Abstract
Objectives:
Urinary incontinence (UI) is more prevalent in the elderly populations with dementia than without dementia, and Alzheimer’s disease (AD) is the most common cause of dementia. Urinary incontinence may complicate AD morbidity and mortality. Therefore, this study aimed to evaluate the prevalence and annual incidence and determine the risk possibility of UI, which is the main type of incontinence in patients with AD in Taiwan.
Methods:
A total of 933 patients with AD were included in the study cohort, and a total of 2799 patients without AD by 1:3 proportion compared to the study cohort were used as a matched cohort. All participants were selected from the National Health Insurance Research Database in 2000 sample population. We utilize Cox proportional hazard regression to evaluate the risk of UI and cumulative incidence ratio curve to analyze the cumulative incidence function. Prevalence and annual incidence rate are calculated in individual medication including rivastigmine, donepezil, galantamine, and memantine only being initiated in patients with AD.
Results:
The risk of UI is higher in AD cohort (hazard ratio: 1.54, 95% confidence interval: 1.13-2.09). The cumulative incidence ratio of UI event between AD cohort and matched cohort presents statistical significance (P < .001). Annual incidence and prevalence of UI in patients with AD are 6.2% and 4.2%, respectively.
Conclusion:
The present results suggest that the risk of UI is higher in patients with AD than in the general population.
Keywords: Alzheimer’s disease, cholinesterase inhibitors, dementia, urge incontinence, urinary incontinence
Introduction
With the increasing aging population, age-related diseases have become more important. Dementia, common in the elderly society, is a disease of mental decline, and it impairs the cognitive ability to deal with daily life. 1 Previous data indicate more than 35 million people worldwide live with dementia, and the number will be expected to double by 2030. 2 Alzheimer’s disease (AD) is the most common cause of dementia with approximately 60% to 70% of all cases of dementia in Taiwan, and the prevalence is increasing with age. 3 Therefore, AD will cause large economic burden about caring. 4 Urinary incontinence (UI) means any involuntary leakage of urine defined by the International Continence Society, which is also common in the elderly population. 5 According to previous studies, UI is more prevalent in the elderly population with dementia than without dementia. 6,7 Although UI is a crucial symptom and common concomitant in patients with AD, study about the association between UI and AD is rare because of the difficult assessment of UI in patients with severe cognitive and physical deterioration. Several studies have indicated that cerebral dysfunction will impact the brain by interfering with the inhibitory function of the micturition reflex, resulting in involuntary detrusor contraction. 7 Actually, UI impacts the quality of life, social activity, and person hygiene and may cause severe complications such as falling down when going to the toilet frequently. 8 The causes of UI in patients with AD may stem from comorbidities, cognitive and functional impairment of neurodegenerative processes, or be medication related. In addition, treatment of AD with cholinesterase inhibitors (ChEIs) by increasing acetylcholine levels in the brain may also increase the incidence of UI. 9 Urinary incontinence is comprised of stress incontinence, urge incontinence, overflow incontinence, and functional incontinence. 10 In order to clarify the actual association of UI and AD precisely, the present study specifically analyzed the prevalence of UI in patients with AD and the ChEI effect about UI separately by adjusting other comorbidities in Taiwan with a population-based cohort study. The purpose was to report the status of UI in patients with AD in relation to the use of ChEIs to provide references for further management and care.
