Abstract
Although some studies point to cognitive stimulation as a beneficial therapy for older adults with cognitive impairments, this area of research and practice is still lacking dissemination and is underrepresented in many countries. Moreover, the comparative effects of different intervention durations remain to be established and, besides cognitive effects, pragmatic parameters, such as cost-effectiveness and experiential relevance to participants, are seldom explored. In this work, we present a randomized controlled wait-list trial evaluating 2 different intervention durations (standard = 17 vs brief = 11 sessions) of a cognitive stimulation program developed for older adults with cognitive impairments with or without dementia. 20 participants were randomly assigned to the standard duration intervention program (17 sessions, 1.5 months) or to a wait-list group. At postintervention of the standard intervention group, the wait-list group crossed over to receive the brief intervention program (11 sessions, 1 month). Changes in neuropsychological, functionality, quality of life, and caregiver outcomes were evaluated. Experience during intervention and costs and feasibility were also evaluated. The current cognitive stimulation programs (ie, standard and brief) showed high values of experiential relevance for both intervention durations. High adherence, completion rates, and reasonable costs were found for both formats. Further studies are needed to definitively establish the potential efficacy, optimal duration, cost-effectiveness, and experiential relevance for participants of cognitive intervention approaches.
Keywords: cognitive intervention, cognitive stimulation, dementia, mild cognitive impairment (MCI)
Introduction
Cognitive impairment in older adults has a considerable prevalence, whether present with or without dementia (eg, approximately 10% for cognitive impairment without dementia 1 and between 5% to 7% for dementia in people with 60 years and older 2 ).
In Portugal, the prevalence of cognitive impairment without dementia in a sample of 55- to 79-year-old patients is estimated to be 12.3% and dementia-related cognitive impairment is 2.7%. 3 In other countries, such as the United States, prevalence is higher with 11% of people aged 65 and older having Alzheimer’s disease (AD). 4
In addition to the significant prevalence, age-related cognitive disorders represent a considerable and increasing burden not only to the affected individuals but the society as a whole as well. For example, estimates show dementia contributing with 11.2% of the years lived with disability in people aged 60 years and older. 4 Furthermore, cognitive deficits in dementia, via their effect on the patients’ capacity for activities of daily living, are associated with patient care costs. 5
Moreover, age-related cognitive disorders are considered to be a growing societal problem. For example, in the United States, between the years 2000 and 2010, the proportion of deaths resulting from Alzheimer’s-type dementia has increased 68% (with a reverse tendency observed in heart disease, stroke, and prostate cancer, which decreased 16%, 23%, and 8%, respectively). 4
Despite the unavoidable progressive nature of the neurodegenerative disorders, pharmacological and psychosocial approaches have been developed in hopes of finding an effective treatment. Pharmacological therapies, used for mild cognitive impairment (MCI) and dementia, show moderate benefits for cognition and quality of life throughout the disease for individuals with dementia. 4 However, since currently there are no disease-modifying drugs, complementary psychosocial approaches, namely, cognitive interventions, have been used for complementing pharmacotherapy.
According to Clare and Woods, 6 the diversity of cognitive interventions has been theoretically grouped in 3 main approaches: cognitive training (computer-based or paper-and-pencil cognitive exercises), cognitive stimulation (cognitive and social group activities), and cognitive rehabilitation (individualized interventions tackling patients key difficulties and goals).
Positive effects of cognitive intervention in normal aging, MCI, and dementia (namely AD) have been reported. 7,8 However, many issues still remain to be clarified. Namely, as Alves and colleagues 8 have mentioned, dosage parameters are yet to be identified and costs and feasibility data are seldom reported. Furthermore, the experience of participants attending cognitive interventions is rarely evaluated. This is an issue of utmost relevance since it is plausible for a study to show significant group effects, in a given intervention, and yet not be clinically significant or not bring any experiential value (eg, engagement, generalization to real life, and utility) to the patient. These considerations are especially relevant during psychosocial therapies since they can lead an intervention being perceived as inadequate or compromising adherence and efficacy. Similarly, when an intervention does not show statistical significance, it might still bring about observable and subjective experiential benefits (eg, engagement in task, enjoyment about activities at hand, and promoting relation with others).
Keeping in mind the importance of developing naturalistic interventions, which are easy to apply, disseminate (eg training of the staff), and made adaptable to the participants’ background and cognitive stage, we developed a cognitive stimulation program adapted to the Portuguese context based on the study by Clare and Woods. 6
To the best of our knowledge, this is the very first study analyzing the effects of a cognitive stimulation program, specifically developed for Portuguese older adults and comparing 2 different durations (dosage study) of cognitive stimulation intervention while exploring its experiential relevance for participants. Cost and feasibility data (completion and adherence) are presented as well.
Methods
Participants
Participants were selected from a day care and long-term older adult care center located in Northern Portugal. Individuals with evidence of cognitive decline (scores defined by a Global Deterioration Scale (GDS) 9 equal or higher than 3 and up to 5) were recruited for this study. All participants, except for 1, kept stable doses of medications during project participation. This participant (from the brief intervention group) required psychoactive medication adjustment (due to behavioral disturbances). One participant (from the standard intervention group) was taking antidementia drugs (an acetylcholinesterase inhibitor—rivastigmine—and a N-methyl-D-aspartate receptor antagonist—memantine).
