Abstract
Gait disturbance results in an increase in the risk of falls in patients with Alzheimer’s disease (AD). The falls are events that might be related to an increase in the number of fractures, loss of mobility, being bedridden, early institutionalization, and increased use of medication. Therefore, the reduction in the number of falls is important for the maintenance of the functional independence of the patients as well as for the prevention of sequelae resulting from those events. Alterations in the gait occur very frequently in AD, and the gait disturbance occurs relatively early in the course of the disease. This study has important implications for public health and clinical practice. This study and previous studies have reported that abnormal gait predicts greater risk of falls, dementia, institutionalization, and death. The high prevalence and incidence of abnormal gait and its association with multiple adverse outcomes in older adults require urgent attention. Our results allow us to identify the risk factors.
Keywords: gait, Tinetti, Alzheimer, neuropsychiatric
Introduction
Gait disorders become increasingly common with advancing age, occurring in 8% to 19% of elderly persons residing in the community. 1,2 Common causes of abnormal gait in elderly persons include neurologic diseases, arthritis, and acquired foot deformities. Neurological gait abnormalities result from focal or diffuse lesions occurring in the neural pathways linking the cortical motor centers to the peripheral neuromuscular systems. 3,4 Gait disorders are more common in dementia than in the context of the physiological aging process. 5 Prevalence of dementia associated with gait disturbances depends on the type of dementia and the severity of cognitive impairment. Although vascular dementia gait disorders occur more frequently in the early stages of the disease, patients with Alzheimer’s disease (AD) usually have stable gait until late disease stages. 6
Many studies have reported that gait abnormalities are commonly observed in AD and increase in frequency and severity over time. These gait abnormalities may result from different underlying mechanisms, although their exact anatomic location is not clear.
Gait disorders result in decreased mobility and an increase in the risk of falling. 7 The consequences of gait disorders and falls can be severe, including fractures, worsening of mobility, loss of independence, and increased cardiovascular morbidity and mortality. 8
Information about the presence of gait abnormalities in AD is important because they may predict a faster cognitive decline, 9 institutionalization, 10 and death. 11 However, there is a large variability in the reported frequencies for the various stages of the disease. For example, in a review of clinical series published over a 10-year period, frequency of gait abnormalities in AD ranged from 6% to >50%. 12 Some of this inconsistency derives from methodological differences including variable definitions of gait abnormalities, inclusion or exclusion of participants who used neuroleptics, use of standardized scales versus just clinical evaluation, inclusion of participants at varying stages of disease, variable levels of participation in the follow-up, and so on. Additionally, most information about gait abnormalities in AD is inferred from cross-sectional studies. Besides, although AD is a progressive disease, relatively little information is available from longitudinal studies tracking changes in gait abnormalities over time. Also, most studies examine gait abnormalities globally, and only few reports have focused on individual subsets of gait abnormalities. 13 Finally, little is known about specific factors that influence the occurrence or the rate of progression of gait abnormalities in AD.
We analyzed data from a cohort of 72 patients with probable AD, which included patients with mild and moderate disease stages. The patients were followed up every 3 to 4 months throughout the study with standardized assessment of gait abnormalities during 5 years. We first attempted to characterize the occurrence of gait abnormalities during the natural course of AD and the onset of gait disturbance as measured by a global score of the Tinetti’s gait. We also investigated the existence of other factors that may predict which of the patients with AD will develop gait abnormalities.
Materials and Methods
Study Population
The province of Segovia is 1 of the 7 provinces of the Castilla-Leon region (Spain). In 1990, it had a population of 155 517 habitants. We conducted a study of gait abnormalities in a cohort of patients with mild and moderate AD in this province in order to document epidemiological data and the natural history based on cases diagnosed in Segovia. A monographic outpatient clinic was designed specifically for this purpose, conducting a detailed monitoring. Patients with AD were recruited from the outpatient clinic of neurology of Segovia General Hospital, and all the patients were diagnosed and followed with a specific protocol, using inclusion and exclusion criteria according to the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA), 14 by a neurologist expert in dementia. We included the first 72 consecutive patients diagnosed with AD. Patients were followed up every 3 to 4 months throughout the study during 5 years.
Case Finding
In 2009, we developed a computerized neurological diagnostic register for dementia disorders in order to document epidemiological data and the natural history of these patients, which were included in a data record.
