Abstract
Objectives
The aim of this study was to compare the applicability of the 1998 consensus diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD) with the recently proposed diagnostic criteria of the International bvFTD Criteria Consortium (FTDC).
Methods
We reviewed each individual item in the 1998 and FTDC criteria in 30 patients with bvFTD followed in a memory clinic (including 2 with the C9orf72 gene repeat expansion).
Results
All patients fulfilled the FTDC criteria (40% possible, 60% probable bvFTD) but only 66.7% fulfilled the 1998 criteria. One of the C9orf72 expansion carriers did not fulfill the 1998 criteria. This discordance was always due to the presence of exclusion features in the 1998 criteria, the most common being spatial disorientation and early severe amnesia.
Conclusion
The new FTDC criteria are less restrictive and hence more sensitive for the diagnosis of bvFTD.
Keywords: behavioral variant frontotemporal dementia, frontotemporal lobar degeneration, frontotemporal dementia, diagnostic criteria, C9orf72, differential diagnosis, diagnosis of neurodegenerative diseases.
Introduction
Frontotemporal dementia (FTD) is a complex neurodegenerative disease that is clinically characterized by progressive behavioral changes, frontal executive deficits and/or language difficulties. 1 It is the second most common early-onset dementia after Alzheimer’s disease (AD) and accounts for 5-10% of all neurodegenerative dementias. 2 The clinical spectrum of FTD encompasses 3 distinct clinical syndromes: the behavioral variant of FTD (bvFTD) and 2 language variants, namely semantic dementia and progressive nonfluent aphasia. Some patients can also present a parkinsonian syndrome, in particular, progressive supranuclear palsy syndrome and corticobasal syndrome (CBS-S), or associate motor neuron disease. 3 The FTD has a high prevalence of familial history compared to other dementias, suggesting a strong genetic component. 4 Mutations in GRN and MAPT genes are the most frequent genetic cause of FTD. A recent breakthrough has been the identification of a hexanucleotide repeat expansion in C9orf72 gene as the cause of some cases of FTD and amyotrophic lateral sclerosis. 5,6
Behavioral variant FTD is the most common of the 3 FTD syndromes, 7 and it is characterized by personality changes, impairment in social interaction, and functional decline. Changes in personality mainly consist of apathy, lack of empathy, emotional blunting, disinhibition, impulsive behavior, lack of concern for personal appearance and hygiene, and altered preference for foods. Special attention has lately been given to the so-called social cognition, particularly theory of mind abilities, 8 which appear to be specifically impaired in bvFTD. 9,10
In 1998, Neary and colleagues published consensus clinical criteria for the 3 syndromes that comprise FTD, 11 criteria that became standard use in the diagnosis of FTD. Over the last decade, however, some limitations have arisen. 12 In particular, the ambiguities in describing behavioral changes and the rigidity of exclusion criteria have led to a low sensitivity, especially in early stages of the disease. 13,14 Consequently, an international bvFTD Criteria Consortium (FTDC) recently proposed revised guidelines for the diagnosis of bvFTD, based on a multicenter sample of patients with pathologically confirmed FTD. 15 The most significant change in these revised criteria is that they are more flexible. The 1998 criteria require fulfillment of 5 core features to establish a diagnosis of bvFTD, whereas FTDC criteria only require any 3 of 6 sets of behavioral/cognitive symptoms to diagnose bvFTD. Furthermore, FTDC exclusion criteria are less restrictive, and patients can be diagnosed as either possible or probable bvFTD. Possible bvFTD is diagnosed when patients fulfill any 3 of the 6 inclusion criteria, while probable bvFTD is considered when patients additionally show functional decline and consistent structural or functional neuroimaging findings. The aim of the present study was to compare these revised criteria with the Neary 1998 criteria in a cohort of patients with clinical diagnosis of bvFTD followed in a memory clinic. We also discuss the application of the revised criteria in patients with bvFTD having a C9orf72 repeat expansion.