Participants were included if they (1) showed cognitive decline ranging from MCI to mild-to-moderate dementia (as assessed according to compatible scores of the GDS—scores between 3 and 5); (2) had some ability to understand and communicate in response to, at least, brief sentences; (3) had sufficient hearing and sight; (4) did not show current psychotic symptoms or existing symptoms were under control (ie, no visual or auditory hallucinations); (5) did not show severe mood disorders or disorders were under control (as assessed through a score lower than 21 in the Geriatric Depression Scale 10 ); (6) no current substance abuse; (7) no current major disruptive or aggressive behavior; and (8) no physical disabilities limiting the participation in the study.
Study Design
The present study was a randomized controlled trial. University of Minho Ethics Committee institutional review board and the local elderly live-in and day care center approved this study. Informed consent was obtained from all participants and, when necessary, from family members.
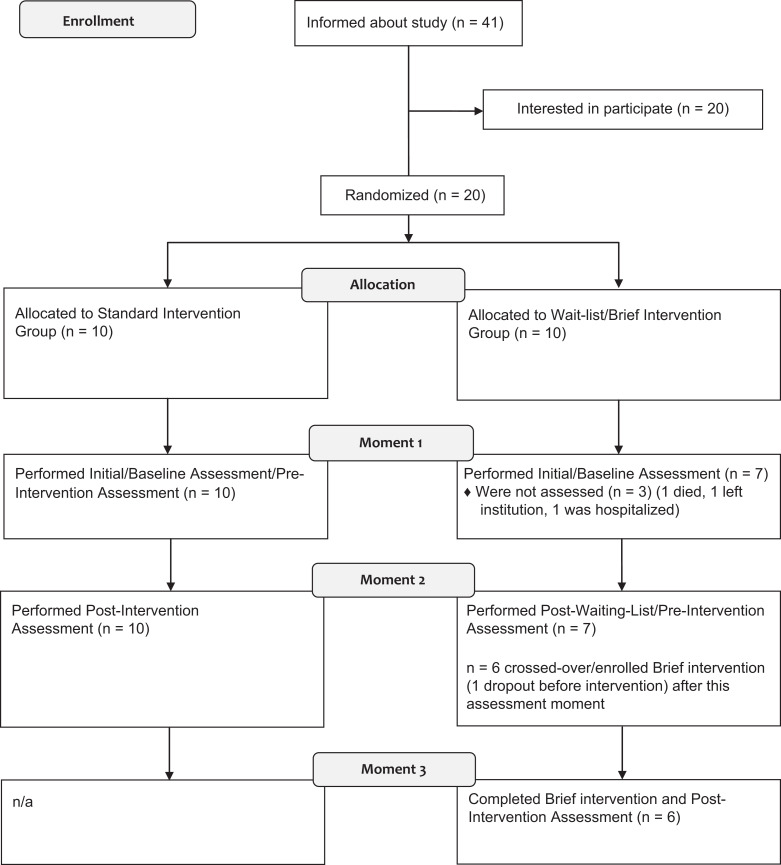
Of 41 elderly members from the center, 20 were interested in participating and met the inclusion criteria. After initial contact, utilizing a computer software, participants were randomly allocated (Random Allocation Software) 11 and distributed into 1 of the following 2 groups (10 participants each): the standard intervention group or the wait-list/brief intervention group. The researcher performing software randomization was blinded to patient’s identity, characteristics, and assessment data. After allocation, due to various reasons (ie died, left the institution, and was hospitalized) 3 of the participants were unable to continue their participation, resulting in 17 participants entering the following moments.
After randomization and allocation, the study was composed of the following moments: moment 1 (M1), moment 2 (M2), and moment 3 (M3; please refer to Figure 1 for further details). At M1, an initial assessment was conducted with the standard intervention and wait-list groups. The standard intervention group (n = 10) was enrolled in the 17-session standard cognitive stimulation program (1.5-month duration) while the wait-list group (n = 7) received no intervention (except for the usual institutional care, namely, daily routines and medications, if applicable). At M2, the standard intervention group received a postintervention assessment, terminating their participation in the study, and the wait-list group received a postwait-list assessment (this assessment would also be used as the prebrief intervention assessment). Next, the wait-list group crossed over to receive the brief intervention. From the 7 participants in the wait-list group, 1 person was not interested in continuing participation. The wait-list group, now with 6 participants, received the brief intervention program (11 sessions, 1 month) and had a postintervention assessment afterward (M3).
Figure 1.
CONSORT flow diagram of progression of participants through the study.
Researchers, directly involved in assessments and interventions, were not blind to the patient groups. However, the remaining research team was only aware of the identity of the groups after the data collection phase was complete. Participants were blind in respect to the received intervention (standard vs brief).
Interventions
Standard cognitive stimulation program
Primarily based on the cognitive stimulation approach, as defined by Clare and Woods, 6 we developed a cognitive stimulation program for Portuguese older adults adapted to their social, cultural, cognitive, and economical background.