Case Definition
All patients had probable AD according to the criteria NINCDS-ADRDA. 14 The diagnosis of AD was confirmed through a structured interview conducted as part of the clinical examination including basic demographic data and information on AD such as age at onset (the age at which the first symptoms of AD were reported by the patient or family preceding the first visit to our department), age at diagnosis, and date of initiating therapy and vascular comorbidity. Also, assessment of psychometric tests to confirm cognitive impairment and conventional tests to exclude other causes of dementia (computerized tomography imaging, biological tests with plasmatic levels of vitamin B12, folic acid, and thyroid-stimulating hormone, and detailed physical and neurological examinations) were collected and recorded. Staging of the disease was based upon the findings of the clinical examination and in accordance with the present standards (Rapid Disability Rating Scale (RDRS-2) that evaluate activities of daily life, with particular emphasis on basic activities (only 1 item of 7 is related to instrumental activities, 15 and Global Deterioration Scale of Reisberg [GDS]). 16 Cognitive functions were assessed by the Mini-Mental State Examination. 17 Caregivers were asked about the patient’s use of current medication (yes/no): psychotropic medication (anxiolytics, neuroleptics, benzodiazepines, or antidepressants), specific AD treatment (acetyl cholinesterase inhibitors or memantine), and other medications. Nutritional assessment included weight, height, body mass index, albumin (g/L), and the Mini-Nutritional Assessment (MNA). 18 The study of psychiatric and behavioral disorders was assessed using Neuropsychiatric Inventory Questionnaire (NPIQ severity and stress), 19 which is composed of 12 subscales that assess behavioral and psychological changes that occur more commonly in patients with dementia: delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria, apathy/indifference, disinhibition, irritability/emotional lability, activity aberrant motor, sleep disturbance, and appetite disturbances. Caregiver burden was assessed using the Zarit scale. 20
Gait and balance were assessed using the Tinetti test. 21 The Tinetti test was developed as a tool to predict risk of falling in elderly people and had the advantage of quantifying gait and disturbances in terms of performance. Only few studies have assessed this tool in population with AD. 22,23 This test has 2 parts: performance-oriented assessment of balance with 16 points (sitting balance, arising from chair, immediate standing balance in the first 3-5 seconds, standing balance, balance with eyes closed, and turning balance [360°]) and performance-oriented assessment of gait with 12 points (initiation of gait, step height, step length, step symmetry, step continuity, path deviation, trunk stability, walk stance, and turning while walking). Less than 19 points means high risk of falls, between 19 and 24 means risk of falls, and between 24 and 28 means no disturbance in gait or balance. Therefore, the cutoff point that predicts moderate or high risk of falling and disturbance in balance and gait was 24. 21,22,23
Statistical Analysis
For qualitative variables, frequency measures were calculated, and for quantitative variables, the mean and the standard deviation. To analyze the time until the onset of gait disturbance, we performed a survival analysis using the Kaplan-Meier method. We calculated the median time to gait disturbance with the appropriate 95% confidence interval (95% CI), and graphics representation of time until the event is presented. We identified the variables that had a high correlation between each other using bivariate correlation analysis. We used a proportional hazards model (Cox regression) to analyze the prognostic factors specified previously: age at onset, sex, comorbid vascular diseases, treatment for dementia, treatment with psychotropic drugs, GDS, MNA, NPIQ severity, NPIQ stress, and time with treatment. We built univariate models and selected the variables that in the crude analysis were P < .2. Also, the variables considered clinically relevant were included following the strategy described, starting from an initial model that included age at onset, sex, onset time of treatment, RDRS, NPIQ-stress, NPIQ-severity, and duration of the specific treatment of dementia. To get to the final model by Cox regression analysis, we followed a backward strategy and calculated hazard ratio (HR) with 95% CIs and keeping in the final model variables with P < .1. Criteria of changes in −2 log likelihood were followed for the construction of the successive models. We used a software program SPSS 20.0 (SPSS, Chicago, Illinois).