Methods
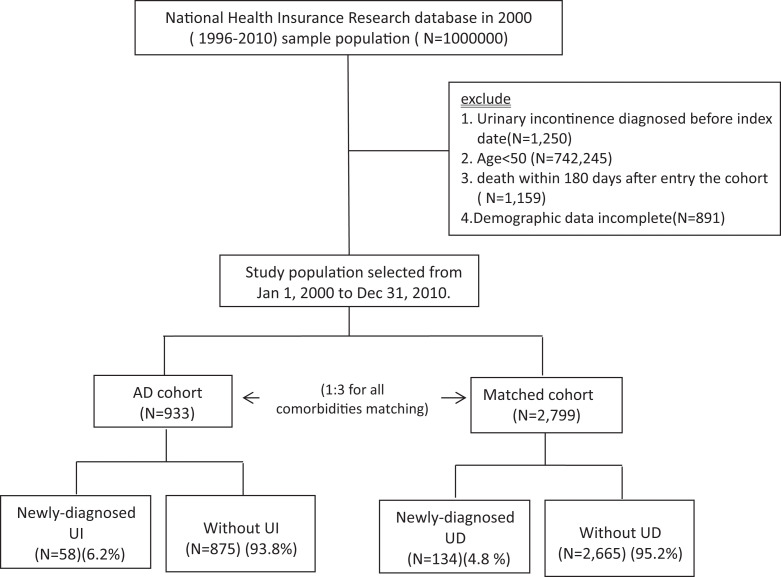
Data for this study were obtained from the National Health Insurance Research Database in 2000 (1996-2010) sample population (N = 1,000,000), which is a large database network designed in 1995 to provide health care for all residents of Taiwan. The study population was selected from those taking rivastigmine, donepezil, galantamine, and memantine from January 1, 2000, to December 31, 2010, longitudinally tracing the use of medical services and excluding UI (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 625.6, 788.30-788.34, and 788.39) diagnosed before January 1, 2000, patients younger than 50 years, death within 180 days after entry into the cohort, and incomplete demographic data. We then choose matched cohort with 1:3 population for all comorbidities matching to AD groups (Figure 1). Independent t test, χ 2 test, or Fisher exact test were used to estimate the distribution of risk factors between the AD and matched cohorts. For considering different mortality rate after index date, Cox proportional hazard regression was conducted to calculate the adjusted hazard ratio (HR) for the risk of UI after being adjusted for age, gender, diabetes, hypertension, chronic kidney disease, and urinary tract infection. Fine-Gray regression was used for evaluating the competing risk end points and Cox proportional hazards model for the composite end point. Cumulative incidence ratio curve of UI events was analyzed by the cumulative incidence function. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Inc, Cary, North Carolina). Statistical significance was set at P < .05.
Figure 1.
Flowchart of study selection.
Results
A total of 933 patients with AD (569 women and 364 men) were included, with a mean age of 75.22 ± 7.72 years. The average AD follow-up duration is 4.26 ± 2.54 years. Table 1 shows the basic characteristics between AD cohort and matched cohort without AD. Age was categorized as 50 to 59, 60 to 69, and ≥70 years in the 2 cohorts with no statistical difference. Over 60% were female, and 80% of the participants were aged ≥70. The gender and comorbidities distribution including diabetes mellitus, hypertension, chronic kidney disease, and urinary tract infection were also similar between them. In the AD cohort, the ratio of individual ChEIs therapy including rivastigmine, donepezil, galantamine, and memantine was 41.5%, 56.3%, 11.3%, and 7.6%, respectively. Compared with the matched cohort, urge incontinence exhibited a higher cumulative annual incidence in patients with AD (P < .001; Figure 2). After adjusting age, gender, diabetes mellitus, hypertension, chronic kidney disease, and urinary tract infection, urge incontinence had a higher risk in patients with AD compared to matched cohorts, as in Table 2 (HR: 1.54, 95% confidence interval [95% CI]: 1.13-2.09). Annual incidence and prevalence of UI in patients with AD were 6.2% and 4.2%, respectively. Annual incidence and prevalence also varied with different drugs prescribed only for patients with AD—rivastigmine (7.8%, 3.4%), donepezil (5.8%, 4.8%), galantamine (2.9%, 5.6%), and memantine (1.4%, 4.4%; Table 3).
Table 1.
Basic Characteristics Between AD Cohort and Matched Cohort.a
| Variables | AD Cohort (n = 933), n (%) | Matched Cohort (n = 2799), n (%) | P Value |
|---|---|---|---|
| Age | |||
| 50-59 | 51 (5.5) | 153 (5.5) | >.999 |
| 60-69 | 158 (16.9) | 475 (17.0) | |
| ≥70 | 724 (77.6) | 2171 (77.6) | |
| Mean ± SD | 75.22 ± 7.72 | 74.92 ± 7.83 | |
| Gender | |||
| Female | 569 (61.0) | 1721 (61.5) | .786 |
| Male | 364 (39.0) | 1078 (38.5) | |
| Comorbidities | |||
| Diabetes mellitus | 274 (29.4) | 820 (29.3) | .967 |
| Hypertension | 597 (64.0) | 1795 (64.1) | .937 |
| Chronic kidney disease | 50 (5.4) | 126 (4.5) | .285 |
| Urinary tract infection | 282 (30.2) | 837 (29.9) | .853 |
| Acetylcholinesterase inhibitors | |||
| Rivastigmine | 387 (41.5) | ||
| Donepezil | 525 (56.3) | ||
| Galantamine | 105 (11.3) | ||
| Memantine | 71 (7.6) | ||
Abbreviations: AD, Alzheimer’s disease; SD, standard deviation.
aN = 3732.
Figure 2.