Our objectives were to develop an experientially relevant intervention, improve cognitive functioning, promote social interaction and engagement, and improve participants’ quality of life.
Sessions were delivered by 1 psychologist and 2 therapeutic assistants (3 group guides) trained in the intervention program. All sessions were manualized in order to improve treatment standardization.
The program consisted of 17 sessions, of 1 hour each, delivered for 1.5 months (3 sessions per week; 2 sessions in the final week). Each session had 2 difficulty levels that could be alternatively used depending on the participants’ cognitive status.
Brief cognitive stimulation program
As stated previously, at M2 the wait-list group was enrolled in a briefer version of the cognitive intervention program described previously. Except for the duration, this program was similar in goals, content, and mode of application to the aforementioned program. The brief intervention program consisted of 11 sessions, of 1 hour each, delivered for 1 month (3 sessions per week; 2 sessions in the final week).
For a detailed comparative summary of the 2 intervention formats please refer to Table 1.
Table 1.
Cognitive Intervention Programs/Sessions Description and Comparison.
| Interventions’ Goals and Program Structure/Activities | |
|---|---|
| Standard Intervention | Brief Intervention |
| Sessions 1-6 | Sessions 1-5 |
| Objectives: stimulating episodic autobiographical memory and oral language expression; building group interaction; and maintenance of social interaction. Description: participants discussed autobiographical past experiences related to games, songs, social and cultural context and events, which occurred throughout their childhood, adulthood, and professional life. Objects, such as games and toys of participants childhood era, were used. (spatial and temporal orientation exercises were performed in every session throughout the program.) | Objectives: stimulating episodic autobiographical memory and oral language expression; building group interaction; and maintenance of social interaction. Description: participants discussed autobiographical past experiences related to games, songs, social and cultural context and events which occurred throughout their childhood, adulthood, and professional life. Objects, such as games and toys of participants childhood era, were used. (spatial and temporal orientation exercises were performed in every session throughout the program.) |
| Sessions 7-14 | Sessions 6-8 |
| Objectives: stimulating verbal skills, visual and auditory attention, working memory, and reasoning. Description: participants performed exercises identifying objects, foods and sounds, identifying object functions, and categorizing foods and sounds. Verbal fluency exercises, visual memory and association exercises related to food, country, and place themes were performed as well. Memory exercises with shopping lists were included as well. | Objectives: stimulating verbal skills, visual and auditory attention, working memory, and reasoning. Description: participants performed exercises identifying objects, foods and sounds, identifying objects functions, and categorizing foods and sounds. Verbal fluency exercises, visual memory, and association exercises related to food, country, and place themes were performed as well. |
| Session 15 | Session 9 |
| Objectives: stimulating emotion identification and expression. Description: participants performed exercises including identification and imitation of facial expressions in photos and exemplified by the group guides in a game/playful way. | Objectives: stimulating emotion identification and expression. Description: participants performed exercises including identification and imitation of facial expressions in photos and exemplified by the group guides in a game/playful way. |
| Session 16 | Session 10 |
| Objectives: stimulating executive functioning (planning and sequencing skills), memory, and expressive language. Description: discussion of gardening activities, plants lifecycles. Participants were prompted to elaborate on the required sequence/order of tasks for gardening activities. (eg, how would you plant a rose? Which are the required steps?) | Objectives: stimulating executive functioning (planning and sequencing skills), memory, and expressive language. Description: discussion of gardening activities, plants lifecycles. Participants were prompted to elaborate on the required sequence/order of tasks for gardening activities. (eg, how would you plant a rose? Which are the required steps?) |
| Session 17 | Session 11 |
| Objectives: program evaluation and program closing. Description: participants were prompted to express their assessment of the program using graphical (ie, drawing), written or oral representations. | Objectives: program evaluation and program closing. Description: participants were prompted to express their assessment of the program using graphical (ie, drawing), written or oral representations. |
Outcome Measures
To verify inclusion criteria, interviews and assessments were performed with all participants. A brief but comprehensive cognitive, neuropsychiatric, and functional assessment was performed by a trained psychologist. Baseline assessment lasted 1 to 2 hours.
Global Deterioration Scale (Portuguese version) 10 was used for assessment of staging cognitive impairment and global deterioration, and Geriatric Depression Scale (Portuguese version) 10 for the assessment of depression. The following instruments were used as outcome measures:
Primary outcome measures: Mini-Mental State Examination (MMSE, Portuguese version) 12 ; Alzheimer Disease Assessment Scale (ADAS-Cog, Portuguese version) 10 ; Instrumental Activities of Daily Living (caregiver version, applied to formal caregivers; Portuguese version) 10 ; and Non-Pharmacological Therapy Experience Scale (NPT-ES) 13 Portuguese version 14 (applied to both the intervention groups; independently rated by 2 researchers).