Results
Seventy-two patients with dementia were recruited, of whom 48 (66.7%) were women and 24 (33.3%) were men, and mean age at disease onset was 76.5 ± 6.3. The mean duration of the disease was 5.04 ± 2.64. The patients were diagnosed with a delay of 1.74 ± 0.63 years since the family observed the first symptoms. In our study, 57 (79.2%) patients live with spouse or a family. Sixty-two (86.1%) patients had mild–moderate dementia (GDS 4-5), and 10 (13.9%) had severe dementia (GDS 6-7). In all, 56 (77.8%) of the patients received specific treatment (acetyl cholinesterase inhibitors, and/or memantine), and 31 (43.1%) received psychotropic medications. Clinical and demographic data referring to the time at enrollment are shown in Table 1.
Table 1.
Demographic and Clinical Data.
| Demographic and Clinical Data | |
|---|---|
| Sex | N (%) |
| Female | 48 (66.66) |
| Male | 24 (33.34) |
| Initial age | (SD) |
| Total | 76.5 (6.27) |
| Female | 76.1 (6.09) |
| Male | 77.2 (6.67) |
| Residence | N (%) |
| Rural | 48 (66.7) |
| Urban | 24 (33.3) |
| Living arrangement | N (%) |
| Alone | 5 (6.9) |
| Family | 57 (79.2) |
| Institutionalized | 10 (13.9) |
| Comorbid vascular diseases | 54 (75.0) |
| Psychiatric symptoms | N (%) |
| Depression | 47 (65.3) |
| Hallucinations and delusion | 14 (19.4) |
| Sleep disorders | 15 (20.8) |
| Initial treatment dementia | 56 (77.8) |
| Psychotropic drugs | 31 (43.1) |
| Time of treatment since diagnosis of dementia | 0.57 (1.02) |
| Duration of diseasea | 5.04 (2.64) |
| Scales | (SD) |
| MMSE | 17.31 (4.53) |
| GDS | 4.69 (0.70) |
| BMI | 27.84 (4.56) |
| RDRS-2 | 34.59 (9.10) |
| NPIQ-stress | 6.06 (4.82) |
| NPIQ-severity | 5.43 (4.03) |
| Tinetti global | 21.47 (5.92) |
| Gait | 8.62 (3.40) |
| Balance | 12.84 (3.11) |
Abbreviations: BMI, body mass index; GDS, Global Deterioration Scale of Reisberg; MMSE, Mini Mental State Examination; N, number; NPIQ, Neuropsychiatric Inventory Questionnaire; RDRS, Rapid Disability Rating Scale; SD, standard deviation; , mean score.
ayears.
In total, 48 (66.7% ± 5.5%) patients have gait disorders and 24 (33.3% ± 5.5%) no reported alterations. The median time to gait disturbance was 4.19 years, within a 95% CI: 3.62 to 4.76. The graphical representation is shown in Figure 1. The patients presenting gait disturbance were elderly patients with a mean age at onset of 76.7 ± 6.3 years, whereas for the group without impairments it was 76.0 ± 6.12 years and was found more frequently in men than in women, 75% versus 62.5%. They presented a significantly increased risk of malnutrition with MNA of 23.1 ± 2.06. We found more patients, 40 (83.3% ± 5.5%), with vascular comorbidities in this group.
Figure 1.
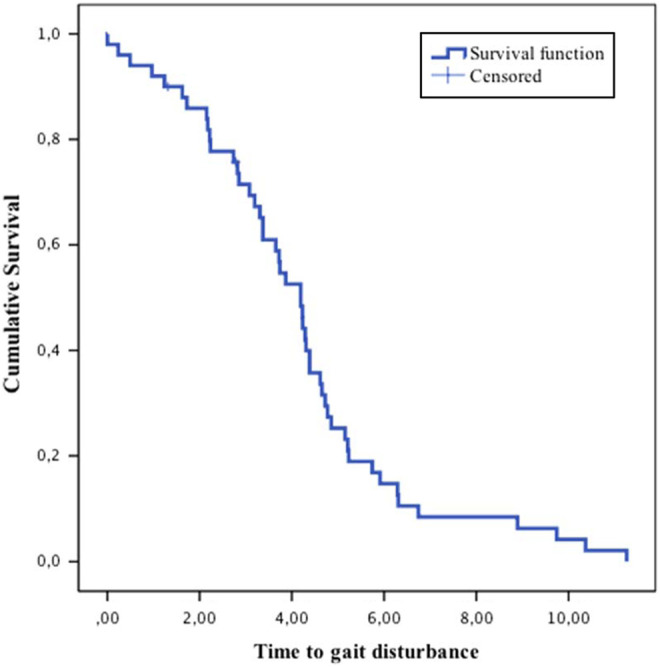
Time to gait disturbance.