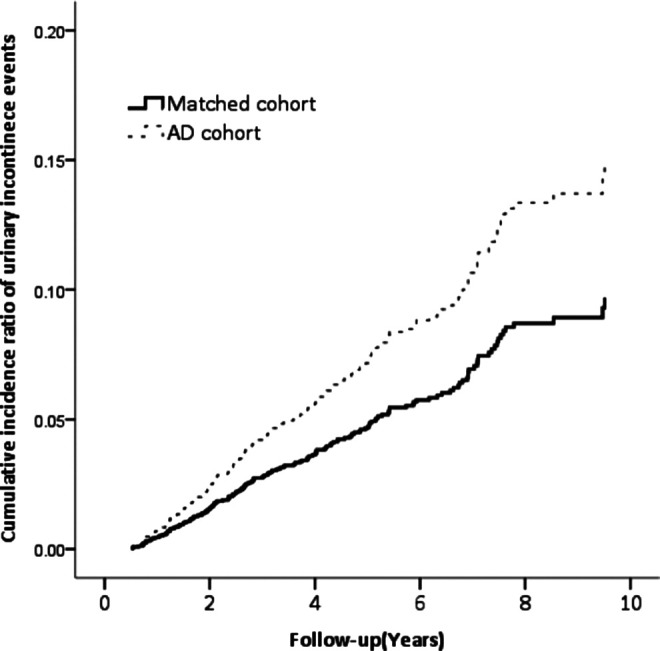
Cumulative incidence ratio curve of urge incontinence.
Table 2.
The Risk of Urinary Incontinence Between AD Cohort and Matched Cohort.a,b
| Number of Cases | Per 1000, Person Year | aHR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Matched cohort | 134 | 9.61 | Ref | 1.13-2.09 | <.001 |
| AD cohort | 58 | 14.61 | 1.54 |
Abbreviations: AD, Alzheimer’s disease; aHR, adjusted hazard ratio; CI, confidence interval; Ref, reference.
aN = 3732.
bAdjusted for age, gender, diabetes, hypertension, chronic kidney disease, and urinary tract infection.
Table 3.
Annual Incidence and Prevalence of Urinary Incontinence in Patients With AD.
| Annual incidence of urinary incontinence in AD | |
| All patients with AD | 6.2% |
| Rivastigmine | 7.8% |
| Donepezil | 5.8% |
| Galantamine | 2.9% |
| Memantine | 1.4% |
| Urinary incontinence prevalence in AD | |
| All patients with AD | 4.2% |
| Rivastigmine | 3.4% |
| Donepezil | 4.8% |
| Galantamine | 5.6% |
| Memantine | 4.4% |
Abbreviation: AD, Alzheimer’s disease.
Discussion
Both UI and AD are common in the elderly society, but the relationship between these 2 diseases as mentioned before is even rare. However, UI in patients with AD will aggravate the risk of adverse effect combining cognitive and physical deterioration. In this study, the prevalence of UI was 4.2% in 933 patients with AD. Compared to the previous studies, the prevalence of UI composed of all kinds of incontinence in dementia ranged from 11% to 90%. 11 -13 The diversity possibly derives from different inclusion criteria of patients and definitions used in the estimation. In order to increase the accuracy and precision, we included patients having UI encoding from the National Health Insurance Research Database of ICD-9. Meanwhile, we specified the exact patients with AD by choosing those taking 1 of the 4 ChEIs, including rivastigmine, donepezil, galantamine, and memantine. Physicians in Taiwan have to strictly follow guidelines, allowing them to prescribe ChEIs only to patients with AD. Therefore, we collected the data from patients with pure AD and used the diagnostic criteria of AD according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition [DSM-IV]) and have the brain neuroimaging and referring to a series of neuropsychological assessments that have been asked by the National Health Insurance. Because of our national insurance policy, only patients with pure AD, not mixed type and vascular dementia, based on DSM-IV criteria can be prescribed with these 4 drugs. We have also reported the rule of treatment of AD in Taiwan elsewhere. 14
In our study, we found a higher risk of UI in patients with AD than in the general population. However, the pathophysiological mechanism for UI in AD has not been well known but is presumed to be multifactorial. In an objective view, detrusor overactivity (DO) identified by urodynamic study was considered as the principle cause of UI in dementia. 7 Due to the injury of the anteromedial frontal cortex, which is thought to be the center of micturition reflex in patients with AD, DO was induced by loss of inhibitory function. 15 From functional image study, prefrontal cortex was activated when the bladder had strong sensation to void and was proposed to be involved in bladder storage function. Therefore, patients will have symptoms of frequency, urgency, and urge incontinence. 16 In a study by Takahashi et al, prefrontal cortex played an important role in lower urinary tract symptoms found in white matter lesions or patients with AD. 17 On the other hand, cognitive and physical ability impairment in patients with AD will increase the UI risk because it is difficult to recognize when or where to void, uncoordinated movement, and mobility and ability to transfer to reach the toilet. Moreover, patients with AD have higher opportunity to have comorbidities and prescribe medications that induce urge incontinence.
