Abstract
The studies on the prevalence of dementia, depression, and mild cognitive impairment (MCI) in Greece are sparse and show major variations of prevalence depending on geographical areas, nutritional habits, and the way of living. The aim of this door-to-door study was to find the prevalence of dementia, depression, and MCI in a rural Greek population. Four hundred and forty-three individuals older than 61years following the application of specific criteria were diagnosed with: normal cognition, depression, MCI with and without depression, and dementia with and without depression. Four diagnostic methods were used, 2 of which included Mungas correction for age and education. After Mungas adjustment, the results were as follows—depression: 33.9%; MCI: 15.3%; MCI with depression: 8.6%; dementia: 2.0%; and dementia with depression: 7.2%. Dementia is less prevalent compared to global data and other Greek areas. Mild cognitive impairment is more prevalent than dementia. High percentages of depression may be related to low education.
Keywords: prevalence, dementia, MCI, depression, Mungas
Introduction
Dementia has been described as a clinical syndrome caused by neurodegeneration (Alzheimer’s disease [AD], Lewy body, and frontotemporal dementia being the most common pathologies) or a secondary syndrome (vascular, metabolic, hormonic, and infectious dementia), characterized by progressive deterioration in cognitive ability, behavior, and capacity for independent living. 1 Typically, it is a condition that usually affects older people. 2 –4 Because of longer life expectancy along with the lack of efficient therapeutic strategies, dementia is increasingly becoming a major public health problem.
The number of patients with dementia has been rapidly growing in last decade. According to the latest data of the World Alzheimer’s Report 2015, there are currently 47.47 million cases and the expected numbers for 2030 and 2050 are 75.63 and 135.46 million, respectively. 5 The prevalence of dementia ranges from 1% in people younger than 60 years to 45% in people older than 85 years. The age-standardized prevalence of dementia for people aged ≥60 years varied between 5% and 7% in most world regions, with a higher prevalence in Latin America (8.5%), and a distinctively lower prevalence in the 4 sub-Saharan African regions (2%-4%). 1 Demographic aging is progressing rapidly in the low- and middle-income countries (LAMICs), such as China, India, and South America, and 58% of all people with dementia live in these countries. 5
Mild cognitive impairment (MCI) is an intermediate state between normal cognitive aging (becoming significantly forgetful) and dementia. The construct of MCI has evolved to capture this predementia phase of cognitive dysfunction. 6,7 Ιt was observed that people who meet the criteria for MCI are likely to progress to dementia at a rate of approximately 12% per year, compared to 1% to 2% for cognitively normal people of the same age. 7,8 However, many people with MCI never develop dementia, and some types of MCI can be static or may in some cases revert to normal cognition. 9,10 Recent data indicated that the percentage of patients with MCI is between 1.7% and 22.6% in Europe, America, and Brazil. 11 Furthermore, the incidence (new cases in a specified period) of MCI ranges between 51 and 76.8 per 1000 person-years. 11 More recent data indicated an essential variation for both incidence (MCI: 21.5-71.3 per 1000 person-years) and prevalence (MCI: 3%-42%) in North America, Europe, Africa, Asia, and Australia. 12 To our knowledge, very few population-based studies have been published regarding LAMICs. A recent study in LAMICs regions indicated that Latin America has an amnestic MCI prevalence ranging between 3.8% and 6.3%, India has a crude amnestic MCI prevalence of 4.3%, and China has the lowest prevalence of 0.8%. 13
Depression is the most common comorbidity in cognitive impairment among the elderly patients. Late-life depression and cognitive impairment have become a global issue that burdens the health-care system and society. The worldwide prevalence of late-life depression was estimated to range from 3% to 30% 14 and is consistently associated with an increased risk of dementia and particular in AD and vascular dementia. 15 –18 However, it remains unclear whether a history of depression is a true risk factor for dementia or rather represents a prodromal clinical phase of the neurodegenerative changes that occur in AD. Depression may be a psychological response to AD or an outcome of the same pathogenic process that lead to the other symptoms of AD (eg, aberrant amyloid-β processing, tau hyperphosphorylation, etc). 19 Furthermore, it has been reported the potential role of depression in the conversion from normal cognition to MCI and from MCI to dementia. 20 –24 Although late-onset depression increases the risk of progression to MCI, chronic depression is associated with only a modest increase in the risk of MCI to AD transition. 25
The studies on the prevalence of dementia and MCI in Southern Europe, 26 –28 and Greece, in particular, are sparse. 29 –31 However, the prevalence of depression or its relation with cognitive decline has examined extensively in Greek area. 31 –36 The aim of this study is to examine the prevalence of cognitive impairment and depression symptoms in a rural Greek population, residing on the island of Crete, the birthplace of Mediterranean diet. We thus seek to estimate the prevalence of (a) normal mental status, (b) depression, (c) dementia with depressive symptoms, (d) dementia without depressive symptoms, (e) MCI with depressive symptoms, and (f) MCI without depressive symptoms.
Materials and Methods
Study Design
This is a door-to-door, home-based questionnaire-type study. It began in July 2006 and ended in January 2007.
Setting Participants
The municipality of Milopotamos is a rural area close to Heraklion, the largest city of Crete. Milopotamos comprises 8 villages whose residents older than 65 years were 1726 according to the municipality data. Some of the residents (468 people) were not tracked, others (170 people) denied to participate in the study, and another 546 people had moved out of the villages and could not be detected, whereas some of them (99 people) were unable to answer the questionnaire or answer some of the questions. This is a not surprising condition due to the population targeted: Elderly patients with low levels of education, often suspicious, reserved and with limited exposure to and familiarity with voluntary participation in research. Finally, 443 residents participated in this study.
Study Procedure
This study was carried out by 2 research assistants, students of the Department of Social Work, Crete, and a group of students of the Department of Social Work after intensive training by a neuropsychologist and a social worker, in collaboration with a neurologist, specialist in dementia, and who is working at the Third Department of Neurology at the Aristotle University of Thessaloniki. The census lists of Milopotamos residents were obtained from the municipality registry office. All municipality-registered residents aged 65 and older were eligible for the study. First, we contacted the mayor of the municipality of Milopotamos, in order to request permission for our research and seek support if required. The study protocol was explained and discussed in meetings with general practitioners, local health authorities, and the residents. We also contacted authorities (presidents, priests, and rural doctors) of each village requesting their assistance in raising awareness concerning dementia among residents. We would not have found the addresses of the residents without the precious help of the municipality employees. All participants were assessed in person at their residence by the research assistants and students who were well trained in completing the questionnaires. Initially, a questionnaire on demographics was administered, whose items concerned age, gender, education, occupation, marital status, health problems (illness, medication), self-care, use of leisure time, lifestyle habits (smoking, alcohol use, exercise), and awareness/perceptions about AD. Subsequently, the neuropsychological battery described below was administered and recorded. The scores and results were forwarded to the neurology specialist in dementia, who interpreted test scores according to established cutoffs and was responsible for the final diagnosis.
Ethical Considerations
The study protocol was approved by the research ethics committee of the School of Health and Welfare Services of the Technological Educational Institute of Crete and was made in accordance with the ethical standards of the Institute and the guidelines outlined in the Declaration of Helsinki. 37 The researchers informed the participants of the study procedures and discussed thoroughly different areas of concern, including data protection. Patients who agreed to participate in the study signed the informed consent document and were provided with the researchers’ contact details.
Neuropsychological Assessment
A neuropsychological test battery was administrated in order to evaluate not only the participants’ cognitive and mood status but also activities of daily living. This battery includes the Mini–Mental State Examination (MMSE) or the Hindi Mental State Examination for illiterates (Hindi), the Instrumental Activity of Daily Living (IADL), and the Geriatric Depression Scale (GDS). In our study, the Greek version of MMSE 38 was administered to people who had completed at least 5 years of basic education. In those with fewer years of education, we used the Hindi test instead. 39 The IADL is an old test which describes instrumental tasks in a person’s everyday life and is used to assess the functional status: using the telephone, going shopping, preparing own meals, doing housework, laundering, using transportation, managing medicine, and handling money. However, in our study, we only used the question items which assessed the ability to use the telephone, transportation, medication, and financial behavior. The choice of these question items was based on the assumption that the male population in Greece is not usually involved in housework. 40,41 The short form of the GDS with 15 closed questions was also used in order to assess the mood and the emotions of the elderly patients. Scores between 0 and 6 are considered normal while scores between 7 and 15 indicate depressive symptoms. 42
Diagnosis
The participants of our study were divided into 6 groups according to appropriate criteria—(1) normal mental status, (2) depressive symptoms, (3) MCI with depressive symptoms, (4) MCI without depressive symptoms, (5) dementia with depressive symptoms, and (6) dementia without depressive symptoms. We used 4 methods of diagnosis in order to compare the prevalence in each of them and discuss their methodological value.