We found that patients with AD evolved progressively toward gait disturbance as measured on the Tinetti scale. The probabilities of reaching such impairment were 25% at 2.8 ± 0.45 years, 50% at 4.19 ± 0.29 years, and 75% at 5.15 ± 0.28 years.
We analyzed the crude HR of gait disorder development in patients with AD on the basis of sex, age at onset of symptoms, comorbid diseases, GDS scale, intake of psychotropic drugs, NPIQ, MNA scales, and duration of treatment. Statistical association was found between gait distrurbance and age at onset of symptoms, NPIQ scale at the time of gait disturbance and duration of the treatment, so the patients who were treated from the earliest phases of the disease took longer to develop abnormal gait. The rest of the variables studied were not significant (Table 2).
Table 2.
Gait Disturbance in the 72 Patients With Alzheimer’s Disease (Crude HR).
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Sex | 1.17 | 0.63-2.14 | .61 |
| Age at onset | 1.03 | 0.98-1.09 | .21 |
| Comorbid vascular diseases | 1.36 | 0.62-3.00 | .44 |
| GDS scale | 0.84 | 0.57-3.009 | .41 |
| Psychotropic drugs | 0.87 | 0.49-1.54 | .63 |
| Time with treatment | 0.79 | 0.68-0.92 | <.01 |
| NPIQ-stress | 1.09 | 1.02-1.16 | .01 |
| NPIQ-severity | 1.10 | 1.02-1.19 | <.01 |
| MNA | 1.08 | 0.94-1.23 | .25 |
Abbreviations: CI, confidence interval; GDS, Global Deterioration Scale of Reisberg; HR, hazard ratio; MNA, Mini-Nutritional Assessment; NPIQ, Neuropsychiatric Inventory Questionnaire.
As observed using Cox regression, patients with AD presented 2 variables as predictors of gait abnormalities, measured by the scale of Tinetti (Table 3). Time with treatment with an HR of 0.72 (95% CI: 0.59-0.86, P < .01) had a protective effect to prevent abnormality gait. Patients who were treated at the onset of the disease took longer to develop gait disturbance, a reduction in 28% per year. And, the NPIQ-stress scale in the early stages of the disease (HR: 1.13, 95% CI: 1.05-1.21, P < .01) also predicted progress toward disability. The early onset of psychiatric symptoms in the course of the disease predicts a more rapid onset of gait disorders.
Table 3.
Cox Regression Model (Crude HR).a
| Variable | HR | 95% CI | P |
|---|---|---|---|
| NPIQ-stress | 1.13 | 1.05-1.21 | <.01 |
| Time with treatment | 0.72 | 0.59-1.21 | <.01 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NPIQ, Neuropsychiatric Inventory Questionnaire.
a−2 log likelihood = 251.53; χ2 = 17.20, with 2 df (dregrees of freedom), P < .01.
Discussion
There are 4 important findings in this study. First, alterations in the gait occur very frequently in AD. Gait impairment is frequent in patients having AD with prevalence estimates varying from 9% to 60%. 22,24 Our results are in concordance with previous studies, although slightly higher, and show that gait abnormalities occur in the 66.7%. Second, the gait disturbance occurs relatively early in the course of the disease, and median time was 4.19 years. It has been reported that gait disorders remain normal until the late stages of AD, 25,26 which are related to the severity of the disease, 24 whereas in vascular dementia, gait disorders present earlier or even precede dementia. 6 However, other studies have reported gait abnormalities in the early stages of AD. 27,28 Pettersson et al 29,30 showed that motor performance is affected already at mild stages of AD, and Cedervall et al 31 studied that a simultaneous deficiency in the cognitive task appears to have a distinct impact on gait function already in mild AD, as it can be observed in the 86% of our patients who were in a mild–moderate stage of GDS.
