Abstract
Cognitive reserve (CR) has been defined as the ability to optimize or maximize performance through differential recruitment of brain networks. In the present study, we aimed at providing evidence for a consistent brain network underpinning CR in healthy and pathological aging. To pursue this aim, we performed a coordinate-based meta-analysis of 17 functional magnetic resonance imaging studies on CR proxies in healthy aging, Alzheimer’s disease (AD), and mild cognitive impairment (MCI). We found that different brain areas were associated with CR proxies in healthy and pathological aging. A wide network of areas, including medial and lateral frontal areas, that is, anterior cingulate cortex and dorsolateral prefrontal cortex, as well as precuneus, was associated with proxies of CR in healthy elderly patients. The CR proxies in patients with AD and amnesic-MCI were associated with activation in the anterior cingulate cortex. These results were discussed hypothesizing the existence of possible compensatory mechanisms in healthy and pathological aging.
Keywords: brain reserve, brain maintenance, fMRI, Alzheimer’s disease, mild cognitive impairment
Introduction
Cognitive reserve (CR) was originally defined by Stern 1 as the ability to optimize or maximize performance through differential recruitment of brain networks. Although this definition is suitable for both healthy individuals and those with brain damage, the concept of CR has found potential applications particularly in the field of brain pathologies. In fact, Katzman, 2 in one of the first studies, noticed that higher rates of Alzheimer’s disease (AD) neuropathology at postmortem examinations were seen in individuals who were not clinically demented but possessed heavier brains and higher counts of large neurons. Later studies tried to investigate CR using direct measures of brain 2 –4 and head or intracranial size, 5,6 finding that reduced intracranial volume or smaller head size by its own or in combination with low education may confer an increased risk for cognitive decline and dementia, 5,7,8 including mild cognitive impairment (MCI) in old age. 9
The more the research went on, the more it became necessary to measure the CR through coded proxies that were universally recognized. The first attempts were made in several studies, which showed that factors such as high levels of education, occupational complexity, and/or premorbid intelligence were associated with lower levels of cognitive impairment in neurological patients such as patients with AD, 10 stroke, 11 and traumatic brain injury. 12,13 In this light, Stern 14 expands the initial definition, adding the distinction between 2 types of “reserve”: the brain reserve (BR) and the CR. The first refers to differences in brain structure, which may results in increasing the brain’s tolerance of disease. The second refers to individuals’ differences in cognitive performances in spite of brain damages. In other words, CR refers to the mind resilience to brain damages. There is also another concept considered complementary to BR and CR, that is, the “brain maintenance” (BM) proposed by Nyberg. 15 According to the author, older adults vary in the degree of age-related cellular damage to basic brain structures, resulting in an age-related increase in variability in cognitive function. In this context, BR and CR may mitigate these variations in BM.
With this distinction, research has taken a new direction in this theoretical context using also new technologies. For instance, several studies used positron emission topography to investigate CR in AD and healthy elders. Particularly, it was found that education and both intellectual and social life activities were inversely correlated with regional brain metabolic activity and/or cerebral blood flow in different cortical and subcortical areas during the resting-state condition, 16 –18 while during a cognitive task, it was noticed that specific brain networks were differentially activated depending on CR. 16,19,20 Other studies have investigated the neural correlates of the proxies of CR through the use of functional magnetic resonance imaging (fMRI). Actually, as stated by Jung and Haier, 21 some measures such as general fluid intelligence are related to variations in blood oxygenation level dependent (BOLD) activity. In this light, studies have been conducted on different pathological conditions, 22 –25 revealing the usage of neural networks that underpin CR mechanisms, during cognitive tasks. Furthermore, few studies were conducted also on healthy young patients, 26,27 showing that the pattern of activation during a nonverbal recognition task was related to individual differences in CR variables.