Secondary outcome measures: Digit Span Subtest (WAIS-III 15 ) for measuring attention and immediate memory (raw scores were used for forward, reverse, and total scores); Quality of Life in Alzheimer’s Disease Scale 16 (caregiver and patient versions; Brazilian version); Zarit Burden Interview (applied to formal caregivers); and Geriatric Depression Scale. 10
Data Analysis
Groups were compared at baseline assessment with the following clinical and demographic characteristics: GDS scores, MMSE scores, Geriatric Depression Scale scores, age, gender, and educational level. These characteristics were analyzed for the standard versus brief intervention comparisons. The IBM SPSS Statistics (version 20) software was used for statistical analysis.
A modified intent-to-treat (MITT) analysis was used. The MITT sample for each analysis consisted of randomized patients who were enrolled in groups and had a pre- and postintervention/wait-list assessment for each outcome (independent of the number of attended intervention sessions).
Additionally, observer-rated assessments were collected with NPT-ES regarding participants’ experiences throughout the intervention sessions for both programs.
The following comparisons were carried out:
1. Standard intervention versus wait-list (M2—M1) comparison and (2) comparison of standard (M2—M1) versus brief intervention (M3—M2) efficacy: In order to investigate whether there were any differences in the average change between the 2 groups, for each comparison (namely, between standard intervention vs wait-list and standard intervention vs brief intervention), change in score for each was first calculated (postcondition score minus precondition, for each participant). Then, due to small sample size, nonparametric tests were used (Mann-Whitney test) to compare these change scores. This approach (change score) was used to account for possible differences between moments.
3. Wait-list/ brief intervention group across moments. In order to explore effects of the brief cognitive stimulation program, outcome score means across different moments (M1, M2, and M3) were compared using the Friedman’s test.
4. Experience during intervention. Mean values of the NPT-ES scores across sessions (using 2 independently rated scores) were calculated for each intervention format and then used for calculating the mean values of each program. Scores were calculated for total NPT-ES and its subdomains. Differences between the 2 intervention groups were compared using the Mann-Whitney U test.
5. Costs and feasibility. We estimated intervention costs (in Euros) when intervention programs were to be run for free and non-free. We additionally calculated the completion rate (percentage of people who effectively started and completed an intervention program) and adherence rate (mean number of sessions attended divided by the total number of program sessions) for each group.
Results
Participants’ Characteristics at Baseline and Cross-over
No significant differences were found between standard intervention and wait-list group characteristics (see Table 2). At crossover (M2), the brief intervention group showed significantly higher global cognitive functioning (MMSE) than that of the standard intervention group (M1). The remaining measures did not show statistically significant differences between the 2 groups (Table 3).
Table 2.
Baseline (M1) Demographic and Clinical Characteristics.
| Variable | Standard Intervention (n = 10) | Wait-List (n = 7) | P Value |
|---|---|---|---|
| Gender | 7 Females | 6 Females | a.45 |
| Age | 79.60 (9.06) | 77.71 (12.38) | .77 |
| Education | 2.40 (2.68) | 1.57 (1.99) | .50 |
| Number of institutionalized participants | 6 | 2 | – |
| Medications, number of participants | |||
| Antidementia drugs | 1 | 0 | – |
| Antidepressants | 3 | 0 | – |
| Hypnotics | 5 | 3 | – |
| Antipsychotics | 3 | 1 | – |
| GDS | 3.80 (0.92) | 3.29 (0.49) | .25 |
| GDS stage, number of participants | |||
| 3 | 5 | 5 | – |
| 4 | 2 | 2 | – |
| 5 | 3 | 0 | – |
| MMSE | 17.40 (4.25) | 18.71 (5.02) | .46 |
| GDS Depression | 11.30 (5.33) | 9 (4.87) | .38 |
Abbreviations: GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; GDS Depression, Geriatric Depression Scale; M1, moment 1.
a Mann-Whitney test was used for all variables except for gender, which was assessed with Pearson chi-square.
Table 3.
Demographic and Clinical Characteristics of Standard (Patients Enrolling Intervention at M1) and Brief Intervention (Patients Enrolling Intervention at M2) Groups.
| Standard Intervention (M1), n = 10 | Brief Intervention (M2/Crossover), n = 6 | P Value | |
|---|---|---|---|
| Gendera | 7 Females | 5 Females | .55 |
| Age | 79.60 (9.06) | 79.00 (12.99) | .91 |
| Education | 2.40 (2.68) | 1.83 (2.04) | .69 |
| Number of institutionalized participants | 6 | 2 | – |
| Medications (number of participants) | |||
| Antidementia drugs | 1 | 0 | – |
| Antidepressants | 3 | 0 | – |
| Hypnotics | 5 | 3 | – |
| Antipsychotics | 3 | 1 | – |
| GDS | 3.80 (0.92) | 3.17 (0.41) | .15 |
| GDS stage, number of participants | |||
| 3 | 5 | 5 | – |
| 4 | 2 | 1 | – |
| 5 | 3 | 0 | – |
| MMSE | 17.40 (4.25) | 24.17 (5.15) | .03 |
| GDS Depression | 11.30 (5.33) | 9.33 (6.15) | .62 |
Abbreviations: GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; GDS Depression, Geriatric Depression Scale; M1, moment 1; M2, moment 2.
a Mann-Whitney test was used for all variables except for gender, which was assessed with Pearson chi-square.
