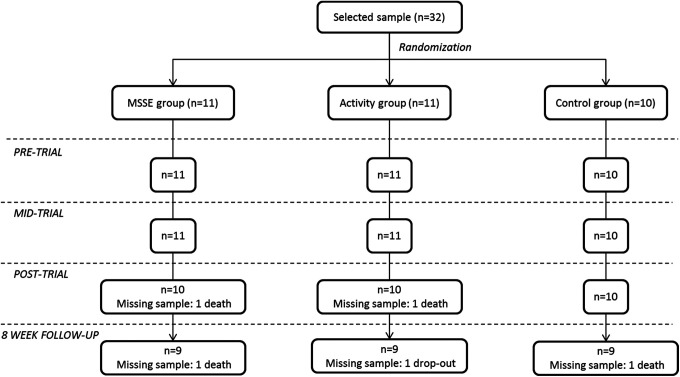
After the clinical psychologist checked the eligibility of participants according to the inclusion and exclusion criteria, the sample consisted of 32 participants. A computer-based random number generator was used to randomly divide the sample into 3 groups of 11, 11, and 10 individuals according to GDS. The initial sample size decreased to 27 during the follow-up period due to participant deaths (n = 4) and dropouts (n = 1). The patients’ progress through the trial is shown in a Consolidated Standards of Reporting Trial (CONSORT) diagram (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trial (CONSORT) diagram. MSSE indicates multisensory stimulation environment.
The study protocol was approved by the Ethics Committee at the University of A Coruña followed the principles of the Declaration of Helsinki. Before beginning data collection, all participants’ proxies were informed about the study. Proxies were used as legally authorized representatives to provide informed consent for the elderly individuals having dementia to participate in the research.
Procedure
People from the MSSE group participated in multisensory sessions in a Snoezelen room. This room, that stimulate all the senses except taste, included several elements such as alternating colors fiber-optic cables, 2 water bubble columns within 2 mirrors, a water bed, a rotating mirror ball with a color light projector, a video, an interactive projecting system, musical selections, aroma therapy equipment with fragrant oils, and a tactile board with various textures, among others.
The activity group participated in a series of one-to-one activity sessions, in which intellectual and/or physical demands were placed on the individual, and the approach used is directive, being the therapist the person responsible to choose the activities to be performed. Participants were asked to take part in simple activities such as looking at photographs or playing games. This group was included in the study to differentiate the specific benefits of the multisensory stimulation from those derived from attending one-to-one therapy sessions. 13
The control group did not participate in any of the aforementioned activities; rather, this group continued with the daily routines of the center, including cognitive stimulation group sessions (GDS 6), training on activities of daily living (GDS 6), education and training of nursing assistants in dementia knowledge, acknowledgment of resident’s experiences, and communication techniques and behavior management (GDS 6-7).
The design of the sessions followed by the MSSE and the activity groups was based on the protocol suggested by Baker et al. 20 Participants from both groups were required to take part in 2 weekly sessions, for a period of 16 weeks, until they complete 32 sessions. These sessions lasted 30 minutes, unless the participant expressed a desire to leave.
The difference between MSSE and activity sessions was given by the characteristics that define the MSSE. In the MSSE group, multisensory unpatterned stimuli were used, the therapist followed a nondirective approach, and the therapy required few intellectual or physical demands. In contrast, during the activity sessions, no intentional special multisensory experiences were introduced, the therapist followed a directive approach, and intellectual and/or physical demands were placed on the patient.
Data on participants’ sensorial preferences and interests were previously collected to design the content of the sessions and to minimize the behavioral problems that some participants could present within the MSSE and the activity contexts. In the MSSE group, sensorial preferences in the Snoezelen room were assessed based on the procedure suggested by Pace et al. 21 Furthermore, relatives of participants of both groups were interviewed to identify participants’ hobbies and interests.
Behavior, mood, cognition, and dementia severity were assessed at baseline (pretrial, week 0), in the middle (midtrial, week 8), at the end of the intervention (posttrial, week 16), and 8 weeks after the intervention (follow-up). Due to ethical reasons, the control group did not remain without intervention for more than 16 weeks; therefore, this group did not participate in the 8-week follow-up.
Assessment Instruments
The validated Spanish version 22 of the Cohen-Mansfield Agitation Inventory (CMAI) 23 was used to assess the frequency of agitated behaviors in the participants. The CMAI consists of 30 items that are each rated on a 7-point scale of frequency, 1 meaning never and 7 several times per hour. The total score is calculated by summing the scores of each of the individual items. Through a factor analysis, Cohen-Mansfield et al 23 found the following 3 factors of agitation in the nursing home: “aggressive behavior” (hitting, kicking, pushing, scratching, tearing things, cursing or verbal aggression, and grabbing); “physically nonaggressive behavior” (pacing, inappropriate robing or disrobing, trying to get to a different place, handling things inappropriately, general restlessness, and repetitious mannerisms); and “verbally agitated behavior” (complaining, constant requests for attention, negativism, repetitious sentences or questions, and screaming). In this study, for each factor the total score was obtained by summing the scores of the corresponding items. The CMAI interrater reliability 23 ranges from .88 to .92 and the internal consistency reliabilities (Cronbach’s α) 24 range from .86 to .91.