In method 1, the participants were divided into groups based on the MMSE/HINDI and GDS scores. The MMSE/HINDI score for MCI was less than 28 and greater than 24. In MCI without depressive symptoms, the GDS score was less than 6, and for MCI with depressive symptoms, the GDS score was greater than 6. We used the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM-IV) criteria 43 for depression diagnosis (MMSE/HINDI score >28), with a GDS score greater than 6. The DSM-IV dementia criteria were applied when the MMSE/HINDI score was less than 24. For dementia without depressive symptoms, the GDS score was lower than 6, and for dementia with depressive symptoms, the GDS score was greater than 6. Finally, in normal mental status diagnosis, group had MMSE/HINDI over 28 and GDS lower than 6.
In method 2, the participants were divided according to first diagnosis criteria plus the Mungas adjustment (MMSEadj), 44 a correction of MMSE/HINDI for age and education. In method 3, we used Petersen criteria 45 for MCI diagnosis with MMSE/HINDI score less than 28 and greater than 24 with no functional impairment according to the IADL scale: All IADL item respondents showed the ability to perform tasks independently or with some help. Besides, GDS score was less than 6 for MCI without depressive symptoms and greater than 6 in MCI with depressive symptoms. We used the DSM-IV criteria 43 for depression diagnosis, with an MMSE/HINDI score greater than 28, no functional problems, or if any they were due to coexisting health problems. In all these participants, the GDS score was greater than 6. Dementia diagnosis was based on the DSM-IV criteria and MMSE/HINDI score was less than 24. For dementia without depression, the GDS score was less than 6 and there were functional problems as well. For dementia with depression, the GDS score was greater than 6 with functional problems as well. Apart from the appliance of diagnostic criteria and neuropsychological tools (MMSE/Hindi, IADL, and GDS) for establishing the diagnosis, we collected information about factors such as the age, gender, current employment, marital status, health problems, and way of living of all participants. Finally, respondents not included in any of the above categories were of normal mental status: They had high score on the MMSE/HINDI (>28), no functional problems, and no depression symptoms.
Method 4 was based on method 3 criteria plus the MMSEadj.
Statistical Analysis
The raw prevalence of each diagnostic category was calculated with respect to the total sample of the study. Raw prevalence and prevalence after MMSEadj were calculated according to age group and gender.
Mungas correction was introduced by Mungas et al in 1996. 44 The authors claimed the MMSE may be biased against MCI and dementia due to demographic value effects, such as age and education. Consequently, Mungas is a safe measure of cognitive state in low educated people. Ιts mathematical operations contributed to the greater sensitivity and specificity of the MMSE test. The Mungas formula, providing an adjustment for age and education for the raw MMSE score, is given as follows:
For each prevalence estimate, 95% confidence intervals (95% CIs) were also calculated. Continuous variables were presented as mean value (SD) and compared using Student t test between groups. One-way analysis of variance with Bonferroni post hoc tests was used to check differences between means of continuous variables for the different diagnostic groups. Pearson χ2 test was used to check the independence between categorical variables. P values <.05 were considered statistically significant. Statistical analysis was performed using SPSS v21.0 (IBM Corp, Armonk, New York).
Results
A total of 443 patients were included in the study with 49.7% of those being females. Considering each diagnosis separately for the raw data and according to method 4, the percentage of females in the normal group (N = 146) was 32.2%. In the depression group (N = 150), the female percentage was 62.7%, in the MCI group (N = 68) 35.3%, in the MCI with depression group (N = 38) 73.7%, for the dementia group (N = 9) 55.6%, and in the dementia with depression group (N = 32), the female percentage was 68.8%.
Frequencies and relative frequencies of each diagnosis status for the raw data before and after MMSEadj are shown in Table 1 and Figure 1. There is an association between the diagnosis and the method used (χ2 = 228.97, degrees of freedom [df] = 15, P < .001). There are more individuals in the normal and depression groups after applying MMSEadj.
Table 1.
Frequencies and Relative Frequencies of the Sample for Each Diagnosis for the Raw Data of Methods 1, 2, 3, and 4.
| Diagnosis | Method 1, n (%) | Method 2, n (%) | Method 3, n (%) | Method 4, n (%) |
|---|---|---|---|---|
| Normal | 109 (24.6) | 186 (42.0) | 99 (22.3) | 146 (33.0) |
| Depression | 94 (21.2) | 157 (35.4) | 61 (13.8) | 150 (33.9) |
| MCI without depression | 73 (16.5) | 26 (5.9) | 89 (20.1) | 68 (15.3) |
| MCI with depression | 57 (12.9) | 28 (6.3) | 97 (21.9) | 38 (8.6) |
| Dementia without depression | 41 (9.3) | 11 (2.5) | 35 (7.9) | 9 (2.0) |
| Dementia with depression | 69 (15.6) | 35 (7.9) | 62 (14.0) | 32 (7.2) |
Abbreviation: MCI, mild cognitive impairment.
Figure 1.
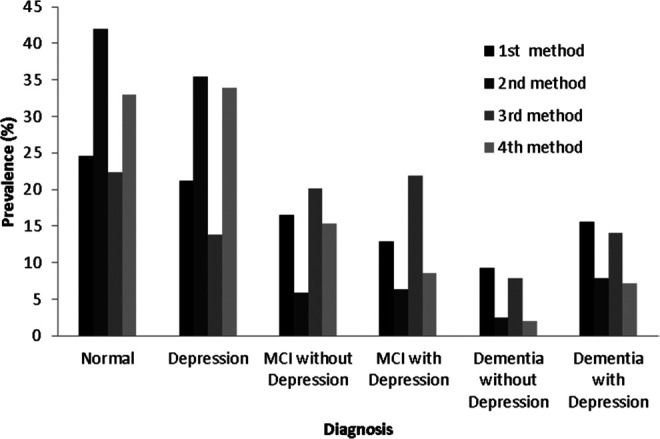
Prevalence of each diagnosis for the 4 different methods.
The mean age of all participants was 75.3 (7.1) and the mean years of education were 3.5 (2.7). There was no difference in age between males and females (P = .347). However, females had significantly fewer years of education (2.7 (2.4)) than males (4.3 (2.9); t[441] = 6.321; P < .001).
Age and Education in Diagnosis Prevalence
Table 2 shows the age and education characteristics according to method 4 of the total data set for males, females, and both. Table 2 also shows the group comparison concerning age and education in the normal group (p1) as well as the comparison between genders (p2).
Table 2.