Efficacy of the Intervention Programs
Standard intervention versus wait-list (M2—M1) comparison
No differences were observed when comparing patient and caregiver outcomes change scores between the standard intervention and the wait-list groups (Table 4).
Table 4.
Efficacy of Standard Intervention Versus Wait-list Change Scores From Baseline (M1) to Postintervention (M2).
| Measure | Standard Intervention Change Score | Wait-List Change Score | P Value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | N | Mean | SD | ||
| MMSE | 9 | 3.00 | 2.12 | 7 | 4.57 | 3.31 | .26 |
| ADAS-Cog | 10 | −4.10 | 6.24 | 7 | −2.14 | 4.22 | .43 |
| Digit Span Forward | 10 | 0.20 | 1.87 | 7 | 1.57 | 2.57 | .49 |
| Digit Span Reverse | 10 | 1.10 | 1.91 | 7 | 0.14 | 1.77 | .25 |
| Digit Span Total | 10 | 1.30 | 2.06 | 7 | 1.71 | 4.03 | .88 |
| GDS (depression) | 10 | 0.20 | 4.96 | 7 | 0.14 | 2.41 | .84 |
| IADL | 10 | −0.90 | 2.28 | 7 | 1.86 | 2.67 | .07 |
| QoL–caregiver | 9 | −0.44 | 2.79 | 0 | – | – | – |
| QoL–patient | 10 | −2.30 | 5.21 | 7 | 0.57 | 4.35 | .15 |
| Zarit (formal caregiver) | 10 | 0.70 | 4.22 | 7 | −2.86 | 5.79 | .19 |
Abbreviations: MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; GDS depression, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; QoL, quality of life in Alzheimer’s Disease Scale; Zarit, Zarit Burden Interview; M1, moment 1; M2, moment 2; SD, standard deviation.
Wait-list/brief intervention group across moments
Considering outcome differences in wait-list/brief intervention group across moments (see Table 5), significant differences were found between Zarit scores of M2 and M3 (Z= −2.03; P = .04), with lower values in M3 (lower scores mean less formal caregiver burden). No further statistical significant differences were detected across moments.
Table 5.
Efficacy of Brief Intervention (Wait-List/Brief Intervention Group Outcome Differences Across Moments).
| Outcome | Moment 1 | Moment 2 | Moment 3 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| MMSE | 6 | 19.00 | 5.44 | 6 | 24.17 | 5.15 | 6 | 23.83 | 4.40 | .06 |
| ADAS-Cog | 6 | 24.67 | 9.40 | 6 | 22.67 | 8.12 | 6 | 23.67 | 11.41 | .69 |
| Digit Span Forward | 6 | 5.00 | 1.27 | 6 | 7 | 2.10 | 6 | 7.5 | 2.74 | .08 |
| Digit Span Reverse | 6 | 1.83 | 1.17 | 6 | 2.17 | 2.04 | 6 | 2.17 | 1.47 | .72 |
| Digit Span Total | 6 | 6.83 | 1.47 | 6 | 9.17 | 3.82 | 6 | 9.67 | 3.39 | .23 |
| GDS (depression) | 6 | 9.67 | 4.97 | 6 | 9.33 | 6.15 | 6 | 7.00 | 3.23 | .24 |
| IADL | 6 | 19.83 | 5.98 | 6 | 22.17 | 4.88 | 6 | 20.33 | 6.86 | .31 |
| QoL—caregivera | 0 | – | – | 6 | 29.50 | 4.64 | 6 | 29.67 | 5.75 | .92 |
| QoL—patient | 6 | 31.67 | 2.81 | 6 | 31.00 | 3.46 | 6 | 31.83 | 4.07 | .82 |
| Zarit | 6 | 8.17 | 6.88 | 6 | 4.17 | 2.14 | 6 | 2.33 | 1.37 | .03 b |
Note: Boldface values highlight significant results. Abbreviations: MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; GDS depression, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; QoL, quality of life in Alzheimer’s Disease Scale; Zarit, Zarit Burden Interview; SD, standard deviation.
a Since Moment 1 data were not available due to lack of manpower, a Wilcoxon test was performed showing no significant differences (Z = −0,11; P = .92) between QoL—Caregiver scores of moment 2 and moment 3.
b Wilcoxon tests were performed showing significant differences (Z = −2.03; P = .04) between Zarit scores of moment 2 and moment 3.
Compared efficacy of standard intervention (M2–M1) versus brief intervention (M3–M2)
The comparison of the 2 intervention groups’ change scores (Table 6) showed statistical significant differences in MMSE (Z = −2.20, P = .03), with higher change scores in standard intervention group (mean = 3.00; standard deviation = 2.12). However, it should be noted that the remaining analyses showed no significant effects in MMSE outcome neither between standard intervention and wait-list groups nor across different moments in the wait-list/brief intervention group. Since no significant differences were detected in ADAS-Cog total, differences in ADAS-Cog subscores were not assessed.
Table 6.