Third, we have found that time with treatment and NPIQ scales were predictors of gait disturbance. Recent studies are investigating the improvement in gait disturbance with cholinesterase inhibitors such as donepezil. 32 And, Henderson et al assess treatment with rivastigmine as a new therapeutic option to ameliorating gait and cognitive deficits in a population at high risk of falls. 33 On the other hand, treatment with rivastigmine has been associated with a faster gait in the Timed-Up-and-Go test and may be indicative of improved mobility, stability, and decrease in risk of fall in patients with gait disorder, 34 but a recent meta-analysis has shown inconclusive results on the effect of drugs that are used in dementia to treat gait impairment. 35
The NPIQ scale assesses behavioral and psychological changes that occur more commonly in patients with dementia. Patients with AD may develop symptoms with frontal dominance, such as personality changes, anxiety, depression, psychotic syndromes, and obsessive compulsive disorder in mild to moderate stages and can be a good predictor of gait disturbance. Several authors relate different gait disorders and falls with depression. 36,37 In our series, patients with gait disorders increased the rates of neuropsychiatric symptoms and took more psychiatric drugs. And finally, in our study, the age at the onset of the disease is not a predictive factor to develop gait disorders, despite the fact that previous studies have related a high prevalence of gait disorders to age, which increased with advancing age. 38 However, this may be due to risk factors such as hypertension, stroke, and arthritis. 4,37 Although in our study, we didn’t find a relationship between gait disturbance and comorbid diseases, this relationship has been valued by Inzitari et al. 39
We found worst scores on the disability scale RDRS in patients presenting gait disturbance. Patients with greater disability in the early stages of the disease reach gait disturbance sooner, so it could be a good predictor of gait disturbance. Besides, we have observed that males develop gait disturbance more frequently than females, unlike other series where it was more common in women, 23 although these differences were not statistically significant either in our series or in other studies. 40
According to Tinetti, 21 gait disturbance results in an increase in the risk of falls in patients with AD. The falls are events that might be related to an increase in the number of fractures, loss of mobility, being bedridden, early institutionalization, and increased use of medication. Therefore, the reduction in the number of falls is important for the maintenance of the functional independence of the patients as well as in the prevention of sequelae resulting from those events.
Our study has several limitations. First and most notably, the sample size, being small, can lead us to a great degree of variability in the results, and to the consideration of other factors, both intrinsic and extrinsic. Second, Tinetti test is valid for the elderly individuals and not for dementia and therefore break points of reference are for seniors, although there are few studies in dementia. And finally, patients were recruited in the neurology clinic and they were at different stages of the disease, with a delay of 1.7 years from the onset of the symptoms. However, due to the active recruitment of patients for the study, 86% of them were in a mild–moderate stage.
Strengths of this study include (1) the application of widely accepted diagnostic criteria for AD, (2) assessment of nutritional status and comorbidity, (3) the use of a valid and reliable system to measure progress, and (4) prospective design of this study with a 5-year follow-up which makes this data set relatively unique. The results of this study should be interpreted in light of its limitations. It was designed as an initial exploration in people with mild to moderate AD to assess gait disorders and identify risk factors, and we believe that the effect of treatment should be assessed with clinical trials.
In conclusion, gait impairment is frequent and it appears early in the course of AD. The results allow us to identify the risk factors. This study has important implications for public health and clinical practice. This study and previous studies have reported that abnormal gait predicts greater risk of falls, dementia, institutionalization, and death. The high prevalence and incidence of abnormal gait and its association with multiple adverse outcomes in elderly adults require urgent attention.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support for the research and authorship of the medical school of Segovia.