Age and Education Characteristics According to Sex and Diagnosis for Method 4.a
| Males | Females | Both | p1 | p2 | |
|---|---|---|---|---|---|
| Age | |||||
| Normal (n = 146) | 73.2 (7.1) | 72.7 (6.0) | 73.0 (6.8) | 0.711 | |
| Depression (n = 150) | 75.4 (6.9) | 75.9 (7.3) | 75.7 (7.1) | 0.001 | 0.705 |
| MCI without depression (n = 68) | 76.6 (5.1) | 74.9 (8.3) | 76.1 (6.4) | 0.003 | 0.299 |
| MCI with depression (n = 38) | 75.3 (6.5) | 78.7 (7.1) | 77.8 (7.0) | <0.001 | 0.192 |
| Dementia without depression (n = 9) | 70.5 (4.5) | 79.8 (5.8) | 75.7 (6.9) | 0.268 | 0.034 |
| Dementia with depression (n = 32) | 78.3 (7.9) | 78.4 (7.6) | 78.4 (7.7) | <0.001 | 0.958 |
| Education | |||||
| Normal (n = 146) | 4.7 (3.1) | 2.6 (1.9) | 4.1 (2.9) | <0.001 | |
| Depression (n = 150) | 3.9 (2.4) | 1.9 (2.0) | 2.7 (2.4) | <0.001 | <0.001 |
| MCI without depression (n = 68) | 3.9 (2.4) | 3.4 (3.0) | 3.8 (2.9) | 0.484 | 0.446 |
| MCI with depression (n = 38) | 3.8 (2.4) | 4.0 (2.8) | 3.9 (2.7) | 0.826 | 0.844 |
| Dementia without depression (n = 9) | 7.5 (3.0) | 3.0 (2.4) | 5.0 (3.5) | 0.304 | 0.042 |
| Dementia with depression (n = 32) | 2.0 (1.3) | 3.7 (2.1) | 3.2 (2.0) | 0.086 | 0.028 |
Abbreviation: MCI, mild cognitive impairment.
ap1 is difference from normal and p2 is difference between males and females.
Gender and Age in Diagnosis Prevalence
Gender
There is a correlation between diagnosis and gender. Females have more depressive symptoms than males. This result was the same for method 1 (Pearson χ2 = 59.94, df = 5, P < .001), method 2 (Pearson χ2 = 49.10, df = 5, P < .001), method 3 (Pearson χ2 = 63.28, df = 5, P < .001), and method 4 (Pearson χ2 = 47.89, df = 5, P < .001; Table 3).
Table 3.
Gender Prevalence of Diagnosis of the Total Sample in Methods 1, 2, 3, and 4.
| Age Groups | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Normal | ||||||||
| 61-70 | 42 (56.0) | 47 (62.7) | 40 (53.3) | 43 (57.3) | 14 (21.5) | 23 (35.4) | 13 (20.0) | 20 (30.8) |
| 71-80 | 33 (30.6) | 63 (58.3) | 29 (26.9) | 41 (38.0) | 12 (12.2) | 29 (29.6) | 9 (9.2) | 23 (23.5) |
| 81-90 | 8 (22.9) | 15 (42.9) | 8 (22.9) | 13 (37.1) | 0 (0.0) | 6 (12.0) | 0 (0.0) | 4 (8.0) |
| 91+ | 0 (0.0) | 3 (60.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| P | 0.373 | 0.338 | ||||||
| Depression | ||||||||
| 61-70 | 15 (20.0) | 17 (22.7) | 12 (16.0) | 17 (22.7) | 16 (24.6) | 26 (40.0) | 12 (18.5) | 26 (40.0) |
| 71-80 | 19 (17.6) | 28 (25.9) | 15 (13.9) | 26 (24.1) | 22 (22.4) | 46 (46.9) | 13 (13.3) | 43 (43.9) |
| 81-90 | 7 (20.0) | 12 (34.3) | 3 (8.6) | 12 (34.3) | 13 (26.0) | 22 (44.0) | 5 (10.0) | 21 (42.0) |
| 91+ | 1 (20.0) | 1 (20.0) | 0 (0.0) | 1 (20.0) | 1 (14.3) | 5 (71.4) | 1 (14.3) | 4 (57.1) |
| P | 0.95 | 0.956 | ||||||
| MCI without depression | ||||||||
| 61-70 | 6 (8.0) | 2 (2.7) | 7 (9.3) | 6 (8.0) | 10 (15.4) | 4 (6.2) | 14 (18.4) | 8 (12.3) |
| 71-80 | 30 (27.8) | 10 (9.7) | 31 (28.7) | 32 (29.6) | 17 (17.3) | 6 (6.1) | 23 (23.5) | 12 (12.2) |
| 81-90 | 7 (20.0) | 2 (5.7) | 7 (20.0) | 5 (14.3) | 3 (6.0) | 1 (2.0) | 5 (10.0) | 3 (6.0) |
| 91+ | 0 (0.0) | 0 (0.0) | 2 (40.0) | 1 (20.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (14.3) |
| P a | 0.977 | 0.827 | ||||||
| MCI with depression | ||||||||
| 61-70 | 4 (5.3) | 3 (4.0) | 6 (8.0) | 3 (4.0) | 13 (20.0) | 4 (6.2) | 18 (27.7) | 5 (7.7) |
| 71-80 | 7 (6.5) | 2 (1.9) | 11 (10.2) | 5 (4.6) | 20 (20.4) | 9 (9.2) | 32 (32.7) | 12 (12.2) |
| 81-90 | 5 (14.3) | 2 (5.7) | 9 (25.7) | 2 (5.7) | 6 (12.0) | 7 (14.0) | 17 (34.0) | 9 (18.0) |
| 91+ | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 2 (28.6) | 1 (14.3) | 3 (42.9) | 2 (28.6) |
| P | 0.961 | 0.825 | ||||||
| Dementia without depression | ||||||||
| 61-70 | 4 (5.3) | 3 (4.0) | 5 (6.7) | 3 (4.0) | 4 (6.2) | 1 (1.5) | 1 (1.5) | 0 (0.0) |
| 71-80 | 11 (10.2) | 1 (0.9) | 14 (13.0) | 1 (0.9) | 9 (9.2) | 3 (3.1) | 6 (6.1) | 3 (3.1) |
| 81-90 | 3 (8.6) | 1 (2.9) | 3 (8.6) | 0 (0.0) | 6 (12.0) | 2 (4.0) | 4 (8.0) | 2 (4.0) |
| 91+ | 3 (60.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| P | 0.294 | 0.975 | ||||||
| Dementia with depression | ||||||||
| 61-70 | 4 (5.3) | 3 (4.0) | 5 (6.7) | 3 (4.0) | 8 (12.3) | 7 (10.8) | 7 (10.8) | 6 (9.2) |
| 71-80 | 8 (7.4) | 4 (3.7) | 8 (7.4) | 3 (2.8) | 18 (18.4) | 5 (5.1) | 15 (15.3) | 5 (5.1) |
| 81-90 | 5 (14.3) | 3 (8.6) | 5 (14.3) | 3 (8.6) | 22 (44.0) | 12 (24.0) | 19 (38.0) | 11 (22.0) |
| 91+ | 1 (20.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 3 (42.9) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| P | 1 | 0.637 | ||||||
Abbreviation: MCI, mild cognitive impairment.
aThe alpha level is equal to 0.05.
Age
There were no significant differences between the prevalence of method 1, the prevalence of method 2, and the prevalence coming from methods 3 and 4. A possible explanation may be the participants’ poor schooling.
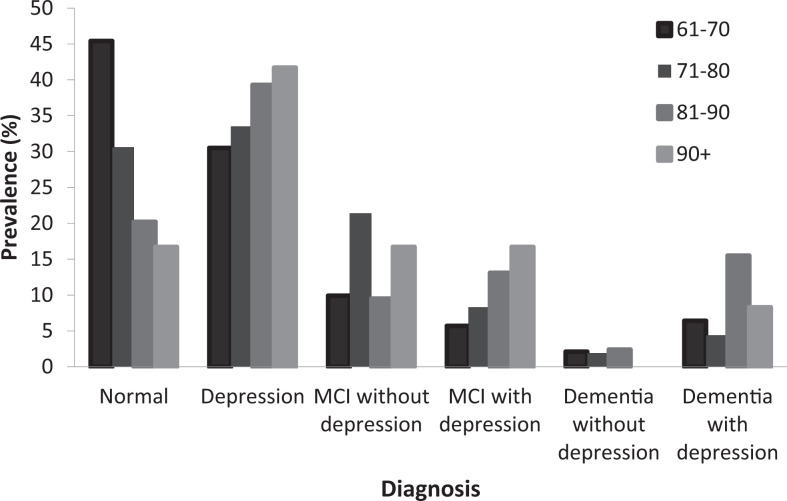
The prevalence of method 4 in the “normal” group was 45% (95% CI [36.8-53.2]) for the 61- to 70-year-old individuals, 31.1% (95% CI [24.8-37.4]) for the 71- to 80-year-old ones, and 20.0% (95% CI [11.5-28.5]) for the 81- to 90-year-old individuals. As people grow older, cognitive impairment and mood disorders are more possible.