Behavior was assessed using the Spanish version 25 of the Neuropsychiatric Inventory (NPI). 26 This scale was developed to assess a wide range of behaviors in patients with Alzheimer’s disease and other dementias. The NPI questionnaire evaluates 12 neuropsychiatric disturbances including delusions, hallucinations, agitation, dysphoria/depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep and night-time behavior disturbances, and changes in appetite and eating behaviors. Neuropsychiatric Inventory is completed according to the answers of the caregivers. A group of screening questions are asked first, which are followed by a series of subsequent questions if the response to the initial screening indicates the presence of neuropsychiatric alterations. The caregiver rates the frequency of the symptoms using scores from 1 to 4 (1 = occasionally, less than once per week; 4 = very frequently, once or more per day or continuously) and also rates the severity using scores from 1 to 3 (1 = mild, 2 = moderate, and 3 = severe). The total score ranges from 0 to 144, with higher values indicating more behavioral and psychological alterations. The Spanish version of the NPI 25 has shown good internal consistence (α = .85) and interrater reliability (.63 to 1.00).
The Spanish version 27 of the Cornell Scale for Depression in Dementia (CSDD) 28 was used to assess mood. This scale was specifically developed to assess signs and symptoms of major depression in patients with dementia. Information is elicited through 2 semistructured interviews: an interview with an informant and an interview with the patient. The CSDD consists of 19 items that are rated for severity on a scale of 0 to 2 (0 = absent, 1 = mild or intermittent, 2 = severe). Total score is obtained by summing the scores for all items, being the minimum score 0 and the maximum score 38. Scores above 10 indicate probable major depression. Scores above 18 indicate definite major depression. In the Spanish population, CSDD has shown good test–retest reliability (.61 to .84) and good internal consistency (α = .81). 27
To assess cognitive function, the Spanish version 29 of the Severe Mini-Mental State Examination (SMMSE) 30 was used. The SMMSE was designed for assessment of severe dementia preventing the floor effect found when using the MMSE. 31 This simple instrument does not require specialized training or foreign material, and it is not tiring for the patient with dementia (takes less than 5 minutes to administer). It consists of 10 items related with autobiographical knowledge (complete name and birth date), constructional praxis tests, phonological loop (spelling), and semantic verbal fluency step (animal category generation). The SMMSE also tests receptive and expressive language skills, along with elementary executive functions and visual–spatial abilities, which are likely to be preserved in patients with severe impairment. The total score ranges from 0 to 30 points, with lower values indicating lower cognitive function. The SMMSE has shown both construct and criterion validity for assessing patients with severely impaired Alzheimer’s disease. 30 In the Spanish population, SMMSE has shown high internal consistency (α = .88), test–retest reliability (.64 to 1.00), interrater reliability (.69 to 1.00), and construct validity in correlation with the Spanish version of the Mini-Mental State Examination (Pearson r coefficient = .59). 29
The overall severity of dementia was measured by the Bedford Alzheimer Nursing Severity Scale (BANS-S). 32 The BANS-S is an observational scale that can be used even with persons who are unable to follow simple commands, uncooperative, or unable to communicate. This is a 7-item scale that combines ratings of interaction ability (speech, eye contact), functional deficits (dressing, eating, and ambulation), and occurrence of pathological symptoms (sleep–wake cycle disturbance, muscle rigidity). 33 Each item is scored on a 4-point scale where a scoring system is specified for each item. The total score ranges from 7 (no impairment) to 28 (most severe impairment). The BANS-S is more sensitive to detecting disease progression beyond the severe stage than scales that measure only cognitive or functional deficits. 32 BANS-S has shown good internal consistence (α = .80), convergent validity with other cognitive and functional scales (r = .62-.79), and discriminant validity in comparison with the NPI (r = .36). 32,34
Statistical Analysis
Sample characteristics were summarized as mean and standard deviation (SD) for the continuous variables and as frequency and percentage for the categorical variables. The Shapiro-Wilk test was used to evaluate the normality of the sample. This test is more appropriate for small sample sizes (< 50 samples). 35 Differences between groups at baseline were compared using chi-square test for proportions and the nonparametric Mann Whitney U test for nonnormally distributed continuous variables. Statistical significance was set at a P value of less than .05.
Finally, repeated measures two-way analysis of variance (two-way mixed ANOVAs) (two-way mixed ANOVAs) was used to assess performance differences in behavior, mood, and cognitive status and dementia severity over the pre-, mid-, and posttrial assessment points. In the first analysis, the within-subject variable was the measures over time (pre-, mid-, and posttrial assessment) and the between-subject variable included the group (MSSE and activity). In the second analysis, the within-subject variable was the measures overtime (pre-, mid-, and posttrial assessment) and the between-subject variable included the group (MSSE and control).
In addition, repeated measures two-way mixed ANOVAs were used to assess performance differences in behavior, mood, and cognition, and dementia severity between the posttrial and the 8-week follow-up. In this case, the within-subject variable was the measures over time (posttrial assessment and follow-up) and the between-subject variable included the group (MSSE and activity).
Differences between groups were tested by a group–time interaction. Eta-squared values (η2) were reported as indicators of effect size. We interpreted the importance of the effect size using the benchmarks for “small” (η2 of .02), “medium” (η2 of .13) and “large” (η2 of .26) offered by Cohen (1988). 36 Statistical significance was set at a P value of less than .05. Statistical analysis was performed using the SPSS version 20.