Patients and Methods
Patient Selection and Diagnostic Criteria
We included 30 patients diagnosed of bvFTD followed in the Memory Unit at Hospital de la Santa Creu i Sant Pau (Barcelona, Spain). These patients had been previously evaluated by a neurologist with experience in neurodegenerative dementias in our tertiary unit (D.A., J.F., M.C., R.B., and A.L.) who considered bvFTD the most likely diagnosis based on clinical, neuropsychological and neuroimaging data. The study had a “pragmatic” approach with the goal of assessing the available criteria for bvFTD. Therefore, we studied all patients diagnosed of bvFTD by judgment of an experienced clinician, independently of the application of available criteria for bvFTD. Between 2009 and 2011, 2937 consecutive patients were evaluated at the Memory Unit and 30 patients with bvFTD were selected. All patients and their caregivers underwent a structured interview with a neurologist (M.S.) who reviewed the medical and neurological history in detail, performed a complete neurological examination, and ascertained the presence of each individual item in the 1998 criteria. After the FTDC criteria were published in 2011, we retrospectively reviewed the clinical records, the neuropsychological tests, and neuroimaging examinations of these patients and determined the presence or absence of each item in the new FTDC criteria (S.C.) during the period 2009 to 2011. Each item was rated as “present” or “not present” and inconclusive items were discussed and rated at consensus meetings with neurologists and neuropsychologists. All patients were followed every 6 months during the study period.
As part of routine examination, all patients were administered the Mini-Mental State Examination (MMSE), the Global Deterioration Scale (GDS), the Clinical Dementia Rating (CDR), and the Neuropsychiatric Inventory (NPI), and patients with mild dementia underwent a formal neuropsychological examination. 16 Disease onset was defined as the year in which the patient’s family noticed the first neurological symptoms. All patients also underwent a brain structural imaging study, either magnetic resonance imaging (MRI, N = 27) or computerized tomography (CT, N = 3). Most patients had undergone brain functional imaging with 99mTc-HMPAO single-photon emission CT (SPECT, N = 7) or 18 -FDG positron emission tomography (PET, N = 18). All neuroimaging evaluations were performed within 6 months of the structured interview. Imaging findings were independently assessed by 2 experienced neurologists. The pattern of agreement between the 2 raters was 93%. Any discrepancies were evaluated by a third neurologist blinded to the clinical information.
Written informed consent was obtained from all participants or their caregivers. The study was approved by the ethics committee at our center.
Genetic Study
All patients had been previously screened for the GGGGCC hexanucleotide repeat expansion in C9orf72 gene and for mutations in GRN and MAPT genes in those with a family history of dementia, as previously described. 17
Statistical Analysis
Categorical variables were compared using the Pearson chi-square test or the Fisher’s exact test. One-way analysis of variance was used to compare continuous variables between groups and nonparametric statistical analyses (Kruskal-Wallis) were used for ordinal variables. Statistical significance for all the analyses was set at 5% (α = 0.05). All data were analyzed using the SPSS version 19.0.
Results
Patient Characteristics
The demographic, clinical, and imaging data of each patient are detailed in Online Appendix 1 at http://aja.sagepub.com/. Overall, there was a male predominance (60%), the mean age was 64.5 ± 10 years, the mean age of onset of the disease was 58.6 ± 8.7 years, and the mean disease duration was 6.0 ± 4.1 years. Mean education was 12 ± 5.6 years. Twenty-four (80%) patients had an onset before 65 years, 16 patients (53.3%) had a positive family history of dementia, and 2 patients had associated motor neuron disease. Median MMSE score was 20.5 (4.5-27.25; interquartile range) and median NPI score was 30.5 (22.75-39.25; interquartile range). Two patients were found to carry a C9orf72 expansion.
Sensitivity of bvFTD Criteria
Twenty patients (66.7%) fulfilled the 1998 criteria, whereas all patients met the FTDC criteria (40% possible and 60% probable). There was no statistical difference in sex, age, education, age of onset or duration of the disease or in scores of the MMSE, GDS, and CDR scales between patients who fulfilled the 1998 criteria and those who did not. However, patients diagnosed as probable bvFTD according to the FTDC criteria had lower scores in the MMSE test and higher scores in GDS and CDR scales than patients with a diagnosis of possible bvFTD. These results indicate that patients with probable bvFTD were likely to be in a more advanced stage of the disease. Conversely, NPI scores were similar between the 2 groups (Online Appendix 2 at http://aja.sagepub.com/).