The prevalence of “depression” was 30.7% (95% CI [23.1-38.3]) in the 61- to 70-year-old group, 33.5% (95% CI [27.1-39.9]) in the 71- to 80-year-old group, 38.8% (95% CI [28.4-49.2]) in the 81- to 90-year-old group, and 41.5% (95% CI [13.6-69.4]) in the 91+ year-old ones. Depression diagnosis is more prevalent in older ages.
The highest prevalence of “MCI without depressive symptoms” appeared to be in the 71- to 80-year-old group, which was 21.4% (95% CI [15.8-27.0]). The other groups had lower percentages. More specifically, in the 61- to 70-year-old group, the prevalence was 10.0% (95% CI [5.0-15.0]), in the 81- to 90-year-old group was 9.4% (95% CI [0.0, 15.6]), and in the 91+ year-old group was 16.7% (95% CI [0.0-37.8]).
The highest prevalence “MCI with depressive symptoms” appeared to be in the 91+ year-old individuals and was equal to 16.7% (95% CI [0.0-37.8]). Then, in the 81- to 90-year-old group, the prevalence was 12.9% (95% CI [5.8-20.0]). In the 71- to 80-year-old group, the prevalence was 8.3% (95% CI [4.5-12.1]) and in the 61- to 70-year-old group was 5.7% (95% CI [0.0-9.5]). The youngest group had the lowest prevalence of MCI with depressive symptoms.
The highest prevalence of “dementia without depressive symptoms” was found in the 81- to 90-year-old individuals and was equal to 2.4% (95% CI [0.0-5.7]), and the lowest prevalence was found in the 91+ year-old group and was equal to 0.0% (95% CI [0.0-0.0]). Moreover, in the 71- to 80-year-old group, the prevalence was 1.9% (95% CI [0.0-3.8]), whereas in the61- to 70-year-old group, the prevalence was 2.1% (95% CI [−0.3 to 4.5]).
The highest prevalence of “dementia with depressive symptoms” appeared in the 81- to 90-year-old group and was equal to 16.5% (95% CI [8.6-24.4]) and then in the 91+year-old group which was 8.3% (95% CI [0.0-23.9]). The rest of age groups had lower prevalence. In the 71- to 80-year-old group, the prevalence was 3.9% (95% CI [1.3-6.5]) and in the 61- to 70-year-old group was 6.4% (95% CI [2.3-10.5]; Table 4 and Figure 2).
Table 4.
Age Prevalence of Diagnosis of the Total Sample in Methods 1, 2, 3, and 4.
| Age Groups Both | Age Groups Both | Age Groups Both | Age Groups Both | Age Groups Both |
|---|---|---|---|---|
| Diagnosis | 1 | 2 | 3 | 4 |
| Normal | ||||
| 61-70 | 56 (40.0) | 70 (50.0) | 53 (37.9) | 63 (45.0) |
| 71-80 | 45 (21.8) | 92 (44.7) | 38 (18.4) | 64 (31.1) |
| 81-90 | 8 (9.4) | 21 (24.7) | 8 (9.4) | 17 (20.0) |
| 91+ | 0 (0.0) | 3 (25.0) | 0 (0.0) | 2 (16.7) |
| P | .207 | |||
| Depression | ||||
| 61-70 | 31 (22.1) | 43 (30.7) | 24 (17.1) | 43 (30.7) |
| 71-80 | 41 (19.9) | 74 (35.9) | 28 (13.6) | 69 (33.5) |
| 81-90 | 20 (23.5) | 34 (40.0) | 8 (9.4) | 33 (38.8) |
| 91+ | 2 (16.7) | 6 (50.0) | 1 (8.3) | 5 (41.5) |
| P | .789 | |||
| MCI without depression | ||||
| 61-70 | 16 (11.4) | 6 (4.3) | 21 (15.0) | 14 (10.0) |
| 71-80 | 47 (22.8) | 16 (7.8) | 54 (26.2) | 44 (21.4) |
| 81-90 | 10 (11.8) | 3 (3.5) | 12 (14.1) | 8 (9.4) |
| 91+ | 0 (0.0) | 1 (8.3) | 2 (16.7) | 2 (16.7) |
| P | .973 | |||
| MCI with depression | ||||
| 61-70 | 17 (12.1) | 7 (5.0) | 24 (17.1) | 8 (5.7) |
| 71-80 | 27 (13.1) | 11 (5.3) | 43 (20.9) | 17 (8.3) |
| 81-90 | 11 (12.9) | 9 (10.6) | 26 (30.6) | 11 (12.9) |
| 91+ | 2 (16.7) | 1 (8.3) | 4 (33.3) | 2 (16.7) |
| P | .973 | |||
| Dementia without depression | ||||
| 61-70 | 8 (5.7) | 4 (2.9) | 6 (4.3) | 3 (2.1) |
| 71-80 | 20 (9.7) | 4 (1.9) | 20 (9.7) | 4 (1.9) |
| 81-90 | 9 (10.6) | 3 (3.5) | 7 (8.2) | 2 (2.4) |
| 91+ | 4 (33.3) | 0 (0.0) | 2 (16.7) | 0 (0.0) |
| P | .819 | |||
| Dementia with depression | ||||
| 61-70 | 12 (8.6) | 10 (7.1) | 12 (8.6) | 9 (6.4) |
| 71-80 | 26 (12.6) | 9 (4.4) | 23 (11.2) | 8 (3.9) |
| 81-90 | 27 (31.8) | 15 (17.6) | 24 (28.2) | 14 (16.5) |
| 91+ | 4 (33.3) | 1 (8.3) | 3 (25.0) | 1 (8.3) |
| P | .845 | |||
Abbreviation: MCI, mild cognitive impairment.
Figure 2.
Prevalence of each diagnosis for method 4 for the different age categories.
Discussion
Our study population eventually consisted of 443 residents from a mountainous area of Heraklion, in Crete. Prevalence varied depending on the diagnostic method we used. Some studies have indicated that the adjusted MMSE has higher sensitivity and specificity than the unadjusted MMSE. 44,46,47 The MMSEadj, used in methods 2 and 4 was compared to the raw MMSE scores in methods 1 and 3 of each diagnosis (Table 1). An interesting finding was that the means of MMSE and GDS scores were higher in normal, depression, MCI, and MCI with depressive symptoms diagnosis after MMSEadj (Table 5). Furthermore, there was a higher prevalence in normal and depression groups and a lower prevalence in the “MCI” and “dementia” groups, after applying the MMSEadj (Table 1). We conclude that after adjusting for age and education, individual cognitive deficits fall within the range of normal or depression. Furthermore, in our findings, methods 3 and 4 of diagnosis were based not only on mental and cognitive state tests (as methods 1 and 2) but also on functionality in a daily life test. Due to the fact that functionality (as measured with IADL tool) is a diagnostic criterion of dementia and MCI and that MMSEadj corrects age and education differences, we took into consideration the raw scores of method 4 of each diagnosis as the most appropriate method to discuss prevalence (Table 6).
Table 5.
MMSE and GDS Scoring Per Diagnosis for the Sample Before and After Mungas Adjustment.
| Diagnosis | MMSE (Mean (SD)) | GDS (Mean (SD)) |
|---|---|---|
| Before Mungas adjustment | ||
| Normal | 29.09 (0.52) | 2.47 (1.58) |
| Depression | 29.11 (0.32) | 8.43 (1.85) |
| MCI | 25.48 (2.31) | 2.92 (1.64) |
| MCI + depression | 25.96 (2.61) | 8.93 (2.33) |
| Dementia | 20.31 (4.74) | 2.97 (1.60) |
| Dementia + depression | 18.15 (4.90) | 9.45 (2.22) |
| After Mungas adjustment | ||
| Normal | 29.81 (0.53) | 2.63 (1.57) |
| Depression | 29.84 (0.41) | 8.70 (2.07) |
| MCI | 28.06 (2.30) | 2.97 (1.68) |
| MCI + depression | 26.45 (1.90) | 9.39 (2.57) |
| Dementia | 18.59 (4.96) | 2.56 (1.81) |
| Dementia + depression | 19.74 (3.38) | 9.50 (2.20) |
Abbreviations: MMSE, Mini–Mental State Examination; GDS, Geriatric Depression Scale; MCI, mild cognitive impairment; SD, standard deviation.