Efficacy of Standard Intervention (Change Score From Baseline/M1 to Postintervention/M2) Versus Brief Intervention (Change Score From Preintervention/M2 to Postintervention/M3).
| Measure | Standard Intervention Change Score | Brief Intervention Change Score | P Value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| MMSE | 9 | 3.00 | 2.12 | 6 | 0.33 | 2.07 | .03 |
| ADAS-Cog | 10 | −4.10 | 6.24 | 6 | 1.00 | 6.39 | .17 |
| Digit Span Forward | 10 | 0.20 | 1.87 | 6 | 0.50 | 2.95 | 1.00 |
| Digit Span Reverse | 10 | 1.10 | 1.91 | 6 | 0.00 | 1.27 | .30 |
| Digit Span Total | 10 | 1.30 | 2.06 | 6 | 0.50 | 3.39 | .38 |
| GDS depression | 10 | 0.20 | 4.96 | 6 | −2.33 | 4.41 | .28 |
| IADL | 10 | −0.90 | 2.28 | 6 | −1.83 | 3.66 | .74 |
| QoL—caregiver | 9 | −0.44 | 2.79 | 6 | 0.17 | 3.71 | .59 |
| QoL—patient | 10 | −2.30 | 5.21 | 6 | 0.83 | 3.13 | .23 |
| Zarit (formal caregiver) | 10 | 0.70 | 4.22 | 6 | −1.83 | 1.47 | .09 |
Abbreviations: MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; GDS depression, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; QoL, Quality of life in Alzheimer's Disease Scale; Zarit, Zarit Burden Interview; SD, standard deviation.
Experience During Intervention
Both the standard and brief intervention groups showed high mean value (higher values mean a more positive experience) in the NPT-ES total scores and in all the subdomain scores (Table 7). The brief intervention group showed statistically significant higher participation values (NPT-ES participation) than the standard intervention group.
Table 7.
Therapy Experience—Standard and Brief Intervention Scores.
| Standard—17 Sessions (M/SD) | Brief—11 Sessions (M/SD) | Statistical Test and P Value | |
|---|---|---|---|
| NPT-ES Total (Portuguese version)—mean of the 2 means of the therapists | 14.18 (0.73) | 14.36 (0.51) | MW; Z = −0.58; P = .56 |
| NPT-ES participation | 2.68 (0.35) | 2.96 (0.15) | MW; Z = −2.34; P = .02 |
| NPT-ES pleasure | 2.53 (0.41) | 2.82 (0.34) | MW; Z = −1.90; P = .58 |
| NPT-ES relation with others | 3.00 (0.00) | 3.00 (0.00) | MW; Z = −0.00; P = 1.00 |
| NPT-ES displeasure | 2.97 (0.12) | 2.96 (0.15) | MW; Z = −0.32; P = .75 |
| NPT-ES rejection | 2.97 (0.12) | 2.96 (0.15) | MW; Z = −0.33; P = .75 |
Note: Boldface values highlight significant results. Abbreviations: NPT-ES, Nonpharmacological Therapy Experience Scale; MW, Mann-Whitney.
Costs
The estimated cost of each group guide was 20 Euros per session, with total cost of materials for each program estimated to be 50 Euros (provided that a computer and the session’s items, such as food items, were already available or borrowed from the institution). This would total to a cost per program of 710 Euros for the brief intervention and 1070 Euros for the standard intervention for 6 to 10 people groups. Assuming a group of 10 people in the standard format, this would result in 107 Euros per person per full 17 sessions program and approximately 6.29 Euros per person per session. Since it seems viable that at least some centers could build on a manual version after staff training, and include the program in the tasks of 3 staff members as therapeutic assistance guides at no extra cost, this would bring the total cost of the intervention to 50 Euros.
Feasibility
In the feasibility analysis, we found the following completion rates: 100% for both the standard intervention group (10 of 10 participants entered and finished the intervention) and the brief intervention group (6 of 6 participants entered and finished the intervention). The following adherence rates were found: 97% for the standard intervention group and 88% for the brief intervention group.
Discussion
To the best of our knowledge, this is the first randomized controlled trial of cognitive stimulation specifically designed for Portuguese older adults with cognitive impairment and the first comparative duration trial simultaneously evaluating the feasibility and efficacy of cognitive stimulation in both neuropsychological and experiential outcomes.
In the present study, we found high values of experiential relevance to participants for both intervention durations. We also found excellent adherence and completion rates, and reasonable costs, for both cognitive stimulation durations.
When comparing different intervention durations, the standard intervention group showed higher change scores in MMSE than that of the brief intervention group. However, it should be highlighted that the brief intervention group had higher values at baseline (and closer to the ceiling score) and therefore did not have enough room for improvement, contrary to what happened with the standard intervention group. Furthermore, since no differences were detected in the remaining comparison between standard intervention and wait-list change scores, this finding was not considered clinically relevant. The aforementioned result implies that although the standard intervention group showed higher change score than the brief intervention group, the change was not enough to imply a statistically significant improvement when compared to the wait-list group (nonintervention condition).