Because aging will impact bladder and pelvic floor function, UI is common in the elderly society. 18 In order to exclude the age effect, the age distribution between AD and matched cohorts is not statistically different. We thought not only age but also other factors including cognitive, functional dysfunction, and drug-induced effects were better associated with UI in patients with AD.
In order to reduce the bias of comorbidities, we adjusted for diseases including diabetes mellitus, hypertension, chronic kidney disease, and urinary tract infection, which may cause UI to strength reliability. In AD, treatment of ChEIs is to reverse a deficiency in cholinergic neurotransmission in the brain, which results in cognitive and physical disability. 19 Since muscarinic receptors have a main role in bladder detrusor contraction, ChEIs may affect bladder storage function and lead to or worsen urge incontinence. Therefore, the use of ChEIs was associated with an increased risk of UI when we were managing AD.20 20,21 We analyzed the annual incidence and prevalence of UI in 4 kinds of drugs separately. In our results, galantamine was discovered with the most prevalence compared to other drugs. It is supposed that galantamine has more actions including acetylcholine esterase inhibitor and nicotine receptor enhancement compared to other drugs. 22 Because both muscarinic and nicotinic acetylcholine receptors are found in urothelium, which influence bladder function, galantamine increases the impact of interaction with receptors. 23,24 Clinicians should advise patients of the possible adverse effect of ChEIs and also anticholinergic agents may cause cognitive impairment. Patients with AD have higher opportunity to take concomitant ChEIs and anticholinergic agents. This result is consistent with previous studies where rivastigmine, donepezil, galantamine, and memantine may increase the risk of UI. 9,20,25 However, some previous studies including animal model research concluded that drugs used for treating patients with AD may not impact lower urinary tract function. 26,27 Therefore, the higher risk of UI in patients with AD induced from disease itself or medication needs further evaluation.
There are some limitations in our study. First, the elderly population are prone to take various medications, which may aggravate voiding dysfunction. However, we did not adjust for other possible drugs. Second, this is a retrospective population-based study, so we did not have objective data including questionnaire, dementia rating scale such as Mini-Mental State Examination (MMSE), and urodynamic study. The MMSE is not necessary for the diagnosis of AD, as it was diagnosed by the DSM-IV criteria. Patients with AD often present cognitive and physical impairment; therefore, they may be unable to express real thoughts or accomplish urodynamic study, which requires cognitively intact patients to obey directions and communicate with an investigator. Because patients with AD are less likely to undergo complex urodynamic study, the accuracy of the results is considered as in doubt. Third, the data did not provide information on differential diagnosis or stage and severity of AD, so we cannot consider its influence to the frequency of urge incontinence. However, the strength of this study lies in its longitudinal database and large population size. In addition, we used more stringent criteria to choose 4 definitive drugs only initiated in patients with clinically confirmed AD. This is the first study to recognize the prevalence and annual incidence of UI. These data can provide information to clinicians. In a recent study, overactive bladder with urge incontinence under medicine treatment had more economic benefit than without treatment in vulnerable elderly patients. 28 In conclusion, the present study shows higher risk of UI in patients with AD compared with the general population. Alzheimer’s disease is a progressive neurological disorder leading to difficult moving and cognitive dysfunction, so UI in patients with AD will aggravate medical problems. In order to decrease the impact of health and economic burden, treatment of UI is an important issue, especially in patients with AD.