Table 6.
Plos and Cons for Each of 4 Diagnosis Method.
| Diagnosis Methods | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Pros | (1) Direct administration for assessing cognitive status and mood behavior, without needing the relatives presence | (1) Direct administration for assessing cognitive status and mood behavior, without needing the relatives presence | (1) A well-established diagnosis with functional criteria | (1) A well-established diagnosis with functional criteria |
| (2) Adjustment for age and education | (2) Adjustment for age and education | |||
| Cons | (1) No data regarding the functionality | (1) No data regarding the functionality | (1) Missing the adjustment of age and education | – |
| (2) Missing the adjustment for age and education |
Summarizing our main findings, people are more likely to develop dementia and MCI with or without depressive symptom as they grow older. These data are consistent with previous studies arguing that dementia affects older people. 1,2 The unique Cretan nutritional habits may account for the low percentages of dementia in our study. In specific, the prevalence of dementia (with and without depression) according to method 4 appeared to be 9.2%. In a similar study population with Polish participants (55-104 years old), the prevalence of dementia after Mungas correction was 12.1%, a percentage much higher than ours. 47 A possible explanation may be the different methodology of studies and different cultures, including Mediterranean nutrition of Cretan residents. The prevalence of MCI (with and without depression) was 23.9% and the prevalence of depression was 33.9%, whereas in the normal groups was 33% with adjustment.
Prevalence of Depressive Symptoms in Normal Cognition, Dementia, and MCI
A significant finding of this study was the increased proportion of individuals with depressive symptoms in all age groups (33.9%). The high prevalence of depressive symptoms in all age group of our study may be not only due to the participants’ low education (3.5 years (2.7)) but also to the restrictive way of living in a village. The indicator of lower education is associated with both odds of dementia and substantial depressive symptoms or with late-life depression, which is in agreement with the findings of other studies. 48 –50 Besides, in our results, lower education seems to be positively related with depression, whereas higher education seems to be a protective factor for depression, MCI, dementia, and dementia and depressive symptoms. The age groups with the highest prevalence of dementia with depressive symptoms were the 81- to 90-year-old group (16.5%). We could hence conclude that depressive symptoms are more likely to coexist with dementia as age increases. Although this finding comes in agreement with many studies, it is substantial lower than in others. In a Canadian Community Health Survey, depression seems highly prevalent (18%-28%) in neurological conditions such as AD/dementia, dystonia, multiple sclerosis, Parkinson disease, stroke, migraine, epilepsy, and spina bifida. 51 Other studies in China and Australia indicate that the prevalence of AD with depression ranges from 25% to 74.9%. 52,53 Moreover, the higher level of depressive symptoms in AD diagnosis was associated with worse clinical outcome such as faster subsequent cognitive and functional decline. 54 Also, findings from a study across 8 Europeans countries with severe demented participants indicated that 30% of the sample had depression. 55
Furthermore, MCI with depressive symptoms prevailed in the 91+ year-old group (16.7%) rather than in the 81- to 90-year-old one (12.9%). Nonetheless, we need to highlight the fact that the number of participants in the 91+ group was very small, only 12 participants, so there is a limitation in our conclusions. These data confirm that depression may lead to or coexist not only with dementia but also with MCI as individuals grow older. Little is known about the prevalence of depressive symptoms in MCI by other studies. Individuals with MCI, especially aMCI, present with more depressive symptoms than the cognitively normal individuals. 56 Another recent systematic review showed that 20% of patients with MCI aged 55 years and older seem to suffer from minor depression. 57 Besides, very recent data provided evidence that depressive symptoms may accelerate the risk of developing dementia in patients with MCI and after 24 months of a follow-up assessment, 2% of participants developed dementia. 58,59 These findings highlight the importance of assessing and treating depressive symptoms in MCI. 56 Nevertheless, a recent meta-analysis stressed that it is unclear whether depressive symptoms is a risk factor for progressing dementia or whether it is an early biomarker which indicates the development of depressive symptomatology in the prodromal stages of the disease. 15 Interestingly, depression, dementia, and MCI share some common pathological features. There are demonstrated links between the development of depression and increased cerebral atrophy, neuroinflammation, oxidative stress, white matter lesions, 60 and vascular changes in the brain. 61,15 Given that these pathological changes are common in depression, dementia, and MCI as a predementia phase, there is a mediating neurobiological mechanism linking them. 62
Prevalence in Dementia
The prevalence of dementia varies around the world, depending on cultural and socioeconomic differences among nations. In the United States, the prevalence of dementia among 856 individuals aged 71 and older was 13.9% in 2002, whereas a recent study in 2010 with 856 individuals older than 70 years indicated 14.7% which is higher than ours (9.2%), probably due to the different methodology. 63,64 A recent review in China, which included 89 studies and 340 247 participants, indicated that the prevalence of dementia in 2010 was 2.6% for the 65- to 69-year age group and 60.5% for the 95- to 99-year age group, 65 a percentage quite different from our findings for the 61- to 70-year age group (8.5%). Another study by Suh and Kee in 2010 showed that the prevalence of people with dementia older than 65 years in Korea, China, Taiwan, Singapore, and Japan varied between 9.7% and 14.3%, a percentage quite similar to our findings (9.2%). Overall prevalence rates in Japan and Singapore were significantly lower than those in Korea, China, and Taiwan. Prevalence rates in older groups (aged 75 and older) were markedly higher than those in younger groups (aged 65-74). 66 The studies in Africa, especially in the sub-Saharan regions, are very sparse. An early study with 2494 participants >65 years old, divided into 2 groups of Africans (African Americans and African Nigerians) in 1995, showed that the rates of dementia were 2.29% with a lower proportion for African Nigerians. 67 More recently in 2013, Prince and colleagues, having used meta-analysis, showed that the prevalence in this area seems to be around the same percentages (2.07%). 1 However, Ramlall and colleagues in 2013 presented a much higher percentage of dementia prevalence (7.9%) in South Africa, but the sample was too small (N = 140). 68 In our view of 14 selected studies from 1988 to 2001 and over 600 participants in each study, the crude prevalence rates of dementia in Europe varied between 5.9% and 9.4% for individuals aged 65 years and older, 69 a range that includes our percentage (9.2%). Another study by Lobo and colleagues in 2000, with 2346 participants aged 65 years and older, showed that the prevalence of dementia in Europe was 6.4%. 70 According to a EUROcODE report in 2006, the European rate of dementia prevalence is 7.1%, with Spain (16.3%, Ν = 1460), Germany (17.4%), France (17.8%), Italy (21.9%), and Sweden (13%) featuring the highest percentages. 71 Nevertheless, in the same report, there are studies with very different results in prevalence such as Spain (9.4%, N = 1954) and Italy (6.5%, N = 7930; 9.8%, N = 968). Limitations in methodology among studies are a possible reason for the lower or the higher prevalence in these areas. Low percentages of dementia recurred in a recent study of an urban Spanish region in 2011 (4.3%, Ν = 480), in line with previous studies, 72 whereas in Portugal, the percentage for 2010 was 2.7%. 73 According to the latest data of 2015, the proportion of new cases of dementia in Europe has dropped, and the crude prevalence is 5.9% with Western Europe featuring the highest prevalence (6.9%). 5