Results
Table 1 shows the sociodemographic characteristics of the sample, differentiated by groups, at baseline. The mean age of the sample (n = 32) was 85.5 years (SD ±8.46). Of participants, 78.1% were women. Regarding marital status, 62.5% of the patients were widowed. Concerning education level, 28.1% had secondary education.
Table 1.
Sociodemographic Characteristics of the Sample at Week 0 (Baseline, Pretrial).
| MSSE (n = 11) | ACT (n = 11) | Control (n = 10) | Total (n = 32) | P Value (MSSE-ACT) | P value (MSSE-Control) | P Value (ACT-Control) | |
|---|---|---|---|---|---|---|---|
| Age (years) | .818 | .339 | .305 | ||||
| Mean (SD) | 86.4 (7.9) | 87.5 (5.6) | 82.3 (11.0) | 85.4 (8.64) | |||
| Age range | 71-96 | 77-97 | 68-102 | 68-102 | |||
| Gender, n (%) | .138 | .122 | .007a | ||||
| Female | 9 (81.8) | 11 (100.0) | 5 (50.0) | 25 (78.1) | |||
| Male | 2 (18.2) | 0 (0.0) | 5 (50.0) | 7 (21.9) | |||
| Marital status, n (%) | .070 | .230 | .010a | ||||
| Single | 0 (0.0) | 4 (36.4) | 0 (0.0) | 4 (12.5) | |||
| Married/partner | 2 (18.2) | 0 (0.0) | 5 (50.0) | 7 (21.9) | |||
| Widowed | 8 (74.7) | 7 (63.6) | 5 (50.0) | 20 (62.5) | |||
| Separated/divorced | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (3.1) | |||
| Educational level, n (%) | .172 | .552 | .021a | ||||
| No formal education | 2 (18.2) | 6 (54.5) | 0 (0.0) | 8 (25.0) | |||
| Primary | 3 (27.3) | 1 (9.1) | 4 (40.0) | 8 (25.0) | |||
| Secondary | 4 (36.3) | 1 (9.1) | 4 (40.0) | 9 (28.1) | |||
| College or higher degree | 2 (18.2) | 3 (27.3) | 2 (20.0) | 7 (21.9) |
Abbreviations: MSSE, multisensory stimulation environment group; ACT, activity group; SD, standard deviation.
aSignificance: P value < .05.
At baseline, the groups were homogeneous. There were no significant differences, neither between the MSSE group and the activity group nor between the MSSE group and the control group in age, gender, marital status, or educational level. Significant differences only between the activity group and the control in gender, marital status, and educational level were found.
Effect on Behavior
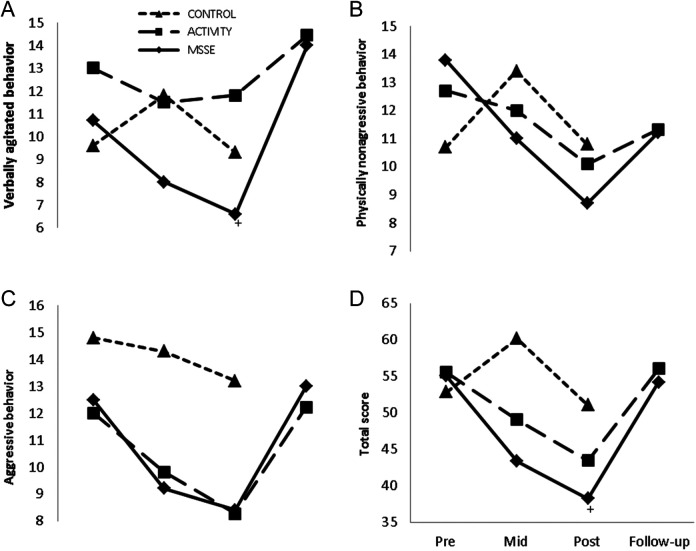
With regard to agitation, as assessed by the CMAI, when comparing the MSSE group and the activity group, an improvement in both groups in the aggressive behavior factor (F 2,38 = 8.200, P = .001, η2 = .300) and in the total score (F 2,36 = 6.990, P = .003, η2 = .277) between pre-, mid-, and postintervention (Figure 2) was observed. However, no significant differences were found between groups. For both groups, the scores worsened in the follow-up period compared to the posttrial assessment in the aggressive behavior factor (F 1,16 = 11.983, P = .003, η2 = .420) and in the CMAI total score (F 1,16 = 14.031, P = .002, η2 = .467), with no significant differences between the groups. With regard the verbally agitated factor, the scores improved from the pretrial to the posttrial in the 2 groups, although the results were not statistically significant. Between the posttrial assessment and the follow-up period, the scores worsened in both groups (F 1,16 = 15.623, P = .042, η2 = .456).
Figure 2.
Cohen-Mansfield Agitation Inventory (CMAI) during the trial and follow-up—verbally agitated behavior (A), physical nonaggressive (B), aggressive behavior (C), and total score (D). Higher scores = worse agitated behavior. +Significant group-time interaction effect from pre- to posttrial (MSSE-control; P < .005). MSSE indicates multisensory stimulation environment.
When comparing the MSSE group and the control group, group-time interactions in verbally agitated behavior and in the CMAI total score were observed. Specifically, an improvement in verbally agitated behavior (F 2,36 = 3.460, P = .042, η2 = .155) and in the CMAI total score (F 2,36= 11.755, P < .001, η2 = .301) from the pretrial to the posttrial in the MSSE in comparison with the control group was found. For the aggressive behavior factor, significant time effects (F 2,36 = 3.632, P = .037, η2 = .160) were also observed among pre-, mid-, and postintervention results, with a decrease in the scores for both groups.