The accomplishment of 1998 criteria differed according to the patient age. Although 76.5% of patients under the age of 65 fulfilled 1998 criteria, only 53.8% of patients over the age of 65 did so.
All patients were screened for genetic mutations and 2 were found to carry the recently described hexanucleotide repeat expansion in C9orf72. 17 One of these patients did not fulfill the 1998 criteria. Unlike 1998 criteria, FTDC criteria include the presence of a known pathogenic mutation as a definitive diagnosis of FTD. Nevertheless, if this were not taken into account, both patients with the expansion would have met criteria for possible but not for probable bvFTD in FTDC criteria. These 2 patients are therefore included in the possible bvFTD group in the statistical analysis.
We also assessed the sensitivity of each set of criteria using compatible neuroimaging as a “gold standard,” either structural (MRI/CT) or functional neuroimaging (SPECT/PET). All patients studied had undergone structural neuroimaging and 66.7% had an MRI/CT pattern suggestive of bvFTD (Online Appendix 1 at http://aja.sagepub.com/). Among them, 70% accomplished the 1998 criteria and 100% the FTDC criteria (10% possible and 90% probable). In addition, 83.3% patients had undergone functional neuroimaging (Online Appendix 1 at http://aja.sagepub.com/). Among them, 44% had a pattern suggestive of bvFTD and 81.8% of these fulfilled the 1998 criteria. All patients with a suggestive imaging pattern of FTD met criteria for probable bvFTD.
Frequency of Individual Items in 1998 Criteria
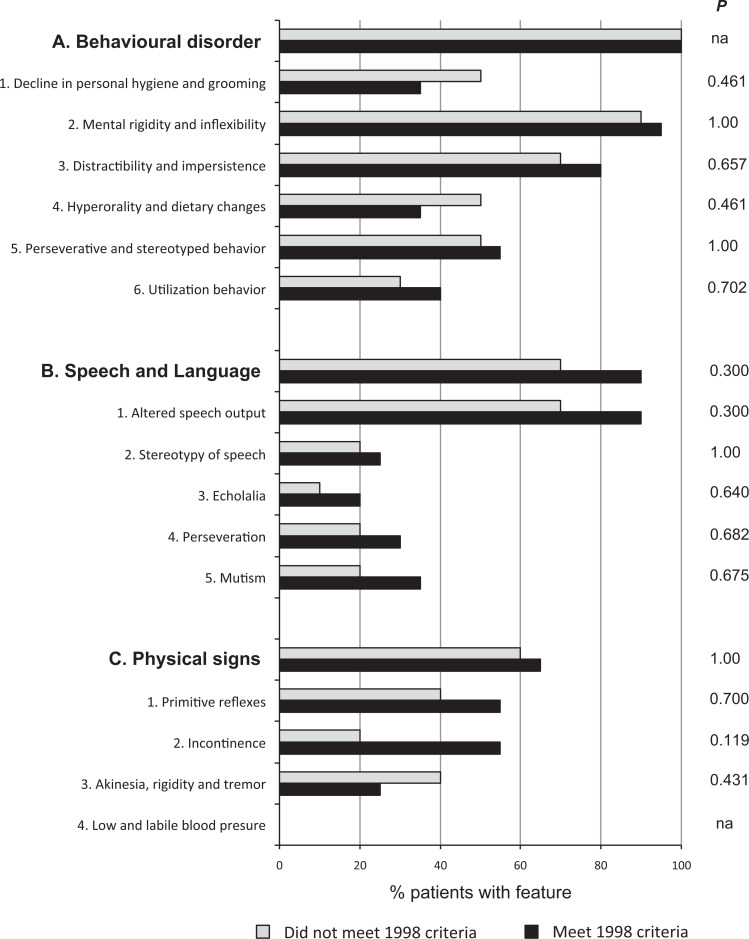
In all, 10 patients (33.3%) failed to meet the 1998 criteria. They all fulfilled the 5 core features but had at least 1 exclusion criteria. The supportive diagnostic features in the 1998 criteria are not mandatory for the diagnosis, but their presence increases the probability of a correct diagnosis. The supportive diagnostic features most frequently found in patients accomplishing 1998 criteria were “behavioral disorder” (100%) and “speech and language disorders” (83%). Regarding behavioral items, 93% of patients had “mental rigidity and inflexibility,” 77% had “distractibility and impersistence,” and 53% had “perseverative and stereotyped behavior.” Regarding the language and speech domains, the item most frequently found was “altered speech output” (83%). We did not find significant differences in the frequency of supportive diagnostic features between patients who met the 1998 criteria and those who did not (Figure 1). Therefore, the presence of exclusion criteria determined the accomplishment of the 1998 criteria but the core or supportive features did not.