With the evolution of research in this field, there was also an increasing compliance on the use of common proxies to properly investigate CR. At present, although research is lacking with regard to creating a full CR model and determining the overall effect this has toward cognitive function and dementia, 28 the most frequently used proxies are those proposed by Valenzuela and Sachdev 29 and include educational/occupational attainment, premorbid intelligence quotient, leisure, cognitive, and mental-stimulating activities. In fact, using a composite score of these factors, a positive correlation between brain volume and CR has been found. 30 Particularly, greater activations have been found in the dorsolateral prefrontal cortex (DLPFC 31 ), which mediates strategic aspects of episodic memory recall 32 and executive functions (EFs 33 ) in healthy elderly patients.
This result is compelling in light of possible effect of CR in mitigating executive and attentional deficits in AD. Actually, executive dysfunction is a prominent deficit in patients with AD 34 and it is associated with memory deficits. 33 Moreover, greater activations have been shown also in frontoparietal regions, 35 which are involved, as DLPFC, in EF and memory recall. Studies in healthy volunteers consistently identified a set of nodes in these regions, which are associated with cognitively demanding tasks that are involved in cognitive control. 36
This created a large amount of controversial data, since, as mentioned above, the lack of a full CR model generated different studies that used different tasks to investigate the same construct, giving, in this way, different results that fall mainly in neural context, with several fMRI activations, including precuneus, anterior cingulate gyrus, bilateral thalamus, right insula, right middle temporal gyrus, bilateral inferior frontal gyrus, and superior frontal gyrus. 26,30,31,37 –40 Anyway, converging and consistent evidence about this network of brain areas is lacking. The main aim of the present study was to draw some definite conclusions and to shed some light about neural networks underpinning CR in healthy and pathological aging, trying to define a red line between the various studies, by verifying converging and consistent evidence in the current literature by means of a meta-analysis. Thus, we adopted a meta-analytic approach based on activation likelihood estimation (ALE) analysis, which allows performing coordinate-based meta-analyses of neuroimaging data and, thus, integrating data from several studies.
Materials and Methods
Inclusion Criteria
The database search on PubMed was performed using the following string: (((“cognitive reserve”[Title/Abstract]) OR “brain reserve”[Title/Abstract]) AND MRI) NOT review [Publication Type]. A total of 108 papers emerged. From this collection, we selected only papers that (1) included whole-brain analysis performed using magnetic resonance imaging (MRI), (2) provided coordinates of activation foci either in Montreal Neurological Institute (MNI) or in Talairach reference space, (3) studied both healthy and elderly participants with amnestic mild cognitive impairment (a-MCI) or AD (studies on young participants were excluded from the meta-analysis), (4) included studies where participants performed a cognitive task during an fMRI acquisition, (5) provided a measurement of CR using defined proxies, and (6) used no pharmacological manipulation. We initially selected 31 papers. Of these papers, 26 were excluded because they did not provide coordinates of activation foci (see Figure 1). In line with the aims of the present meta-analysis, individual experimental studies from selected papers were divided according to 2 main axes: papers reporting (1) fMRI studies during a cognitive task in elderly healthy participants and (2) fMRI studies during a cognitive task in elderly participants who were diagnosed with a-MCI or AD. Note that the fMRI studies (see Table 1) adopted different paradigms. A meta-analytic approach, which models the probability distributions centered at the coordinates of each activation focus, allows obtaining a general picture of functional neural modifications underpinning CR proxies (see Table 2) and the differences between healthy and a-MCI participants when they are faced with a cognitive task. We included 10 individual fMRI experimental studies in healthy aging (151 participants, 37 foci) and 7 fMRI experiments in patients who are diagnosed with AD or a-MCI (99 participants, 16 foci).
Figure 1.
Flowchart of literature screening.
Table 1.