Prevalence in MCI
Worldwide findings indicate that the MCI prevalence ranged between 3% and 42% in Europe, the United States, Asia, Australia, and Africa from 1984 to 2008. Specifically, the prevalence of MCI participants older than 60 years in Europe was 42.9%, 1.4% in North America, 17.1% in Asia, 5.7% in Australia, and 2.9% in Africa. According to the latest data, the MCI prevalence ranges between 5.0% and 36.7% in the United States, Europe, Asia, and Australia, 74 that is compatible with our findings (23.9%). An early study showed that the prevalence of MCI in individuals aged 65 years and older was 28.3% in an urban community of the United States. 75 A few years later, another study by Plassman and colleagues corroborated this prevalence in the entire United States (22.2%), a percentage lower than ours. 76 Nevertheless, in 2012, the prevalence of people older than 60 years with MCI in the southern regions of the United States and Mexico appeared to be much lower (6.45%) probably because of the different culture and different education. 77 Moreover, in a recent meta-analysis, it was shown that the prevalence of people older than 60 years with MCI in China was 12.7%, 78 a percentage much lower than ours (23.9%), with Eastern China reaching 9.6% and Western China 14.7%. Poor education seems to be a catalytic factor for the high prevalence in Western China. Additionally, there seems to be a different prevalence depending on the residing area (rural vs urban) of Chinese people. The prevalence of people older than 65 years with MCI in Japan seems to be quite higher 18.8%. 79 Then, the prevalence of MCI participants older than 65 years in Korea was 32.9% in an earlier study, a higher percentage than ours. 80 In North India, the percentages are much higher, with the prevalence of dementia reaching 19.26%, 81 whereas in the region of Kolkata (India), the percentages of MCI prevalence seem to be much lower (6%). 82 Studies in Southern Europe and Mediterranean countries are of particular interest to our study. In Bulgaria, in MCI participants older than 65 years, the prevalence was found to be 6.7%. 83 In Italy, MCI prevalence for people older than 60 years was 6%. 84 In Spain, MCI prevalence ranged between 13.8% and 35.2%. 85 However, the prevalence of MCI found in our study is lower than the one reported for participants older than 65 years with MCI in France (42%), possibly because of the much lower means of schooling years in education (for men: odds ratio [OR] = 2.3, 95% CI [1.3-4.1] and women: OR = 2.2, 95% CI [1.3-3.6]). 86,37
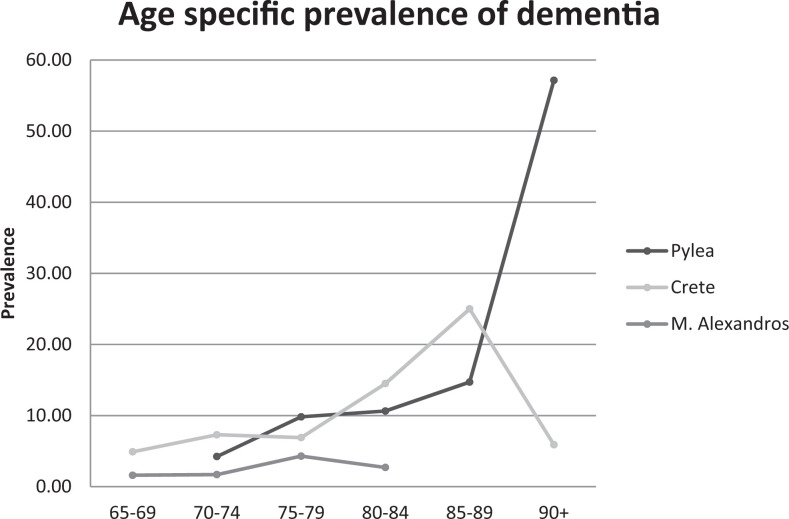
Few studies have been conducted in Greece concerning the prevalence of dementia and MCI. In a study at a rural area of Northern Greece, called M. Alexandros, the prevalence of MCI according to the age groups was 1.6% for the 65- to 69-year age group, 1.7% for the 70- to 74-year age group, 4.3% for the 75- to 79-year age group, and 2.7% for those aged 80+ group. 30 In the same study, the prevalence of MCI due to depression was 8.8%, a percentage similar to ours (8.6%), and 5.9% had only depression, a percentage much lower than ours (33.9%). A previous study in the municipality of Pylaia, an urban area in Thessaloniki, had indicated higher numbers of prevalence of dementia: 4.24% for the 70- to 74-year age group, 10.7% for the 75- to 79-year age group, 10.64% for the 80- to 84-year age group, 11.8% for the 85- to 89-year age group, and 36.7% for those aged 90+ group (Figure 3). 29 The urban versus rural way of living may account for the difference in findings between the latter study and the present one. Moreover, the application of Mungas correction in the present study and its absence in the previous one might explain the different findings. Another study from the Northern Greece showed that 37.6% of the men and 41.6% of the women had cognitive impairment. 31
Figure 3.
Prevalence of dementia in Crete according to method 3 related to those in Pylea and M. Alexandros.
Despite the fact that there only few studies in cognitive impairment and depressive symptoms, several studies about prevalence in depression have been conducted in Greece area. In a rural area, Velestino, the prevalence of mild depression in late life was higher (27%) and much lower (12%) in moderate to severe depression than in our study. 34 Besides, a recent study in Ikaria island, which is reported to be 1 of the places where people have the highest life expectancies in the world, indicates a very low prevalence of depression. 36 Thus, elders in Ikaria have much lower rates in depressive symptoms as compared with their peers from the other Greek islands or Cyprus 87 (in Cyprus, 25% of elderly men and 35% of elderly women had severe depressive symptoms, whereas 54% of men and 70% had mild depressive symptoms). Few years ago, another study in Athens indicated a quite high prevalence of depressed mood (27.1%) but only a 9.5% have clinical types of depression. 32 Another study in Athens indicated that 29.9% of the women and 19.6% of the men had mild to moderate depression, 31 a percentage higher than ours. 31 To our knowledge, this is 1 of the few studies in Greece which has attempted to make direct comparisons of prevalence estimates of dementia and MCI taking into consideration the cultural background and the nutritional habits of the participants. The Mediterranean diet is based on olive oil, fruit, vegetables, and fish. There are worldwide studies investigating the association between the Mediterranean diet and a slower cognitive decline. 88,89
Conclusion
Our aim was to demonstrate the prevalence of depression, MCI, and dementia in a small area in Crete island, Greece. We used 4 different methods to establish the diagnosis. We suggest that functionality in daily life and adjustment for education and age in MMSE score are the appropriate criteria to establish a dementia diagnosis. After Mungas correction, there was a high prevalence in normal and depression groups and a lower prevalence in the MCI and dementia groups. We conclude that after adjusting for age and education, individuals’ cognitive deficits fall within the range of normal or depression.
Study Limitations
This is not a clinical study and there were no laboratory results or neuroimaging data to analyze. Therefore, it was impossible to discriminate the type of dementia for each participant. Besides, this is a nonblinding study procedure. There was a door-to-door research and participants wanted to know about the study aim. Also, the selected villages are isolated and the citizens may be poorly informed about dementia, depression, and its prevention. Besides, an important limitation was the sample size, which was small because of participants’ refusal or suspiciousness to answer. Furthermore, this sample compounds a small proportion of a specific region of Greece and may not be representative of the Greek population. The methodology for the clinical diagnosis may have resulted in differential outcomes. Methods 1 and 2 for MCI diagnosis were based on the MMSE and a depressive scale, whereas methods 3 and 4 were based on Petersen criteria. Although we compared our results according to method 4 in discussion, we must take into account the different methodology used in other researches.
The response rate in this survey is 25.6%. However, it is very common in Greece that a person is registered in a village but they actually leave in urban areas. Considering this fact and deducting the 546 individuals who could not be found, the response rate increases to 37.5%. Moreover, there are studies that show that surveys with a low response rate are not necessarily low in validity. A study from Visser et al showed that a response rate of as low as 20% can give more accurate results than a survey with a response rate of 60% or more. 90
Footnotes
Author’s note: The author Vassiliki Pattakou-Parasyri at the time of this research was affiliated to Nicosia University, Nicosia, Cyprus, Greece and now the author is associated with Department of Social Work, School of Health and Welfare Services, University of Crete, Heraklion, Greece.
The author Eirini Kavalou, at the time of this research was affiliated to Zoniana, Crete, Greece and now the author is associated with Department of Social Work, School of Health and Welfare Services, University of Crete, Heraklion, Greece.