With regard to physically nonaggressive behavior, no significant time effects or intergroup differences were found.
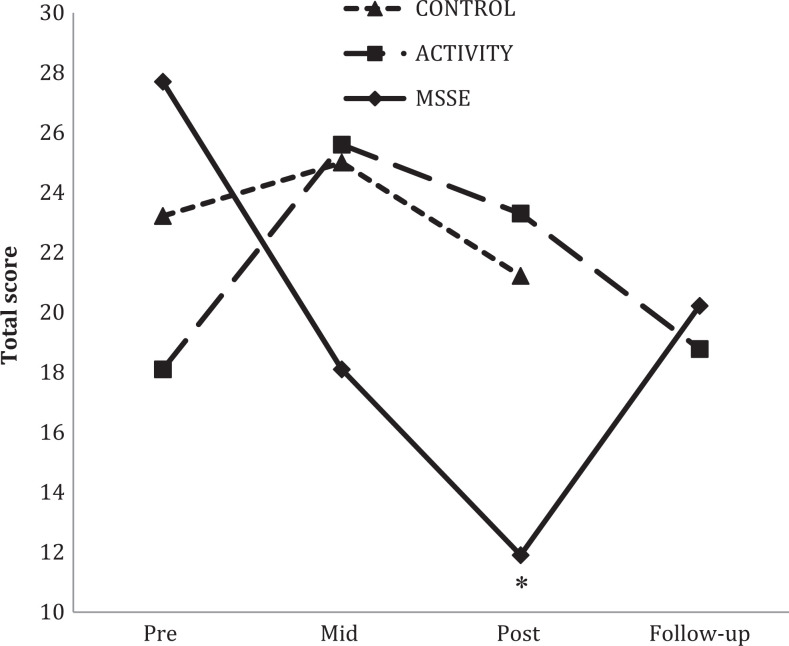
A significant group–time interaction effect was also found when comparing the NPI scores in the MSSE group and in the activity group (Figure 3). Patients in the MSSE group showed a significant higher improvement than the activity group from pretrial to posttrial (F 2,36 = 6.2121, P = .005, η2 = .238), with no significant differences between MSSE and control groups.
Figure 3.
Neuropsychiatric Inventory (NPI) total scores during the trial and follow-up (higher scores = worse behavior). *Significant group—time interaction effect from pre- to posttrial (MSSE-activity; P < .005). MSSE indicates multisensory stimulation environment.
Effect on Mood
The CSDD scores remained stable during the intervention period in the 3 groups (Figure 4). In the follow-up period, the CSDD scores in the MSSE and in the activity group worsened compared to posttrial assessment. However, the results were not significant.
Figure 4.
The Cornell Scale for Depression in Dementia (CSDD) total scores during the trial and follow-up (higher scores = worse mood). MSSE indicates multisensory stimulation environment.
Effect on Cognitive Status
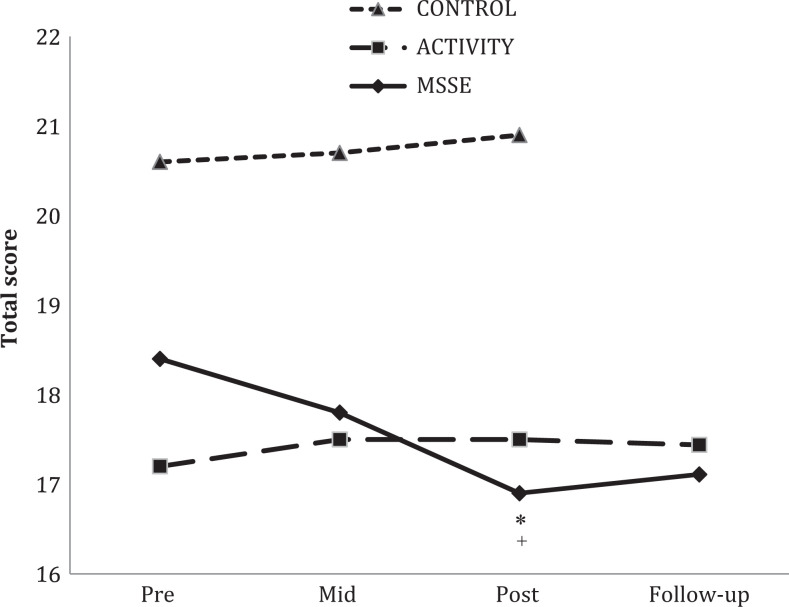
With regard to SMMSE, when comparing the MSSE group and the activity group (Figure 5), both groups displayed an improvement in their scores during intervention. Between the posttrial and the follow-up period, a significant time effect was observed, with an important decrease in the scores of both groups (F 1,15 = 7.276, P = .017, η2 = .324), with no significant differences between the 2 groups.
Figure 5.
Severe Mini-Mental State Examination (SMMSE) total scores during the trial and follow-up (lower scores = worse cognitive state). MSSE indicates multisensory stimulation environment.
When comparing the MSSE group and the control group, no significant time effects or intergroup differences were found.