Paper on Cognitive Reserve Included in the Meta-Analysis.
| Paper | N | Experiments | Contrast | fMRI Task | CR Proxies |
|---|---|---|---|---|---|
| Ansado et al, 2013 37 | HC = 32; AD = NA; MCI = NA | 5 | LL 3 Let vs Ref; older | VLMT | VLMT |
| Ansado et al, 2013 | HC = 32; AD = NA; MCI = NA | 5 | LL 3 Let vs Ref; older > younger | VLMT | VLMT |
| Ansado et al, 2013 | HC = 32; AD = NA; MCI = NA | 5 | HL 5 Let vs Ref; older | VLMT | VLMT |
| Ansado et al, 2013 | HC = 32; AD = NA; MCI = NA | 5 | HL 5 Let vs Ref; older > younger | VLMT | VLMT |
| Ansado et al, 2013 | HC = 32; AD = NA; MCI = NA | 5 | 5 Let vs 3 Let; older | VLMT | VLMT |
| Beeri et al, 2011 38 | HC = 29; AD = NA; MCI = NA | 2 | +MMS | RMT | RMT |
| Beeri et al, 2011 | HC = 29; AD = NA; MCI = NA | 2 | −MMS | RMT | RMT |
| Solé-Padullés et al, 2009 30 | HC = 16; AD = 16; MCI = 12 | 2 | HC; CCR, neg correlation | VET | VS (WAIS III), E-O Var, customized QOLO |
| Solé-Padullés et al, 2009 | HC = 16; AD = 16; MCI = 12 | 2 | HC; E-O attainment, neg correlation | VET | VS (WAIS III), E-O Var, customized QOLO |
| Solé-Padullés et al, 2009 | HC = 16; AD = 16; MCI = 12 | 2 | Mild AD; CCR, pos correlation | VET | VS (WAIS III), E-O Var, customized QOLO |
| Solé-Padullés et al, 2009 | HC = 16; AD = 16; MCI = 12 | 2 | Mild AD; CR questionnaire, Pos correlation | VET | VS (WAIS III), E-O Var, customized QOLO |
| Bartrés-Faz et al, 2009 39 | HC = 15; AD = NA; MCI = NA | 1 | Neg correlation between fMRI signal and CR | WMT | E-O attainments, premorbid IQ records of LSCSA |
| Bosch et al, 2010 40 | HC = 15; AD = 15; MCI = 15 | 5 | Activation: a-MCI more pos than HC | SCT | VS (WAIS III), E-O Var, customized QOLO |
| Bosch et al, 2010 | HC = 15; AD = 15; MCI = 15 | 5 | Activation: AD more pos than CTR | SCT | VS (WAIS III), E-O Var, customized QOLO |
| Bosch et al, 2010 | HC = 15; AD = 15; MCI = 15 | 5 | Activation: AD more pos than a-MCI | SCT | VS (WAIS III), E-O Var, customized QOLO |
| Bosch et al, 2010 | HC = 15; AD = 15; MCI = 15 | 5 | Deactivation: a-MCI more neg than HC | SCT | VS (WAIS III), E-O Var, customized QOLO |
| Bosch et al, 2010 | HC = 15; AD = 15; MCI = 15 | 5 | Deactivation: AD more neg than HC | SCT | VS (WAIS III), E-O Var, customized QOLO |
Abbreviations: AD, Alzheimer’s disease; a-MCI, amnestic mild cognitive impairment; CCR, composite cognitive reserve; E-O, education–occupation; HC, healthy controls; HL, high load; Let, letters; LL, low load; LSCSA, leisure, social and cognitively stimulating activities; MMS, Mini Mental State; NA, not applicable; neg, negative; pos, positive; QOLO, questionnaire of lifetime occupation; Ref, reference; RMT, recognition memory task; SCT, speech comprehension task; Var, variable; VET, visual encoding task; VLMT, visual letter matching task; VS, vocabulary subtest; WMT, working memory task.
Table 2.
Summary Table.
| Paper | Education | Occupation | Leisure Activities |
|---|---|---|---|
| Ansado et al, 2013 | 18 | NA | NA |
| Beeri et al, 2011 | 15.5 | NA | NA |
| Solé-Padullés et al, 2009 | HC = 4.56a; AD = 2.42a; MCI = 2.75a | HC = 4.56a; AD = 2.42a; MCI = 2.75a | NA |
| Bartrés-Faz et al, 2009 | NA | NA | NA |
| Bosch et al, 2010 | HC = 3.53a; AD = 2.60a; MCI = 3.81a | HC = 3.53a; AD = 2.60a; MCI = 3.81a | NA |
Abbreviations: AD, Alzheimer’s disease; HC, healthy controls; MCI, mild cognitive impairment; NA, not applicable.
aThis score refers to a composite education–occupation rate.