Figure 1.
Frequency of individual 1998 supportive features in patients that fulfill these criteria compared with those that do not. Bars indicate the percentage of patients in each group that present the feature. na indicates not applicable.
Frequency of Individual Items in FTDC Criteria
The most recent FTDC criteria require the presence of 3 of 6 behavioral/cognitive core symptoms to make a diagnosis of possible bvFTD. The most frequent behavioral/cognitive abnormalities in our sample were “early loss of sympathy or empathy” (100%), “early behavioral disinhibition” (97%), and “early apathy or inertia” (93%). Less frequent items were “early perseverative, stereotyped, or compulsive/ritualistic behavior” (47%) and “hyperorality and dietary changes” (40%). The diagnosis of probable bvFTD in the FTDC criteria requires a diagnosis of possible bvFTD and the presence of a significant functional decline and compatible structural or functional imaging findings. In our series, 80% of our patients showed significant functional decline and 67% had compatible structural or functional imaging findings. No differences in the presence of frontotemporal atrophy or hypoperfusion/hypometabolism were detected between patients who met the 1998 criteria and those who did not.
The frequency of the 6 core features did not differ between the possible and probable bvFTD groups. However, we found some statistical trends (Figure 2). “Early perseverative, stereotyped or compulsive/ritualistic behavior” and “hyperorality and dietary changes” were more frequent in probable bvFTD than in possible patients with bvFTD (P = .072 and P = .058, respectively). Subitems “stereotypy of speech,” “binge eating, increased consumption of alcohol or cigarettes” were significantly more prevalent in the probable bvFTD group than in the possible bvFTD group (P < .05; Figure 2).
Figure 2.
Frequency of FTDC criteria items. Comparison between patients that meet criteria for “possible bvFTD” and those that meet criteria for “probable bvFTD.” Bars indicate the percentage of patients in each group that present the feature. * denotes statistically significant difference (P < .05). bvFTD indicates behavioral variant of frontotemporal dementia; FTDC, bvFTD Criteria Consortium; na, not applicable.
A neuropsychological profile compatible with FTD was found in 59% of cases and was not different between possible and probable bvFTD groups. Nevertheless, these data should be interpreted with caution since most patients (66%) with probable bvFTD could not be fully evaluated due to the advance stage of the disease.
Discordance Between 1998 and FTDC Criteria
Of the 30 patients in our study, 10 (33%) did not meet the 1998 criteria but fulfilled the FTDC Criteria (5 for possible and 5 for probable bvFTD). Detailed analysis of these patients showed that in all cases the failure to accomplish the 1998 criteria was due to the presence of at least 1 exclusion feature. Failure to meet the 1998 criteria was not influenced by the presence of core or supportive features (Figure 1). Exclusion features from the 1998 criteria are summarized in Table 1. One patient with an expansion in C9orf72 had a spatial disorientation at the very beginning of the disease course and did not consequently accomplish the 1998 criteria.
Table 1.
Exclusion Criteria in Patients that did not Fulfill the 1998 Criteria.a
| Possible bvFTD | Probable bvFTD | |
|---|---|---|
| Diagnostic exclusion features | ||
| Early, severe amnesia | 2 | 1 |
| Spatial disorientation | 2b | 2 |
| Myoclonus | 0 | 2 |
| Brain imaging: predominant postcentral brain atrophy or hypoperfusion/hypometabolism deficit | 2 | 0 |
| Relative diagnostic exclusion features | ||
| Typical history of chronic alcoholism | 1 | 1 |
Abbreviation: bvFTD, behavioral variant of frontotemporal dementia.
aData express the number of patients that present the feature. Some patients may have more than 1 exclusion feature.
bOne patient with an expansion in C9orf72 gene had spatial disorientation at the onset of the disease.