Effect on Dementia Severity
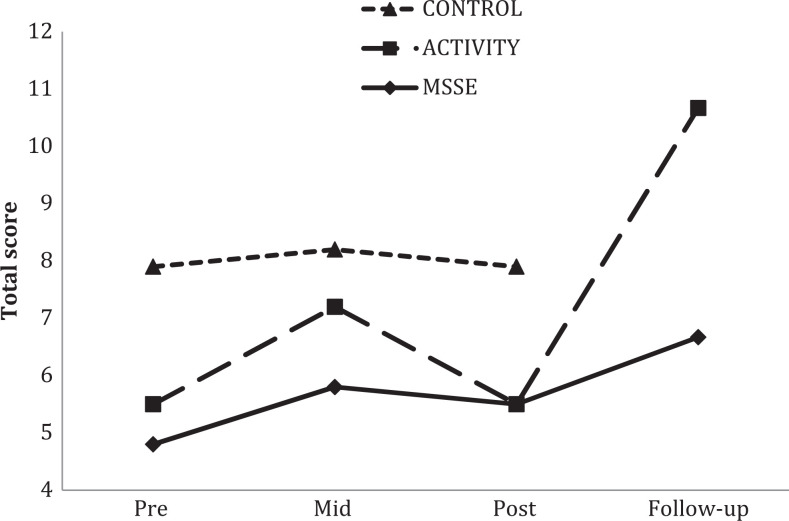
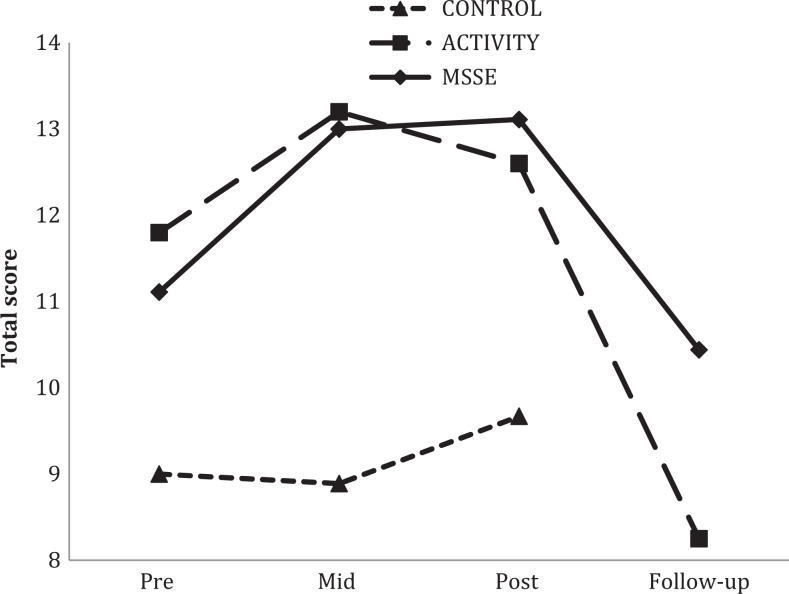
For the BANS-S (Figure 6), there was an improvement in the scores between pre-, mid-, and postintervention assessments in the MSSE group but not in the other groups. Significant interaction effects between group and time when comparing the MSSE group with the activity group (F 2,36 = 4.126, P = .024, η2 = .171) and with the control group (F 2,36 = 11.578, P < .001, η2 = .334) were observed.
Figure 6.
Bedford Alzheimer Nursing Severity Scale (BANS-S) total scores during the trial and follow-up (higher scores = more severe impairment). *Significant group–time interaction effect from pre- to posttrial (MSSE-activity; P < .005); +Significant group–time interaction effect from pre- to posttrial (MSSE-control; P < .005). MSSE indicates multisensory stimulation environment.
Discussion
Effect on Behavior
In the current study the NPI scores improved significantly after 16 weeks of intervention in the MSSE group in comparison with the individualized activities group. For interpreting the effect sizes, we have not found previous studies to compare with, so the benchmarks of Cohen (1988) 36 have been used. According to them, the effect size was large. Previous studies 10,13,17 found that both MSSE sessions as one-to-one preference-based activity interventions could have positive long-term effects (that is, the maintenance of the positive effects of the MSSE outside of the session time and place) on neuropsychiatric symptoms of older adults with moderate to severe cognitive impairment living in nursing homes. In patients with moderate cognitive impairment benefits of intervention can be attributed to the individualized attention rather a specific treatment modality. However, people in the later stages of the disease, with very low level of functioning, may benefit more from sensory interventions, like MSSE, than from more cognitive demanding activities.
As cognitive function deteriorates, the world is experienced at a sensory level and the environment needs to be managed carefully to make it understandable and comfortable. 37 Consequently, individuals with severe dementia specially need an appropriate environmental structure and stimulation, which can be achieved by sensory interventions. 38 The Guideline on Supporting People with Dementia and Their Carers in Health and Social Care developed by the National Institute for Health and Clinical Excellence and the Social Care Institute for Excellence (NICE-SCIE) 39 highlighted that in the late stages of dementia, sensory stimulation is the primary form of psychological intervention to reduce neuropsychiatric symptoms. In this sense, Baker et al 13 studied the effects of an Snoezelen intervention in comparison with a control group that followed one-to-one activity sessions and found that, in the severe cognitive range, the Snoezelen group was significantly less apathetic after 8 weeks of intervention. Indeed, there is evidence that others forms of sensory interventions, like music interventions 40 or aromatherapy with essential oils, 41 also show good results in reducing neuropsychiatric symptoms of people with severe dementia.