Activation Likelihood Estimation
Activation likelihood estimation analyzes the probability that a voxel will contain at least one of the activation foci; it is calculated at each voxel and results in a thresholded ALE map. In other words, ALE assesses the overlap between foci by modeling the probability distributions centered at the coordinates of each one. Our aim was to provide a general picture of areas associated with CR proxies in healthy and pathological aging. Thus, we carried out 2 ALE analyses of fMRI studies on functional activations during cognitive tasks in (1) healthy elderly patients (10 experiments, 321 participants, 37 foci) and (2) patients with a-MCI or AD (7 experiments, 313 participants, 16 foci). The ALE meta-analysis was performed using GingerALE 2.3.5 (http://brainmap.org/) with MNI coordinates (Talairach coordinates were automatically converted into MNI coordinates by GingerALE). Following Eickhoff et al’s modified procedure, 41 the ALE values of each voxel in the brain were computed and a test was performed to determine the null distribution of the ALE statistic of each voxel. The full-width half-maximum value was automatically computed because this parameter is empirically determined. The thresholded ALE map was computed using P values from the previous step and a cluster-level inference at the .01 level of significance. The statistical image of uncorrected voxel-wise P values (P < .001) was first cut off by the cluster-forming threshold. Then, the size of the suprathreshold clusters was compared against a null distribution of cluster sizes derived from simulating 1000 data sets with the same properties. 41 The ALE results were registered on an MNI-normalized template (http://brainmap.org/) using Mricro (http://www.mccauslandcenter.sc.edu/mricro/index.html).
Results
Neural Correlates of CR in Healthy Elderly Patients
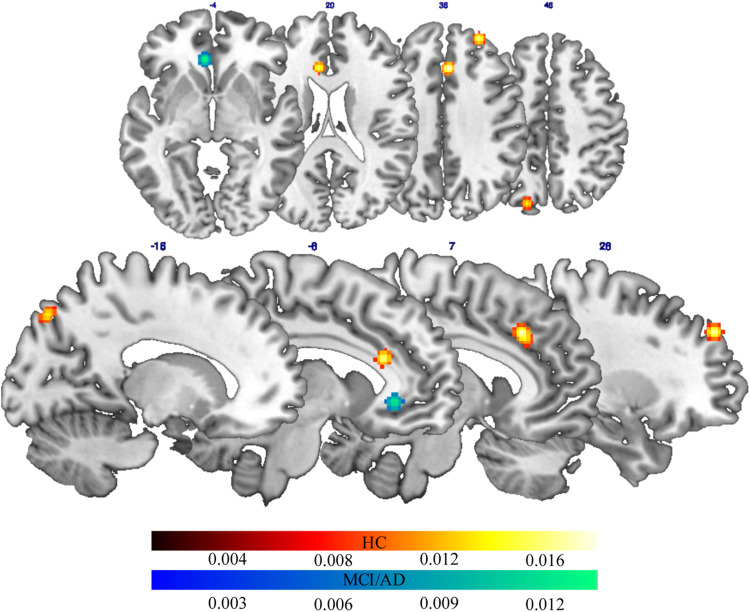
The ALE meta-analysis of fMRI studies carried out during a cognitive task on healthy elderly patients (see Table 3) revealed a bilateral frontoparietal network of areas, which activity highly correlated with proxies of CR. This network included on the medial surface, the anterior cingulate and the precuneus in the left hemisphere, and the cingulate gyrus in the right hemisphere. It also included the superior frontal gyrus in the right DLPFC (Figure 2).
Table 3.