Discussion
This study compares the sensitivity of the recently proposed FTDC criteria side by side with the 1998 criteria. We found that up to one-third of patients who met a diagnosis of possible or probable bvFTD according to FTDC criteria failed to comply with the 1998 criteria. This result agrees with the 53% sensitivity described by Rascovsky et al, 15 which even decreased to ~ 30% in cases with a disease onset over 65 years. However, both these results diverge from other studies that reported higher sensitivities, ranging from 85 to 100%. 18,19 This discrepancy between studies may be explained by the fact that both Rascovsky et al 15 and our study applied the 1998 criteria item by item, allowing no flexibility in the interpretation of criteria, especially regarding the exclusion criteria. In contrast, Knopman et al 18 and Snowden et al 19 made the diagnosis based on all the existing clinical and neuroimaging data and successively applied the 1998 criteria using a more flexible approach. Other studies have assessed the accuracy of 1998 criteria using the follow-up as the gold standard, 13,14,20 with reported sensitivities ranging from 36.5% to 79%. When we use structural or functional neuroimaging as a “gold-standard,” sensitivities of 1998 criteria were 70% and 81.8%, respectively. These values are slightly higher than Piguet et al, 14 who studied 45 well-characterized patients with frontal atrophy on MRI and progressive decline and reported a sensitivity of 56% at initial presentation and 73% at follow-up.
Taken together, the variability in sensitivities between studies suggests that antemortem diagnosis of FTD can be made accurately, but a rigid interpretation of the 1998 criteria may have led to some FTD cases being overlooked. In our series, failure to meet the 1998 criteria was in all cases due to the presence of exclusion features. This was not unexpected, however, since the required behavioral/cognitive symptoms of the FTDC are similar to the core and supportive features of the 1998 criteria, and the main novelty resides in the flexible way these items are grouped.
A more detailed analysis of the exclusion criteria reveals their restrictive nature. Alcoholism is a relative exclusion criterion that was present in 2 patients. It is known that an excessive consumption of alcohol may lead to behavioral changes and frontal lobe atrophy that may mimic bvFTD, but, in turn, bvFTD itself can also be the cause of this increased consumption of alcohol. 21,22 When applying the FTDC criteria, one of these patients even met the diagnosis of probable bvFTD due to a compatible neuroimaging. Presence of myoclonus is another exclusion criterion in the 1998 criteria. Myoclonus is a common neurological sign in numerous neurodegenerative diseases. 23,24 It is particularly frequent in CBS-S, which is currently considered a syndrome under the FTD umbrella and that often overlaps with bvFTD. 25 –27 The 2 patients with myoclonus in our study were both in the advanced stage of the disease and had parkinsonism but without any other cortical sign suggestive of CBS-S. The 1998 criteria stated that early severe amnesia and spatial disorientation should rule out the diagnosis of bvFTD, since they are typical symptoms of AD. However, some subsequent reports noticed that these 2 symptoms are not rare in bvFTD, and a diagnosis of bvFTD should not be ruled out based only on these features. 14,18,28,29 In our patients, the most frequently found exclusion criteria were early severe amnesia and spatial disorientation. These exclusion criteria are more likely to be found in elderly patients, which may explain the higher sensitivity of 1998 criteria when applied to younger patients (<65 years old) compared to elderly patients (≥65 years old). Taken together, these data reinforce the importance of considering these symptoms as possible features in bvFTD.
Another advantage of FTDC criteria is their flexible structure. Symptoms are organized in 6 sets and any 3 of these sets must be present for the diagnosis of “possible bvFTD.” As no single set is mandatory, the clinical variability of bvFTD is better represented. This is particularly important in the early stages when not all typical symptoms may be present. Some patients with bvFTD may present a more “apathetic” or “inert” clinical picture, while others may show “disinhibition” as the main prominent clinical feature. Moreover, the behavioral profile may change during the course of the disease. 30,31 In our study, some of the features were more commonly encountered, such as “early loss of sympathy or empathy,” “early behavioral disinhibition” and “early apathy or inertia,” and thus they may be considered as more sensitive to bvFTD. Others, such as “stereotypy of speech” and “binge eating, increased consumption of alcohol or cigarettes” were less common but more frequently found in the probable bvFTD group. Although our study cannot address the specificity of these criteria, it is likely that these features may be more useful in differentiating bvFTD from other dementias. 14 Stereotypy of speech and distractibility have been reported to differentiate progressors from nonprogressors of bvFTD. A previous study found that stereotypic behavior, changes in eating preference, disinhibition, and poor social awareness, best discriminated between bvFTD and AD. 32 We should emphasize that in our study patients with probable bvFTD were in a more advanced stage of the disease. Although this result was expected because a diagnosis of probable bvFTD in the FTDC criteria requires compatible imaging findings and functional decline, we cannot rule out that these symptoms simply appear later in the dementia process. In any case, the division of possible and probable bvFTD in the FTDC criteria will likely accommodate patients at different stages of disease. Furthermore, NPI scores were similar among patients with probable and possible bvFTD, perhaps because different symptoms may appear at different stages of the disease but with equal severity and impact on caregiver stress.