Caregiver burden (as assessed by the Zarit burden interview) significantly decreased from preintervention to postintervention moment in the brief intervention group. However, it should be noted that burden scores were relatively low across the analyzed 3 moments of the wait-list/brief intervention group, and no further differences were found in the standard intervention versus wait-list comparison. The observed absence of burden could be related to the fact that respondents to this self-report instrument were formal caregivers.
The findings of the present study are in line with prior research 17 showing improvements in a related parameter of experiential relevance (ie, goal performance and satisfaction, as measured by the Canadian Occupational Performance Measure) and no cognitive improvements after an 8-week cognitive rehabilitation intervention in patients with dementia. Another study 18 with a patient with MCI reported similar findings. There is also preliminary evidence that patients with moderate to severe dementia can successfully participate and engage in psychosocial intervention programs. 19
Nonetheless, at present, in the area of cognitive intervention for older adults with cognitive impairments (whether MCI or dementia), experiential relevance parameters (such as experience during intervention, goal performance and satisfaction, and meeting of needs) have been seldom evaluated or reported.
Although some studies have reported cognitive benefits, currently there are a modest number of high-quality/randomized controlled trial (RCT) studies with contradictory findings and issues, which have been illustrated and detailed by reviews in the area such as Clare and colleagues 20 who found neither positive nor negative effects of cognitive training. More recently, Alves et al, 8 using only high-quality RCTs in patients with AD, found improvements only in MMSE scores. Additionally, a review article on cognitive intervention (mainly targeting episodic memory functioning) for MCI 21 reported possible improvements in nonstandardized cognitive and subjective measures despite limited findings in standardized neuropsychological outcomes. Although the majority of studies focused on cognitive training approaches, cognitive stimulation and cognitive rehabilitation remain relatively underrepresented. Nevertheless, recent cognitive stimulation trials 22,23 show cognitive and quality-of-life benefits in people with dementia.
In sum, the evidence remains mixed and inconclusive, with only some studies finding cognitive benefits while preliminary evidence suggesting cognitive intervention being a relevant experience for participants even in the absence of cognitive improvements. 17
Concerning intervention duration, although to our knowledge no previously published study directly compared different intervention durations in patients with MCI or dementia, both short 17 and long 24 formats have shown positive effects. In the present study, we found high values of experiential relevance to participants in both groups. Moreover, concerning cognitive effects, the standard intervention group showed higher change scores in MMSE than the brief intervention group, which could point to possible higher benefits from longer interventions. However, no cognitive effects were detected in the comparison between standard intervention and wait-list change scores. It is possible that a longer intervention duration could have led to cognitive effects reaching a detectable threshold. As stated previously, it should also be mentioned that the brief intervention group presented MMSE values closer to the ceiling score which could have potentially precluded capturing improvements.
Feasibility parameters are seldom reported despite their importance. However, in a recent meta-analysis, Alves et al 8 calculated intervention groups adherence and completion rate values (for the studies included in a meta-analysis of cognitive intervention for patients with AD) and found completion rates ranging from 85% to 100% and adherence rates ranging from 96.7% to 100%. In a recent study, Vidovich and colleagues 25 also found older adults with MCI reliably attended and displayed satisfaction concerning the cognitive intervention programs.
A prior study 26 also reported reasonable costs similar to our cost findings. Namely, it was found that cognitive stimulation could be a cost-effective therapy with estimated costs of £90 per session, which, for groups of 5 people, yielded the cost of the intervention per person per week equaling to £31.50 (as of 2001). Although providing a valuable experience for participants, the present study found even lower costs, with the cost per person per week (3 weekly sessions) equaling to 18.87 Euros per week (assuming 10 people per group in the standard format—6.29 Euros per person per session).
Together with the experiential relevance findings, the feasibility data might be a proxy indicator for participant engagement, suggesting cognitive intervention being well accepted by participants in meeting the needs of older adults with cognitive impairments.
Limitations and Future Research
It is necessary to take into account some limitations of the present study when considering our results. First, the small sample size of our study could have prevented reaching statistical significance in detecting clinically relevant results or led to the detection of clinically irrelevant results. Second, since this was a naturalistic setting study, the participants included in the present study showed different GDS scores although the majority (10 of 17) of patients were in the MCI stage (due to a small N, no analyses according to different GDS stage subsets of participants were performed).
Conclusion
The findings of this study suggest that cognitive stimulation can lead to high values of experiential relevance even in the absence of cognitive or functionality improvements. Results also show that cognitive stimulation can provide excellent adherence and completion rates and present reasonable costs. The major novelty of the present study was the evaluation of the experiences of participants while undergoing intervention.
Future studies should adopt the randomized controlled trial design and assess not only the efficacy of intervention in cognition, functionality, quality of life, and mood. It would also be relevant to evaluate the experiential relevance, participant engagement as well as establish the optimal intervention durations (and other optimal intervention parameters such as frequency, duration and intensity, and format—individual or group based). Moreover, possible differential effects of each cognitive approach (training, stimulation, and rehabilitation) in different types (different etiologies), subtypes (clinical presentation of the same pathological process), and stages of cognitive impairment (such as severe cognitive decline) need to be addressed.
Acknowledgments
The authors thank the elderly live-in and day care center for taking part in the present project and wish to especially acknowledge Miss G. Carvalho and Miss S. Pinto for their assistance. The authors also acknowledge discussions about aging with Dr Agavni Petrosyan.