And the author Niki Galoutzi is now affiliated to Department of Social Work, School of Health and Welfare Services, University of Crete, Heraklion, Greece.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2. doi:10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2. Sousa RM, Ferri CP, Acosta D, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet (London England). 2009;374(9704):1821–1830. doi:10.1016/S0140-6736(09)61829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi:10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sousa RM, Ferri CP, Acosta D, et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: a 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010;10(1):1–12. doi:10.1186/1471-2318-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince M, Wimo A, Guerchet M, Gemma-Claire A, Wu YT, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia – An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 6. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 7. Petersen RC, ed. Mild Cognitive Impairment: Aging to Alzheimer’s Disease. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 8. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi:10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 9. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761–767. doi:10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of the 0.5s: predictors of 2-year outcome in mild cognitive impairment. J Int Neuropsychol Soc. 2011;17(2):277–288. doi:10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2010;29(2):164–175. doi:10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- 12. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8(1):14–21. doi:10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13. Sosa AL, Albanese E, Stephan BCM, et al. Prevalence, distribution, and impact of mild cognitive impairment in latin America, China, and India: a 10/66 population-based study. PLoS Med. 2012;9(2):e1001170. doi:10.1371/journal.pmed.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):249–265. [DOI] [PubMed] [Google Scholar]
- 15. Cherbuin N, Kim S, Anstey KJ. Dementia risk estimates associated with measures of depression: a systematic review and meta-analysis. BMJ Open. 2015;5(12):e008853. doi:10.1136/bmjopen-2015-008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. [DOI] [PubMed] [Google Scholar]
- 17. Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF, III. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. doi:10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim S, Woo SY, Kang HS, et al. Factors related to prevalence, persistence, and incidence of depressive symptoms in mild cognitive impairment: vascular depression construct. Int J Geriatr Psychiatry. 2016;31(7):818–826. doi:10.1002/gps.4400. [DOI] [PubMed] [Google Scholar]
- 19. Mintzer J, O’Neill C. Depression in Alzheimer’s disease: consequence or contributing factor? Expert Rev Neurother. 2011;11(11):1501–1503. doi:10.1586/ern.11.145. [DOI] [PubMed] [Google Scholar]
- 20. Panza F, Capurso C, D’Introno A, et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian longitudinal study on aging. Int J Geriatr Psychiatry. 2008;23(7):726–734. doi:10.1002/gps.1967. [DOI] [PubMed] [Google Scholar]
- 21. Unverzagt FW, Ogunniyi A, Taler V, et al. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in African Americans. Alzheimer Dis Assoc Disord. 2011;25(1):4–10. doi:10.1097/WAD.0b013e3181f1c8b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravaglia G, Forti P, Lucicesare A, et al. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. Am J Geriatr Psychiatry. 2008;16(10):834–843. doi:10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- 23. Kohler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychol Med. 2010;40(4):591–602. doi:10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- 24. Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–1293. doi:10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 25. Spalletta G, Caltagirone C, Girardi P, Gianni W, Casini AR, Palmer K. The role of persistent and incident major depression on rate of cognitive deterioration in newly diagnosed Alzheimer’s disease patients. Psychiatry Res. 2012;198(2):263–268. doi:10.1016/j.psychres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 26. Ravaglia G, Forti P, Maioli F, et al. Education, occupation, and prevalence of dementia: findings from the Conselice study. Dement Geriatr Cogn Disord. 2002;14(2):90–100. doi:64930. [DOI] [PubMed] [Google Scholar]
- 27. Tognoni G, Ceravolo R, Nucciarone B, et al. From mild cognitive impairment to dementia: a prevalence study in a district of Tuscany, Italy. Acta Neurol Scand. 2005;112(2):65–71. doi:10.1111/j.1600-0404.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 28. de Pedro-Cuesta J, Virues-Ortega J, Vega S, et al. Prevalence of dementia and major dementia subtypes in Spanish populations: a reanalysis of dementia prevalence surveys, 1990-2008. BMC Neurol. 2009;9:55. doi:10.1186/1471-2377-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsolaki M, Fountoulakis C, Pavlopoulos I, Chatzi E, Kazis A. Prevalence and incidence of Alzheimers disease and other dementing disorders in Pylea, Greece. Am J Alzheimers Dis Other Dement. 1999;14(3):138–148. doi:10.1177/153331759901400308. [Google Scholar]
- 30. Tsolaki M, Kakoudaki T, Tsolaki A, Verykouki E, Pattakou V. Prevalence of mild cognitive impairment in individuals aged over 65 in a rural area in North Greece. Adv Alzheimers Dis. 2014;3:11–19. doi:10.4236/aad.2014.31002. [Google Scholar]
- 31. Argyriadou S, Melissopoulou H, Krania E, Karagiannidou A, Vlachonicolis I, Lionis C. Dementia and depression: two frequent disorders of the aged in primary health care in Greece. Fam Pract. 2001;18(1):87–91. doi:10.1093/fampra/18.1.87. [DOI] [PubMed] [Google Scholar]
- 32. Madianos MG, Gournas G, Stefanis CN. Depressive symptoms and depression among elderly people in Athens. Acta Psychiatr Scand. 1992;86(4):320–326. [DOI] [PubMed] [Google Scholar]
- 33. Fountoulakis KN, O’Hara R, Iacovides A, et al. Unipolar late-onset depression: a comprehensive review. Ann Gen Hosp Psychiatry. 2003;2(1):11. doi:10.1186/1475-2832-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papadopoulos FC, Petridou E, Argyropoulou S, et al. Prevalence and correlates of depression in late life: a population based study from a rural Greek town. Int J Geriatr Psychiatry. 2005;20(4):350–357. doi:10.1002/gps.1288. [DOI] [PubMed] [Google Scholar]
- 35. Fountoulakis KN, Siamouli M, Magiria S, Kaprinis G. Late-life depression, religiosity, cerebrovascular disease, cognitive impairment and attitudes towards death in the elderly: interpreting the data. Med Hypotheses. 2008;70(3):493–496. doi:10.1016/j.mehy.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 36. Panagiotakos DB, Chrysohoou C, Siasos G, et al. Sociodemographic and lifestyle statistics of oldest old people (>80 years) living in Ikaria island: the Ikaria study. Cardiol Res Pract. 2011;2011:679187. doi:10.4061/2011/679187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Medical Association. World medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. http:10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 38. Fountoulakis KN, Tsolaki M, Chantzi H, Kazis A. Mini mental state examination (MMSE): a validation study in Greece. Am J Alzheimers Dis Other Dement. 2000;15(6):342–345. doi:10.1177/153331750001500604. [Google Scholar]
- 39. Tsolaki M, Iakovidou V, Navrozidou H, Aminta M, Pantazi T, Kazis A. Hindi Mental State Examination (HMSE) as a screening test for illiterate demented patients. Int J Geriatr Psychiatry. 2000;15(7):662–664. doi:10.1002/1099-1166(200007)15:7<662::AID-GPS171>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40. Millán-Calenti JC, Tubío J, Pita-Fernández S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50(3):306–310. doi:10.1016/j.archger.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 41. Allen SM, Mor V, Raveis V, Houts P. Measurement of need for assistance with daily activities: quantifying the influence of gender roles. J Gerontol. 1993;48(4):S204–S211. PubMed PMID: 8315244. [DOI] [PubMed] [Google Scholar]
- 42. Fountoulakis KN, Tsolaki M, Iacovides A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin Exp Res. 1999;11(6):367–372. doi:10.1007/BF03339814. [DOI] [PubMed] [Google Scholar]
- 43. American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 44. Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of mini-mental state examination for english- and spanish-speaking elderly. Neurology. 1996;46(3):700–706. doi:10.1212/WNL.46.3.700. [DOI] [PubMed] [Google Scholar]
- 45. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatrics. 1997;9(suppl 1):65–69. [DOI] [PubMed] [Google Scholar]