Regarding agitated behavior, an improvement in the verbally agitated behavior factor and in the CMAI total score in the MSSE group in comparison with the control group was found. An improvement with the intervention in both MSSE and activity groups in the aggressive behavior factor and in the CMAI total score was also observed. As stated earlier, according to the benchmarks of Cohen (1988), 36 the effect sizes were large. These results indicate that although MSSE may be an appropriate intervention to reduce agitation in advanced dementia, its efficacy is equivalent to the individualized one-to-one activities.
In this study, the improvements observed in the behavior during the intervention disappeared in the follow-up in both groups. This is consistent with the previous studies comparing the effect of MSSE and activity groups in moderated to severe dementia, 13,17 . In this regard, O’Connor et al 42,43 in a systematic review about the psychosocial treatments in people with dementia concluded that the benefits of the intervention on behavioral symptoms of dementia defrayed quickly with time. Therefore, to hope that repeated exposures to a treatment would consolidate benefits in the follow-up could be a too ambitious approach.
Effect on Mood
Although intervention in Snoezelen rooms has demonstrated to improve the mood of people with dementia at short term, long-term effects were not so evident. 16 The current results found neither benefit after 16 weeks of intervention in the CSDD scores of people with severe dementia nor significant differences between the MSSE group and the other 2 groups. In another previous study with people with moderate to severe dementia, 17 neither an improvement in the CSDD scores during the intervention, nor differences between the groups was found. In the study of Baker et al, 13 MSSE was not found to be more effective that one-to-one activities in changing the mood neither in people with moderate dementia nor in those with severe dementia.
However, with the MSSE integrated in the daily care, better results have been observed on the mood of people with dementia. In the MSSE integrated in the daily care, the intervention was carried out during morning care, when the staff were engaged with residents in activities relating to bathing, grooming, dressing, and toileting. 44 Van Weert et al, 45 observed that patients with moderate to severe dementia receiving this type of intervention demonstrated a significant improvement in their level of depression after 15 months of treatment in comparison to the control group who received the usual care. Therefore, for future work it would be interesting to investigate the effect of MSSE integrated in daily care specifically in people with severe dementia.
Effect on Cognitive Status
Previous studies in people with moderate to severe dementia 13,17 have not found significant effects of the MSSE on the cognitive level or significant differences between MSSE and one-to-one activity sessions. The present study showed an improvement in the SMMSE scores during the intervention in MSSE and activity groups, although it was not significant. However, for both groups, a significant worsening in the scores was found between the posttrial and the follow-up period. Consequently, in people with severe dementia, one-to-one attention could have a positive efficacy on the maintenance of cognitive function.
Effect on Dementia Severity
With regard to BANS-S scores, patients in MSSE group showed a significant improvement during the intervention, which was not observed in the other 2 groups. According to the benchmarks of Cohen, 36 the effect sizes were large. There are limited studies that analyze the effect of MSSE on functional status of people with dementia. In patients with moderate to severe dementia, it has also been observed that a more structured approach of multisensory stimulation 46,47 has short-term positive effects on the functional status, however there are very room on the balance in individuals with dementia after 6 weeks of intervention few data on the long-term effectiveness. Klages et al 48 found no significant effects of a Snoezelen nor significant differences compared with the control group, who received one-to-one visits by volunteers. In another previous study, 17 long-term benefits of MSSE on the functional status (Barthel index score) in people with moderate to severe dementia were not found. A possible reason for that could be that the Barthel index no assesses well the impact of the intervention in the functional status, especially in people with severe dementia. In the current study, this limitation was solved by focusing in people with severe dementia, using the BANS-S, a tool more sensitive to detect disease progression for this group of people.
These results highlight that MSSE could be an appropriate intervention in people with severe dementia, and support the MIS, according to which balancing time periods of high arousal and low arousal in people with dementia can delay their functional decline.
Limitations and Recommendations for Future Research
The sample may seem small but it should be considered the difficulty of getting more participants, especially taking into account their homogeneity and the possibility to be randomized. Therefore, future empirical studies with larger samples are needed.
The use of MSSE in a Snoezelen room requires the investment of economic resources greater than those required in other sensory therapies for people with dementia. The future research should conduct randomized control trials to examine whether the Snoezelen benefits are better than those provided by other sensory interventions, like individualized music or aromatherapy interventions.
Further, it would be interesting to compare the traditional MSSE in a Snoezelen room, which follows a nondirectional approach, with a more structured MMSE, like the functional analytic multisensory environmental therapy, 49 for people with severe dementia.
The MSSE integrated in daily care could be also an adequate intervention for people with severe dementia, because of the high frequency of neuropsychiatric symptoms during morning care in patients in the final phase of dementia. 50 Therefore, it would be interesting that future studies analyze the effect of MSSE integrated in daily care in people with severe dementia and compare it with the MSSE carried out in a Snoezelen room. 51,52
Conclusions
These results support the idea that MSSE could be more effective than one-to-one activity sessions for reducing symptoms in patients with severe dementia. In the current study, a positive effect on neuropsychiatric symptoms and dementia severity in the MMSE treatment was observed in comparison with the activity group. With regard to agitation, the improvement in the MSSE and the activity group was similar, with no significant differences between the 2 types of intervention. However, in general, the improvements found during the intervention were lost in the follow-up period, indicating that it is necessary to continue with the intervention over time to maintain the positive effects.