One of the major advances in the FTDC criteria is the incorporation of structural and functional neuroimaging data in the inclusion criteria. The 1998 criteria do not accept cases with predominant postcentral brain atrophy or functional deficits, and this may have led to the exclusion of cases with advanced stages of the disease in whom brain atrophy is more generalized. In support of this possibility, 1 of our 2 patients who failed to meet the 1998 criteria for this reason was in advance stage of the disease (GDS: 7).
We also included 2 patients carrying the recently described repeat expansion in C9orf72 gene. 5,6 Although the full clinical characterization of these patients is still incomplete, many reports have highlighted the clinical heterogeneity at presentation and the occurrence of atypical symptoms. These include psychotic symptoms, early amnesia and spatial disorientation. 33 –36 Of interest, one bvFTD C9orf72 expansion carrier did not fulfill the 1998 criteria due to early spatial disorientation. This observation reinforces the notion that genetic cases do not always fit the standard criteria and that these criteria are usually better suited for nongenetic cases. If mutation status is not taken into account, both patients would be classified as possible bvFTD according to FTDC criteria. However, FTDC criteria reserve the diagnosis of definite diagnosis for cases with known genetic defects.
This study has some limitations. First, it has a reduced sample size, cases did not include neuropathological confirmation, and, consequently, misdiagnosis may have occurred. Second, the study was conducted in a highly selected population and it may be subject to selection bias, and the assessment of FTDC criteria was retrospective. Finally, the study did not include patients with other diagnosis, precluding the assessment of specificity of the criteria.
In summary, we conclude that the strict application of 1998 criteria in patients with suspected bvFTD may exclude up to one-third of patients in a memory clinic setting. Some symptoms considered atypical for bvFTD, such as spatial disorientation and amnesic deficits, may be present in bvFTD, particularly in advanced stages but also in carriers of the C9orf72 expansion. FTDC criteria appear to improve these issues, mainly due to the inclusion of less restrictive exclusion features. Future prospective studies are needed to further evaluate the sensitivity and specificity of these newly proposed diagnostic guidelines.
Acknowledgments
The authors are indebted to all the patients and their families for their participation. They thank Carolyn Newey for editorial help.
Footnotes
Author Contributions: Sónia Costa and Marc Suárez-Calvet contributed equally to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Marc Suárez-Calvet is supported by a grant from the Fondo de Investigación Sanitaria (FI09/00732), Instituto Carlos III, Madrid, Spain.