Results of ALE Meta-Analysis on Healthy Patients and Patients With a-MCI and AD.
| Region | Hema | BAb | X | Y | Zc | Volumed | Peak ALE Valuee |
|---|---|---|---|---|---|---|---|
| HC | |||||||
| Cingulate gyrus | R | 32 | 4 | 26 | 36 | 952 | 0.017 |
| Superior frontal gyrus | R | 8 | 28 | 48 | 36 | 560 | 0.015 |
| Anterior cingulate | L | 24 | −8 | 28 | 22 | 488 | 0.014 |
| Precuneus | L | 7 | −16 | −78 | 46 | 440 | 0.012 |
| a-MCI and AD | |||||||
| Anterior cingulate | L | NA | −6 | 34 | −4 | 720 | 0.014 |
Abbreviations: AD, Alzheimer’s disease; ALE, activation likelihood estimation; a-MCI, amnestic mild cognitive impairment; HC, healthy controls; L, left; MNI, Montreal Neurological Institute; NA, not applicable; R, right.
aHemisphere.
bBroadmann’s area (if applicable).
cMNI coordinates of each foci.
dVolume of cluster (mm3).
eALE value of each peak.
Figure 2.
Brain areas with activations significantly correlated with CR proxies in healthy aging (red-to-yellow patches) and pathological aging (blue-to-light blue patches) (Color figure can be viewed in the online issue). AD indicates Alzheimer’s disease; CR, cognitive reserve; HC, healthy controls; MCI, mild cognitive impairment.
Neural Correlates of CR in Patients With a-MCI and AD
We found that the proxies of CR in elderly participants with a-MCI and AD (see Table 3) were associated with high activation in the anterior cingulate in the left hemisphere during cognitive tasks (Figure 2).
Discussion
Studies about CR leave open the possibility that differences in developing dementia could be attributed to individual differences in environmental and social factors not only in adult or late life but also in childhood and youth. 42 Furthermore, education, occupation, and leisure activities could enhance the CR capabilities and as a consequence to postpone the effects of neurodegeneration. 43 However, brain mechanisms underlying CR are still matter of debate.
Considering CR as the ability to utilize more efficient and flexible cognitive strategies, which can be trained by means of a continuous mental exercise, it makes sense to consider the possibility of exploiting this capacity in the context of AD rehabilitation. The idea is that CR is not a fixed factor but is rather continuously modified by environment and life experiences through the whole life course. In a recent review, Liberati et al 44 hypothesized that an intensive mental training or stimulation could provide a greater resilience in facing the neuropathology, possibly recruiting alternate networks. These authors stressed the idea that CR is not a fixed factor but can be continuously modified by life experiences, even when the brain is already affected by neuropathology.
The main aim of the present study was to identify functional brain network associated with CR proxies in both healthy and pathological aging. We found that different brain regions were associated with CR proxies in healthy and pathological aging. Actually, CR proxies in patients with AD and a-MCI were associated with functional activation in the anterior cingulate cortex. Instead, a wide network of areas, including medial and lateral frontal areas, that is, anterior cingulate cortex and DLPFC, as well as precuneus, was associated with proxies of CR in healthy elderly patients (see Figure 1). Such a result deserves feasible consideration.
Although research in this field is more oriented to the brain pathologies, some studies investigated the activations related to CR proxies in healthy elderly patients, 30,45 reporting greater activations in brain areas involved in EF and memory recall, with DLPFC and frontoparietal regions playing a key role in these processes. 31,35 Those brain areas, in fact, seem the most impaired in brain pathology such as AD or MCI. In fact, other authors 46 identified brain regions where activity represents situations where the increasing level of CR is associated with opposite direction of activation in healthy elders and AD groups. These results led Dhanjal and Wise 35 to suggest that AD affects frontoparietal networks exercising domain-general task-related cognitive control. In fact, our results confirm (see Figure 1) that the activations showed by healthy elderly patients in brain areas related to CR proxies tend to decay in elderly patients with AD and a-MCI. Moreover, in elderly patients with AD and a-MCI, we found an activation of the anterior cingulate cortex. This can be read as a brain compensatory process, due to the fact that both the EF and the memory recall’s network are impaired by the pathology. In this light, Scarmeas 46 asserts that CR-related changes of activation directionality may indicate compensatory reorganization of function in the face of pathological changes in different brain regions. According to the author, if a coordinated activity of many brain areas (ie, a network) is usually required for performance of most cognitive tasks, it is reasonable to hypothesize that perturbations in a single site of this network would result in changes and rearrangements of the response of other regions participating in the network. In this context, the differences in brain activations that we found through the ALE meta-analysis could be explained by the fact that while in healthy elderly patients, neural networks that underlie CR proxies are still intact, in elderly patients with AD and a-MCI, these networks are no longer functional, so that the brain activate other networks, in order to create, using a compensation mechanism, a reorganization of brain resources to cope with a cognitive task that otherwise would be too difficult. In line with our meta-analysis, Belleville et al 47 found that memory training can result in significant neural changes also in people with MCI demonstrating that their brains remain highly plastic.