Future research should conduct specific studies with people in advanced stages of dementia to compare the effect of MSSE in a Snoezelen room with other types of intervention, like individualized music or functional analytic multisensory environmental therapy.
Acknowledgments
We thank the users and staff of the Gerontology Complex La Milagrosa (managed by the Association of Pensioners and Retired People (UDP) from A Coruña, Spain), without whom the study would not have been possible. We are truly grateful to Prof Roger Baker (Poole Hospital NHS Trust and Dorset HealthCare NHS Trust, Poole, United Kingdom) for providing us information to design a randomized control trial of the multisensory stimulation environment for patients with dementia.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Honig LS, Mayeux R. Natural history of Alzheimer’s disease. Aging (Milano). 2001;13(3):171–182. [DOI] [PubMed] [Google Scholar]
- 2. Kverno KS, Black BS, Blass DM, Geiger-Brown J, Rabins PV. Neuropsychiatric symptom patterns in hospice-eligible nursing home residents with advanced dementia. J Am Med Dir Assoc. 2008;9(7):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrmann N, Gauthier S. Diagnosis and treatment of dementia: 6. Management of severe Alzheimer disease. CMAJ. 2008;179(12):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vernooij-Dassen M, Vasse E, Zuidema S, Cohen-Mansfield J, Moyle W. Psychosocial interventions for dementia patients in long-term care. Int Psychogeriatr. 2010;22(7):1121–1128. [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Kelly L, Lewis-Holmes E, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry. 2014;205(6):436–442. [DOI] [PubMed] [Google Scholar]
- 7. Kverno KS, Black BS, Nolan MT, Rabins PV. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998-2008: a systematic literature review. Int Psychogeriatr. 2009;21(5):825–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moffat N, Barker P, Pinkney L. Snoezelen: An Experience for People with Dementia. Chesterfield, UK: Rompa; 1993. [Google Scholar]
- 9. Milev RV, Kellar T, McLean M, et al. Multisensory stimulation for elderly with dementia: a 24-week single-blind randomized controlled pilot study. Am J Alzheimers Dis Other Demen. 2008;23(4):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker R, Bell S, Baker E, et al. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. Br J Clin Psychol. 2001;40(pt 1):81–96. [DOI] [PubMed] [Google Scholar]
- 11. Lykkeslet E, Gjengedal E, Skrondal T, Storjord MB. Sensory stimulation - a way of creating mutual relations in dementia care. Int J Qual Stud Health Well-being. 2014;9:23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edvardsson D, Winblad B, Sandman PO. Person-centred care of people with severe Alzheimer’s disease: current status and ways forward. Lancet Neurol. 2008;7(4):362–367. [DOI] [PubMed] [Google Scholar]
- 13. Baker R, Holloway J, Holtkamp CC, et al. Effects of multi-sensory stimulation for people with dementia. J Adv Nurs. 2003;43(5):465–477. [DOI] [PubMed] [Google Scholar]
- 14. Kovach CR. Sensoristasis and imbalance in persons with dementia. J Nurs Scholarsh. 2000;32(4):379–384. [DOI] [PubMed] [Google Scholar]
- 15. Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, Silva-Smith AL. Effect of the BACE intervention on agitation of people with dementia. Gerontologist. 2004;44(6):797–806. [DOI] [PubMed] [Google Scholar]
- 16. Sánchez A, Millán-Calenti JC, Lorenzo-López L, Maseda A. Multisensory stimulation for people with dementia: a review of the literature. Am J Alzheimers Dis Other Demen. 2013:28(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maseda A, Sánchez A, Marante MP, et al. Effects of multisensory stimulation in a sample of institutionalized elderly people with dementia diagnosis: a controlled longitudinal trial. Am J Alzheimers Dis Other Demen. 2014;29(5):463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maseda A, Sánchez A, Marante MP, et al. Multisensory stimulation on mood, behavior, and biomedical parameters in people with dementia: is it more effective than conventional one-to-one stimulation? Am J Alzheimers Dis Other Demen. 2014;29(7):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reisberg B, Ferris SH, de León MJ, Crook T. Global Deterioration Scale (GDS). Psychofarmacol Bull. 1988;24(4):661–663. [PubMed] [Google Scholar]
- 20. Baker R, Bell S, Assey J, et al. A Randomized Controlled Trial of the Snoezelen Multi-Sensory Environment for Patients with Dementia. Dorset, United Kingdom: Research and Development Support Unit, Poole Hospital; 1998. [Google Scholar]
- 21. Pace GM, Ivancic MT, Edwards GL, Iwata BA, Page TJ. Assessment of stimulus preference and reinforcer value with profoundly retarded individuals. J Appl Behav Anal. 1985;18(3):249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cervilla JA, Rodríguez Cano T, Gurpegui M. Prevalencia de Conductas Agitadas en Ancianos. An Psiquiatr. 1995;11(suppl I):5–6. [Google Scholar]
- 23. Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol A Biol. 1989;44(3):M77–M84. [DOI] [PubMed] [Google Scholar]
- 24. Finkel SI, Lyons JS, Anderson RL. Reliability and validity of the Cohen–Mansfield agitation inventory in institutionalized elderly. Int J Geriatr Psychiatry. 1992;7(7):487–490. [Google Scholar]