References
- 1. Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4(11):771–780. [DOI] [PubMed] [Google Scholar]
- 2. Bird T, Knopman D, VanSwieten J, et al. Epidemiology and genetics of frontotemporal dementia/Pick’s disease. Ann Neurol. 2003;54(5):S29–S31. [DOI] [PubMed] [Google Scholar]
- 3. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(9):1996–2005. [DOI] [PubMed] [Google Scholar]
- 4. Stevens M, van Duijn CM, Kamphorst W, et al. Familial aggregation in frontotemporal dementia. Neurology. 1998;50(6):1541–1555. [DOI] [PubMed] [Google Scholar]
- 5. Renton AE, Majounie E, Waite A, et al. A Hexanucleotide repeat expansion in C9ORF72 is the cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62(6):925–930. [DOI] [PubMed] [Google Scholar]
- 8. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. [DOI] [PubMed] [Google Scholar]
- 9. Gregory C, Lough S, Stone V, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002:125(pt 4):752–764. [DOI] [PubMed] [Google Scholar]
- 10. Adenzato M, Cavallo M, Enrici I. Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48(1):2–12. [DOI] [PubMed] [Google Scholar]
- 11. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 12. Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21(4):S14–S18. [DOI] [PubMed] [Google Scholar]
- 13. Mendez MF, Shapira JS, McMurtray A, Licht E, Miller BL. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol. 2007;64(6):830–835. [DOI] [PubMed] [Google Scholar]
- 14. Piguet O, Hornberger M, Shelley BP, Kipps CM, Hodges JR. Sensitivity of current criteria for the diagnosis of behavioral variant frontotemporal dementia. Neurology. 2009;72(8):732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sala I, Belén Sánchez-Saudinós M, Molina-Porcel L, et al. Homocysteine and cognitive impairment. Relation with diagnosis and neuropsychological performance. Dement Geriatr Cogn Disord. 2008;26(6):506–512. [DOI] [PubMed] [Google Scholar]
- 17. Dols-Icardo O, Suárez-Calvet M, Hernández I, et al. Expansion mutation in C9ORF72 does not influence plasma progranulin levels in frontotemporal dementia. Neurobiol Aging. 2012;33(8):2011–2013. [DOI] [PubMed] [Google Scholar]
- 18. Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol. 2005;57(4):480–488. [DOI] [PubMed] [Google Scholar]
- 19. Snowden JS, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134(9):2478–2492. [DOI] [PubMed] [Google Scholar]
- 20. Pijnenburg YAL, Mulder JL, van Swieten JC, et al. Diagnostic accuracy of consensus diagnostic criteria for frontotemporal dementia in a memory clinic population. Dement Geriatr Cogn Disord. 2008;25(2):157–164. [DOI] [PubMed] [Google Scholar]
- 21. Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36(5):357–368. [DOI] [PubMed] [Google Scholar]
- 22. Brun A, Andersson J. Frontal dysfunction and frontal cortical synapse loss in alcoholism-the main cause of alcohol dementia? Dement Geriatr Cogn Disord. 2001;12(4):289–294. [DOI] [PubMed] [Google Scholar]
- 23. Lleó A, Rey MJ, Castellví M, Ferrer I, Blesa R. Asymmetric myoclonic parietal syndrome in a patient with Alzheimer’s disease mimicking corticobasal degeneration. Neurologia. 2002;17(4):223–226. [PubMed] [Google Scholar]
- 24. Caviness JN. Myoclonus and neurodegenerative disease-what’s in a name? Parkinsonism Relat Disord. 2003;9(4):185–192. [DOI] [PubMed] [Google Scholar]
- 25. Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dement Geriatr Cogn Disord. 2004;17(4):282–286. [DOI] [PubMed] [Google Scholar]
- 26. Sha S, Hou C, Viskontas IV, Miller BL. Are frontotemporal lobar degeneration, progressive supranuclear palsy and corticobasal degeneration distinct diseases? Nat Clin Pract Neurol. 2006;2(12):658–665. [DOI] [PubMed] [Google Scholar]
- 27. Boeve BF. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis Assoc Disord. 2007;21(4): S31–S38. [DOI] [PubMed] [Google Scholar]
- 28. Graham A, Davies R, Xuereb J, et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain. 2005;128(3):597–605. [DOI] [PubMed] [Google Scholar]
- 29. Hornberger M, Piguet O. Episodic memory in frontotemporal dementia: a critical review. Brain. 2012;135(3):678–692. [DOI] [PubMed] [Google Scholar]
- 30. Diehl-Schmid J, Pohl C, Perneczky R, Förstl H, Kurz A. Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22(4):352–357. [DOI] [PubMed] [Google Scholar]
- 31. Shinagawa S, Toyota Y, Ishikawa T, et al. Cognitive function and psychiatric symptoms in early- and late-onset frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;25(5):439–444. [DOI] [PubMed] [Google Scholar]
- 32. Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135(3):693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsiung GY, Dejesus-Hernandez M, Feldman HH, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135(3):709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahoney CJ, Beck J, Rohrer JD, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135(3):736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boeve BF, Boylan KB, Graff-Radford NR, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135(3):765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]