Acknowledgments
All authors are very grateful for the cooperation and interest of all authors.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Nowrangi MA, Rao V, Lyketsos CG. Epidemiology, assessment, and treatment of dementia. Psychiatr Clin North Am. 2011;34(2):275–294. [DOI] [PubMed] [Google Scholar]
- 2. Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3. Fuh JL, Wang SJ. Dementia in Taiwan: past, present, and future. Acta Neurol Taiwan. 2008;17(3):153–161. [PubMed] [Google Scholar]
- 4. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. [DOI] [PubMed] [Google Scholar]
- 6. Ouslander JG, Zarit SH, Orr NK, Muira SA. Incontinence among elderly community-dwelling dementia patients. Characteristics, management, and impact on caregivers. J Am Geriatr Soc. 1990;38(4):440–445. [DOI] [PubMed] [Google Scholar]
- 7. Skelly J, Flint AJ. Urinary incontinence associated with dementia. J Am Geriatr Soc. 1995;43(3):286–294. [DOI] [PubMed] [Google Scholar]
- 8. Alcorn G, Law E, Connelly PJ, Starr JM. Urinary incontinence in people with Alzheimer’s disease. Int J Geriatr Psychiatry. 2014;29(1):107–109. [DOI] [PubMed] [Google Scholar]
- 9. Kröger E, Van Marum R, Souverein P, Carmichael PH, Egberts T. Treatment with rivastigmine or galantamine and risk of urinary incontinence: results from a Dutch database study. Pharmacoepidemiol Drug Saf. 2015;24(3):276–285. [DOI] [PubMed] [Google Scholar]
- 10. Na HR, Park MH, Cho ST, et al. Urinary incontinence in Alzheimer’s disease is associated with Clinical Dementia Rating-Sum of boxes and Barthel Activities of Daily Living. Asia Pac Psychiatry. 2015;7(1):113–120. [DOI] [PubMed] [Google Scholar]
- 11. Teasdale TA, Taffet GE, Luchi RJ, Adam E. Urinary incontinence in a community-residing elderly population. J Am Geriatr Soc. 1988;36(7):600–606. [DOI] [PubMed] [Google Scholar]
- 12. Yap P, Tan D. Urinary incontinence in dementia—a practical approach. Aust Fam Physician. 2006;35(4):237–241. [PubMed] [Google Scholar]
- 13. Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Dementia and lower urinary dysfunction: with a reference to anticholinergic use in elderly population. Int J Urol. 2008;15(9):778–788. [DOI] [PubMed] [Google Scholar]
- 14. Yuanhan Yang. Dementia in Taiwan area. Transl Neurosci Clin. 2016;2(1):38–45. [Google Scholar]
- 15. Kavia RBC, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493(1):27–32. [DOI] [PubMed] [Google Scholar]
- 16. Gao Y, Liao L, Blok BF. A resting-state functional MRI study on central control of storage: brain response provoked by strong desire to void. Int Urol Nephrol. 2015;47(6):927–935. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi O, Sakakibara R, Panicker J. White matter lesions or Alzheimer’s disease: which contributes more to overactive bladder and incontinence in elderly adults with dementia? J Am Geriatr Soc. 2012;60(12):2370–2371. [DOI] [PubMed] [Google Scholar]
- 18. Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136(5):1022–1025. [PubMed] [Google Scholar]
- 19. Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–813. [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto M, Imamura T, Tanimukai S, Kazui H, Mori E. Urinary incontinence: an unrecognised adverse effect with donepezil. Lancet. 2000;356(9229):568. [DOI] [PubMed] [Google Scholar]
- 21. Hemingway-Eltomey JM, Lerner AJ. Adverse effects of donepezil in treating Alzheimer’s disease associated with Down’s syndrome. Am J Psychiatry. 1999;156(9):1470. [DOI] [PubMed] [Google Scholar]
- 22. Nakano Y, Matsuzono K, Yamashita T, et al. Long-term efficacy of galantamine in Alzheimer’s disease: the Okayama Galantamine Study (OGS). J Alzheimers Dis. 2015;47(3):609–617. [DOI] [PubMed] [Google Scholar]
- 23. Takata K, Kitamura Y, Saeki M, et al. Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem. 2010;285(51):40180–40191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290(1):F103–F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossom R, Adityanjee Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother. 2004;2(4):303–312. [DOI] [PubMed] [Google Scholar]
- 26. Sakakibara R, Uchiyama T, Yoshiyama M, Yamanishi T, Hattori T. Preliminary communication: urodynamic assessment of donepezil hydrochloride in patients with Alzheimer’s disease. Neurourol Urodyn. 2005;24(3):273–275. [DOI] [PubMed] [Google Scholar]
- 27. Ozkürkçügil C, Kömür O, Ozkan L. Effect of memantine on overactive detrusor in rats with spinal cord injury. Kaohsiung J Med Sci. 2010;26(5):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin L, Luo X, Zou KH, Snedecor SJ. Economic impact of using fesoterodine for the treatment of overactive bladder with urge urinary incontinence in a vulnerable elderly population in the United States. J Med Econ. 2016;19(3):229–235. [DOI] [PubMed] [Google Scholar]