- 25. Vilalta-Franch J, Lozano-Gallego M, Hernández-Ferrándiz M, Llinàs-Reglà J, López-Pousa S, López OL. Neuropsychiatric inventory. Propiedades psicométricas de su adaptación al español [Neuropsychiatric inventory. The psychometric properties of its adaptation to Spanish]. Revista Neurológica. 1999;29(1):15–19. [PubMed] [Google Scholar]
- 26. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2214. [DOI] [PubMed] [Google Scholar]
- 27. Pujol-Doménech J, de Azpiazu P, Salamero M, Cuevas R. Sintomatología depresiva de la demencia. Escala de Cornell: validación de la versión en castellano. Rev Neurol. 2001;33(4):397–398. [PubMed] [Google Scholar]
- 28. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depresion in Dementia. Biol Psychiatry. 1988;23(3):271–284. [DOI] [PubMed] [Google Scholar]
- 29. Buiza C, Navarro A, Díaz-Orueta U, et al. Evaluación breve del estado cognitivo de la demencia en estadios avanzados: resultados preliminares de la validación española del Severe Mini Mental State Examination. Rev Esp Geriatr Gerontol. 2011;46(3):131–138. [DOI] [PubMed] [Google Scholar]
- 30. Harrell LE, Marson D, Chatterjee A, Parrish JA. The Severe Mini-Mental State Examination: A new neuropsychologic instrument for the bedside assessment of severely impaired patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(3):168–175. [DOI] [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. Mini-Mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 32. Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49(5):M223–M226. [DOI] [PubMed] [Google Scholar]
- 33. Volicer L, Hurley AC, Blasi ZV. Scales for evaluation of End-of-Life Care in Dementia. Alzheimer Dis Assoc Disord. 2001;15(4):194–200. [DOI] [PubMed] [Google Scholar]
- 34. Bellelli G, Frisoni GB, Bianchetti A, Trabucchi M. The Bedford Alzheimer nursing severity scale for the severely demented: validation study. Alzheimer Dis Assoc Disord. 1997;11(2):71–77. [DOI] [PubMed] [Google Scholar]
- 35. Razali N, Wah YB. Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J Stat Model Anal. 2012;2(1):21–33. [Google Scholar]
- 36. Cohen J. Statistical Power Analysis for the Behavioural Sciences. (2nd ed.). Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 37. Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas. 2014;77(4):305–310. [DOI] [PubMed] [Google Scholar]
- 38. Tariot PN. Medical management of advanced dementia. J Am Geriatr Soc. 2003;51(5 suppl dementia):S305–S313. [DOI] [PubMed] [Google Scholar]
- 39. National Collaborating Centre for Mental Health (UK). Dementia: A NICE–SCIE Guideline on Supporting People with Dementia and Their Carers in Health and Social Care - National Clinical Guidelines N. 42. Leicester, England: British Psychological Society; 2007. [PubMed] [Google Scholar]
- 40. Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr. 2013;25(5):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry. 2002;63(7):553–558. [DOI] [PubMed] [Google Scholar]
- 42. O’Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of behavior symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr. 2009;21(2):225–240. [DOI] [PubMed] [Google Scholar]
- 43. O’Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of psychological symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr. 2009;21(2):241–251. [DOI] [PubMed] [Google Scholar]
- 44. van Weert JC, van Dulmen AM, Spreeuwenberg PM, Ribbe MW, Bensing JM. Effects of snoezelen, integrated in 24 h dementian care, on nurse-patient communication during morning care. Patient Educ Couns. 2005;58(3):312–326. [DOI] [PubMed] [Google Scholar]
- 45. van Weert JC, van Dulmen AM, Spreeuwenberg PM, Ribbe MW, Bensing JM. Behavioral and mood effects of snoezelen integrated into 24-hour dementia care. J Am Geriatr Soc. 2005;53(1):24–33. [DOI] [PubMed] [Google Scholar]
- 46. Collier L, McPherson K, Ellis-Hill C, Staal J, Bucks R. Multisensory stimulation to improve functional performance in moderate to severe dementia–interim results. Am J Alzheimers Dis Other Demen. 2010;25(8):698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Staal JA, Sacks A, Matheis R, et al. The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med. 2007;37(4):357–370. [DOI] [PubMed] [Google Scholar]
- 48. Klages K, Zecevic A, Orange JB, Hobson S. Potential of Snoezelen room multisensory stimulation to improve balance in individuals with dementia: a feasibility randomized controlled trial. Clin Rehabil. 2011;25(7):607–616. [DOI] [PubMed] [Google Scholar]
- 49. Staal JA. Functional analytic multisensory environmental therapy for people with dementia. Int J Alzheimers Dis. 2012;2012:294801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koopmans RT, van der Molen M, Raats M, Ettema TP. Neuropsychiatric symptoms and quality of life in patients in the final phase of dementia. Int J Geriatr Psychiatry. 2009;24(1):25–32. [DOI] [PubMed] [Google Scholar]
- 51. Cruz J, Marques A, Barbosa AL, Figueiredo D, Sousa L. Effects of a motor and multisensory-based approach on residents with moderate-to-severe dementia. Am J Alzheimers Dis Other Demen. 2011;26(4):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marques A, Cruz J, Barbosa A, Figueiredo D, Sousa LX. Motor and multisensory care-based approach in dementia: long-term effects of a pilot study. Am J Alzheimers Dis Other Demen. 2013;28(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]