Limitations
One of the major limitations is the number of papers taken into account for this meta-analysis. Furthermore, the experiments included in the present meta-analysis if often taken from the same paper and thus individual features are repeated between studies. This limitation is mainly due to the paucity of studies fitting with the inclusion criteria for the meta-analysis (ie, providing fMRI coordinates) and due to difficulties in finding a large amount of studies that used the same CR proxies. It has to be noted that a great number of studies have been excluded from the meta-analysis because they did not provide coordinates of activation. Furthermore, studies included in the present meta-analysis tap on different cognitive domains. Even if this allows for depicting a general picture of the task-induced activations underpinning CR, it does not allow for disentangling the neural correlates of CR in specific cognitive domains. It would be interesting comparing more studies that present the same cognitive task, in order to have a clear overview of the specific functional networks that underpin CR. To better clarify, these mechanisms should help in developing training activities to enhance CR for postponing neurodegeneration in elderly patients.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Stern Y. What is cognitive reserve? Theory and research applicatione on the reserve concept. J Int Neurpsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 2. Katzman R, Terry R, DeTeresa R, et al. Clinical pathological and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. [DOI] [PubMed] [Google Scholar]
- 3. Kidron D, Black SE, Stanchev P, et al. Quantitative MR volumetry in Alzheimer’s disease. Topographic markers and the effects of sex and education. Neurology. 1977;49(6):1504–1512. [DOI] [PubMed] [Google Scholar]
- 4. Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53(1):189–196. [DOI] [PubMed] [Google Scholar]
- 5. Schofield PW, Mosesson RE, Stern Y, Mayeux R. The age at onset of Alzheimer’s disease and intracranial area measurement: a relationship. Arch Neurol. 1995;52(1):95–98. [DOI] [PubMed] [Google Scholar]
- 6. Tisserand DJ, Bosma H, Van Boxtel PJ, Jolles J. Head size and cognitive ability in non-demented older adults are related. Neurology. 2001;56(7):969–971. [DOI] [PubMed] [Google Scholar]
- 7. Mortimer JA, Snowdown DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25(5):671–679. [DOI] [PubMed] [Google Scholar]
- 8. Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurology. 1997;49(1):30–37. [DOI] [PubMed] [Google Scholar]
- 9. Wolf H, Julin P, Gertz HJ, Winblad B, Wahlund LO. Intracranial volume in mild cognitive impairment, Alzheimer’s disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19(10):995–1007. [DOI] [PubMed] [Google Scholar]
- 10. Roe CM, Mintun MA, Ghoshal N, et al. Alzheimer disease identification using amyloid imaging and reserve variables. Neurology. 2010;75(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ojala-Oksala J, Jokinen H, Kopsi V, et al. Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke. 2012;43(11):2931–2935. [DOI] [PubMed] [Google Scholar]
- 12. Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol. 2003;10(3):153–162. [DOI] [PubMed] [Google Scholar]
- 13. Grafman J. The relationship of brain tissue loss volume and lesion location to cognitive deficit. J Neurosci. 1986;6(2):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. [DOI] [PubMed] [Google Scholar]
- 16. Scarmeas N, Zarahn E, Anderson KE, et al. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. Neuroimage. 2003;19(3):1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perneczky R, Drzezga A, Diehl-Schmid J, et al. Schooling mediates brain reserve in Alzheimer’s disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry. 2006;77(9):1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander GE, Furey ML, Grady CL, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154(2):165–172. [DOI] [PubMed] [Google Scholar]
- 19. Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease. Arch Neurol. 2003;60(3):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stern Y, Habeck C, Moeller J, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30(2):135–154. [DOI] [PubMed] [Google Scholar]