- 46. Pedraza O, Clark JH, O’Bryant SE, et al. Diagnostic validity of age and education corrections for the mini-mental state examination in older African Americans. J Am Geriatr Soc. 2012;60(2):328–331. doi:10.1111/j.1532-5415.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klich-Rączka A, Piotrowicz K, Mossakowska M, et al. The assessment of cognitive impairment suspected of dementia in Polish elderly people: results of the population-based PolSenior Study. Exp Gerontol. 2014;57:233–242. doi:10.1016/j.exger.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 48. Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25(4):289–304. doi:10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. [DOI] [PubMed] [Google Scholar]
- 50. Davydow DS, Zivin K, Langa KM. Hospitalization, depression and dementia in community-dwelling older Americans: findings from the national health and aging trends study. Gen Hosp Psychiatry. 2014;36(2):135–141. doi:10.1016/j.genhosppsych.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bulloch AGM, Fiest KM, Williams JVA, et al. Depression – a common disorder across a broad spectrum of neurological conditions: a cross-sectional nationally representative survey. Gen Hosp Psychiatry. 2015;37(6):507–512. doi:10.1016/j.genhosppsych.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 52. Li XL, Hu N, Tan MS, Yu JT, Tan L. Behavioral and psychological symptoms in Alzheimer’s disease. Biomed Res Int. 2014;2014:927804. doi:10.1155/2014/927804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162(11):2086–2093. doi:10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 54. Zahodne LB, Devanand DP, Stern Y. Coupled cognitive and functional change in Alzheimer’s disease and the influence of depressive symptoms. J Alzheimers Dis. 2013;34(4):851–860. doi:10.3233/JAD-121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giebel C, Sutcliffe C, Verbeek H, et al. Depressive symptomatology and associated factors in dementia in Europe: home care versus long-term care. Int Psychogeriatr. 2016;28(4):621–630. doi:10.1017/S1041610215002100. [DOI] [PubMed] [Google Scholar]
- 56. Shahnawaz Z, Reppermund S, Brodaty H, et al. Prevalence and characteristics of depression in mild cognitive impairment: the Sydney memory and ageing study. Acta Psychiatr Scand. 2013;127(5):394–402. doi:10.1111/acps.12008. [DOI] [PubMed] [Google Scholar]
- 57. Polyakova M, Sonnabend N, Sander C, et al. Prevalence of minor depression in elderly persons with and without mild cognitive impairment: a systematic review. J Affect Disord. 2014;152-154:28–38. doi:10.1016/j.jad.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 58. Mourao RJ, Mansur G, Malloy-Diniz LF, Castro Costa E, Diniz BS. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int J Geriatr Psychiatry. 2016;31(8):905–911. doi:10.1002/gps.4406. [DOI] [PubMed] [Google Scholar]
- 59. Makizako H, Shimada H, Doi T, et al. Comorbid mild cognitive impairment and depressive symptoms predict future dementia in community older adults: a 24-month follow-up longitudinal study. J Alzheimers Dis. 2016;54(4):1473–1482. doi:10.3233/JAD-160244. [DOI] [PubMed] [Google Scholar]
- 60. Hickie I, Naismith S, Ward PB, et al. Vascular risk and low serum B12 predict white matter lesions in patients with major depression. J Affect Disord. 2005;85(3):327–332. doi:10.1016/j.jad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 61. Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J Affect Disord. 2004;81(1):1–16. doi:10.1016/j.jad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 62. Hermida AP, McDonald WM, Steenland K, Levey A. The association between late-life depression, mild cognitive impairment and dementia: is inflammation the missing link? Expert Rev Neurother. 2012;12(11):1339–1350. doi:10.1586/ern.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. doi:10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi:10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 2013;381(9882):2016–2023. doi:10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 66. Suh GH, Kee BS. Prevalence of dementia in Asia: report of ASIADEM collaborative studies. Alzheimers Dement. 2010;6(4):S124. doi:10.1016/j.jalz.2010.05.390. [Google Scholar]
- 67. Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152(10):1485–1492. doi:10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 68. Ramlall S, Chipps J, Pillay BJ, Bhigjee AL. Mild cognitive impairment and dementia in a heterogeneous elderly population: prevalence and risk profile. Afr J Psychiatry. 2013;16(6):456–465. doi:10.4314/ajpsy.v16i6.58. [DOI] [PubMed] [Google Scholar]
- 69. Berr C, Wancata J, Ritchie K. Prevalence of dementia in the elderly in Europe. Eur Neuropsychopharmacol. 2005;15(4):463–471. doi:10.1016/j.euroneuro.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 70. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology. 2000;54(11 suppl 5):S4–S9. [PubMed] [Google Scholar]
- 71. Reynish E, Fratiglioni L, Prince M, Bickel H, Kiejna A, Georges J. EUROCODE: Report of WP7 2006. Prevalence of Dementia in Europe. Alzheimer Europe; 2006. [Google Scholar]
- 72. Rodríguez-Sánchez E, Mora-Simón S, Patino-Alonso MC, et al. Prevalence of cognitive impairment in individuals aged over 65 in an urban area: DERIVA study. BMC Neurol. 2011;11(1):147. doi:10.1186/1471-2377-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nunes B, Silva RD, Cruz VT, Roriz JM, Pais J, Silva MC. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010;10:42. doi:10.1186/1471-2377-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sachdev PS, Lipnicki DM, Kochan NA, et al. ; Cohort Studies of Memory in an International Consortium (COSMIC). The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10(11):e0142388. doi:10.1371/journal.pone.0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739–1746. doi:10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 76. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Juarez-Cedillo T, Sanchez-Arenas R, Sanchez-Garcia S, et al. Prevalence of mild cognitive impairment and its subtypes in the Mexican population. Dement Geriatr Cogn Disord. 2012;34(5-6):271–281. doi:10.1159/000345251. [DOI] [PubMed] [Google Scholar]
- 78. Nie H, Xu Y, Liu B, et al. The prevalence of mild cognitive impairment about elderly population in China: a meta-analysis. Int J Geriatr Psychiatry. 2011;26(6):558–563. doi:10.1002/gps.2579. [DOI] [PubMed] [Google Scholar]
- 79. Shimada H, Makizako H, Doi T, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc. 2013;14(7):518–524. doi:10.1016/j.jamda.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 80. Choi SJ, Jung SS, You YS, et al. Prevalence of Alzheimer’s dementia and its risk factors in community-dwelling elderly Koreans. Psychiatry Investig. 2008;5(2):78–85. doi:10.4306/pi.2008.5.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Singh VB. The prevalence of mild cognitive impairment in a north Indian elderly population: correlation with diabetes mellitus and socio-economic status. Alzheimers Dement J Alzheimers Assoc. 2016;10(4):P526. doi:10.1016/j.jalz.2014.05.825. [Google Scholar]
- 82. Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurol. 2007;68(23):2019–2026. doi:10.1212/01.wnl.0000264424.76759.e6. [DOI] [PubMed] [Google Scholar]
- 83. Dimitrov I, Tzourio C, Milanov I, Deleva N, Traykov L. Prevalence of dementia and mild cognitive impairment in a bulgarian urban population. Am J Alzheimers Dis Other Demen. 2012;27(2):131–135. doi:10.1177/1533317512442371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moretti F, De Ronchi D, Palmer K, et al. Prevalence and characteristics of mild cognitive impairment in the general population. Data from an Italian population-based study: the Faenza Project. Aging Ment Health. 2013;17(3):267–275. doi:10.1080/13607863.2012.732034. [DOI] [PubMed] [Google Scholar]
- 85. Rodr’iguez-Sánchez E, Mora-Simón S, Patino-Alonso MC, et al. Prevalence of cognitive impairment in individuals aged over 65 in an urban area: DERIVA study. BMC Neurol. 2011;11(1):1–13. doi:10.1186/1471-2377-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79(9):979–984. doi:10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 87. Mamplekou E, Bountziouka V, Psaltopoulou T, et al. Urban environment, physical inactivity and unhealthy dietary habits correlate to depression among elderly living in Eastern Mediterranean islands: the MEDIS (MEDiterranean ISlands Elderly) study. J Nutr Health Aging. 2010;14(6):449–455. [DOI] [PubMed] [Google Scholar]
- 88. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi:10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66(10):1210–1215. doi:10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Visser PS, Krosnick JA, Marquette J, Curtin M. MAIL surveys for election forecasting? An evaluation of the columbus dispatch poll. Public Opin Q. 1996;60(2):181–227. doi:10.1086/297748. [Google Scholar]