Authors’ Note: The present study was approved by the University of Minho Ethics Committee Review board and by the elderly live-in and day care center board. Informed consent was obtained from all participants and, when necessary, from family member.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Portuguese Foundation for Science and Technology, with individual grants (SFRH/BD/64457/2009 and SFRH/BD/65213/2009, co-funded by FSE/POPH) and project grant PIC/IC/83290/2007, supported by FEDER (POFC—COMPETE).
References
- 1. Fei M, Qu YC, Wang T, Yin J, Bai JX, Ding QH. Prevalence and distribution of cognitive impairment no dementia (CIND) among the aged population and the analysis of socio-demographic characteristics: the community-based cross-sectional study. Alzheimer Dis Assoc Disord. 2009;23(2):130–138. [DOI] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e62. [DOI] [PubMed] [Google Scholar]
- 3. Nunes B, Silva RD, Cruz VT, Roriz JM, Pais J, Silva MC. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thies W, Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. [DOI] [PubMed] [Google Scholar]
- 5. Leicht H, Konig HH, Stuhldreher N, et al. Predictors of costs in dementia in a longitudinal perspective. PLoS One. 2013;8(7):e70018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil. 2004;14:385–340. [Google Scholar]
- 7. Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev. 2013;12(1):263–275. [DOI] [PubMed] [Google Scholar]
- 8. Alves J, Magalhaes R, Thomas RE, Goncalves OF, Petrosyan A, Sampaio A. Is there evidence for cognitive intervention in Alzheimer disease? a systematic review of efficacy, feasibility, and cost-effectiveness. Alzheimer Dis Assoc Disord. 2013;27(3):195–203. [DOI] [PubMed] [Google Scholar]
- 9. Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 10. Grupo de Estudos de Envelhecimento Cerebral e Demência. Escalas e Testes na Demência. 2nd ed. GEECD; 2008. [Google Scholar]
- 11. Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morgado J, Rocha CS, Maruta C, Guerreiro M, Martins IP. New normative values of mini-mental state examination. Sinapse. 2009;9(2):10–16. [Google Scholar]
- 13. Muñiz R, Olazarán J, Poveda S, Lago P, Peña-Casanova J. NPT-ES: A measure of the experience of people with dementia during non-pharmacological interventions. Non Pharmacol Ther Dement. 2011;1(3):1–11. [Google Scholar]
- 14. Alves J, Núñez-Navarro A. Non pharmacological therapy experience scale (NPT-ES) - Authorized Portuguese adaptation. 2013 Unpublished instrument. Available from http://www.mariawolff.org/_pdf/NPT_ES_portugues.pdf.
- 15. Rocha AM. WAIS-III, Wechsler Adult Intelligence Scale - Third Edition - Adaptação Portuguesa da WAIS-III. Lisboa: CEGOC-TEA; 2008. [Google Scholar]
- 16. Novelli MM, Dal Rovere HH, Nitrini R, Caramelli P. Cross-cultural adaptation of the quality of life assessment scale on Alzheimer disease. Arq Neuropsiquiatr. 2005;63(2A):201–206. [DOI] [PubMed] [Google Scholar]
- 17. Clare L, Linden DE, Woods RT, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry. 2010;18(10):928–939. [DOI] [PubMed] [Google Scholar]
- 18. Clare L, van Paasschen J, Evans SJ, Parkinson C, Woods RT, Linden DE. Goal-oriented cognitive rehabilitation for an individual with Mild Cognitive Impairment: behavioural and neuroimaging outcomes. Neurocase. 2009;15(4):318–331. [DOI] [PubMed] [Google Scholar]
- 19. Cruz J, Marques A, Barbosa A, Figueiredo D, Sousa LX. Making sense(s) in dementia: a multisensory and motor-based group activity program. Am J Alzheimers Dis Other Dement. 2013;28(2):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2003;(4):CD003260. [DOI] [PubMed] [Google Scholar]
- 21. Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic mild cognitive impairment: a systematic review. Neurosci Biobehav Rev. 2012;36(4):1163–1178. [DOI] [PubMed] [Google Scholar]
- 22. Spector A, Thorgrimsen L, Woods B, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;183:248–254. [DOI] [PubMed] [Google Scholar]
- 23. Yamanaka K, Kawano Y, Noguchi D, et al. Effects of cognitive stimulation therapy Japanese version (CST-J) for people with dementia: a single-blind, controlled clinical trial. Aging Ment Health. 2013;17(5):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olazaran J, Muniz R, Reisberg B, et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. 2004;63(12):2348–2353. [DOI] [PubMed] [Google Scholar]
- 25. Vidovich MR, Lautenschlager NT, Flicker L, Clare L, Almeida OP. Treatment fidelity and acceptability of a cognition-focused intervention for older adults with mild cognitive impairment (MCI). Int Psychogeriatr. 2013;25(5):815–823. [DOI] [PubMed] [Google Scholar]
- 26. Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry. 2006;188:574–580. [DOI] [PubMed] [Google Scholar]