- 22. Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(pt 5):1096–1112. [DOI] [PubMed] [Google Scholar]
- 23. Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129(pt 2):527–537. [DOI] [PubMed] [Google Scholar]
- 24. Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59(9):1343–1349. [DOI] [PubMed] [Google Scholar]
- 25. Bartres-Faz D, Marti MJ, Junque C, et al. Increased cerebral activity in Parkinson’s disease patients carrying the DRD2 TaqIA A1 allele during a demanding motor task: a compensatory mechanism? Genes Brain Behav. 2006;6(6):588–592. [DOI] [PubMed] [Google Scholar]
- 26. Habeck C, Hilton HJ, Zarahn E, Flynn J, Moeller J, Stern Y. Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of non-verbal memory. Neuroimage. 2003;20(3):1723–1733. [DOI] [PubMed] [Google Scholar]
- 27. Stern Y, Zarahn E, Hilton HJ, Flynn J, DeLaPaz R, Rakitin B. Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):691–701. [DOI] [PubMed] [Google Scholar]
- 28. Harrison SL, Sajjad A, Bramer W, Ikram MA, Tiemeier H, Stephan BCM. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol. 2015;37(3):253–264. [DOI] [PubMed] [Google Scholar]
- 29. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2005;36(4):441–454. [DOI] [PubMed] [Google Scholar]
- 30. Solé-Padullés C, Bartres-Faz D, Junque C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30(7):1114–1124. [DOI] [PubMed] [Google Scholar]
- 31. Wong S, Flanagan E, Savage G, Hodges J, Hornberger M. Contrasting prefrontal cortex contributions to episodic memory dysfunction in behavioural variant frontotemporal dementia and Alzheimer’s disease. PLoS One. 2014;9(2):e87778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Becker S, Lim J. A computational model of prefrontal control in free recall: strategic memory use in the California Verbal Learning Task. J Cogn Neuorsci. 2003;15(6):821–832. [DOI] [PubMed] [Google Scholar]
- 33. Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L. Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol. 2006;21(1):15–21. [DOI] [PubMed] [Google Scholar]
- 34. Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Executive dysfunction in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60(1):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhanjal NS, Wise RJ. Frontoparietal cognitive control of verbal memory recall in Alzheimer’s disease. Ann Neurol. 2014;76(2):241–251. [DOI] [PubMed] [Google Scholar]
- 36. Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ansado J, Monchi O, Ennabil N, et al. Coping with task demand in aging using neural compensation and neural reserve triggers primarily intra-hemispheric-based neurofunctional reorganization. Neurosci Res. 2013;75(4):295–304. [DOI] [PubMed] [Google Scholar]
- 38. Beeri MS, Lee H, Cheng H, Wollman D, Silverman JM, Prohovnik I. Memory activation in healthy nonagenarians. Neurobiol Aging. 2011;32(3):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartrés-Faz D, Solé-Padullés C, Junqué C, et al. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol. 2009;80(2):256–259. [DOI] [PubMed] [Google Scholar]
- 40. Bosch B, Bartrés-Faz D, Rami L, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46(4):451–461. [DOI] [PubMed] [Google Scholar]
- 41. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation revisited. Neuroimage. 2012; 59: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp. 2003;25(5):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(2):112–117. [DOI] [PubMed] [Google Scholar]
- 44. Liberati G, Raffone A, Olivetti Belardinelli M. Cognitive reserve and its implications for rehabilitation and Alzheimer’s disease. Cogn Process. 2012;13(1):1–12. [DOI] [PubMed] [Google Scholar]
- 45. McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 46. Scarmeas N, Zarahn E, Anderson KE, et al. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Arch Neurol. 2004;61(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Belleville S, Clément F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134(pt 6):1623–1634. [DOI] [PubMed] [Google Scholar]