References
- 1. Dawson D, Hendershot G, Fulton J. Aging in the Eighties: Functional Limitations of Individuals Age 65 and Over. Advance Data From Vital and Health Statistics. No.133. Hyattsville, MD: National Center for Health Statistics; 1987:1–11. (DHHS publication no. (PHS) 87-1250). [Google Scholar]
- 2. Sudarsky L. Geriatrics: gait disorders in the elderly. N Engl J Med. 1990;322(20):1441–1446. [DOI] [PubMed] [Google Scholar]
- 3. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12(4):689–703. [PubMed] [Google Scholar]
- 4. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–1768. [DOI] [PubMed] [Google Scholar]
- 5. Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15(5):365–375. [DOI] [PubMed] [Google Scholar]
- 6. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. [DOI] [PubMed] [Google Scholar]
- 7. Djaldetti R, Lorberboym M, Melamed E. Primary postural instability: a cause of recurrent sudden falls in the elderly. Neurol Sci. 2006;27(6):412–416. [DOI] [PubMed] [Google Scholar]
- 8. Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58(12):1815–1819. [DOI] [PubMed] [Google Scholar]
- 9. Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229-230:89–93. [DOI] [PubMed] [Google Scholar]
- 10. Drachman DA, O’Donnell BF, Lew RA, Swearer JM. The prognosis in Alzheimer’s disease. “How far” rather than “how fast” best predicts the course. Arch Neurol. 1990;47(8):851–856. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell SL, Rockwood K. The association between parkinsonism, Alzheimer’s disease, and mortality: a comprehensive approach. J Am Geriatr Soc. 2000;48(4):422–425. [DOI] [PubMed] [Google Scholar]
- 12. Allan LM, Ballard CG, David J, Burn DJ, Kenny RA. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc. 2005;53(10):1681–1687. [DOI] [PubMed] [Google Scholar]
- 13. van Iersel MB, Hoefsloot W, Munneke M, Bloem BR, Olde Rikkert MG. Systematic review of quantitative clinical gait analysis in patients with dementia. Z Gerontol Geriatr. 2004;37(1):27–32. [DOI] [PubMed] [Google Scholar]
- 14. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. [DOI] [PubMed] [Google Scholar]
- 15. Linn MW. A rapid disability rating scale. J Am Geriatr Soc. 1967;15(2):211–214. [DOI] [PubMed] [Google Scholar]
- 16. Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psych. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 18. Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–122. [DOI] [PubMed] [Google Scholar]
- 19. Cummings JL, Mega M, Rosenberg-Thompson S, Carusi DA, Gornhein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 20. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. [DOI] [PubMed] [Google Scholar]
- 21. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. [DOI] [PubMed] [Google Scholar]
- 22. Allan LM, Ballard CG, Burn DJ, et al. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc. 2005;53(10):1681–1687. [DOI] [PubMed] [Google Scholar]
- 23. Mazoteras Muños V, Abellan Van Kan G, Cantet C, et al. Gait and balance impairments in Alzheimer disease patients. Alzheimer Dis Assoc Disord. 2010;24(1):79–84. [DOI] [PubMed] [Google Scholar]
- 24. Thomas VS, Vandenberg EV, Potter JF. Non-neurological factors are implicated in impairments in gait and mobility among patients in a clinical dementia referral population. Int J Geriatr Psychiatry. 2002;17(2):128–133. [DOI] [PubMed] [Google Scholar]
- 25. Cummings JL, Benson DF. Dementia of the Alzheimer type: an inventory of diagnostic clinical features. J Am Geriatr Soc. 1986;34(1):12–19. [DOI] [PubMed] [Google Scholar]
- 26. Roller WC, Wilson RC, Glass SL, Fox JH. Motor signs are infrequent in dementia of the Alzheimer type. Ann Neurol. 1984;16(4):514–515. [DOI] [PubMed] [Google Scholar]
- 27. Becker JT, Boiler F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer’s disease: description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51(6):585–594. [DOI] [PubMed] [Google Scholar]
- 28. O’Keeffe ST, Kazeem H, Philpott RM, Playfer JR, Gosney M, Lye M. Gait disturbance in Alzheimer’s disease: a clinical study. Age Ageing. 1996;25(4):313–316. [DOI] [PubMed] [Google Scholar]
- 29. Pettersson AF, Engardt M, Wahlund LO. Activity level and balance in subjects with mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;13(4):213–216. [DOI] [PubMed] [Google Scholar]
- 30. Petersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5-6):299–304. [DOI] [PubMed] [Google Scholar]
- 31. Cedervall Y, Halvorsen K, Aberg AC. A longitudinal study of gait function and characteristics of gait disturbance in individuals with Alzheimer’s disease. Gait Posture. 2014;39(4):1022–1027. [DOI] [PubMed] [Google Scholar]
- 32. Montero-Odasso M, Muir-Hunter SW, Oteng-Amoallo A, et al. Donepezilo improves gaits performance in older adults with mild Alzheimer’s disease: a phase II clinical trial. J Alzheimer Dis. 2015;43(1):193–199. [DOI] [PubMed] [Google Scholar]
- 33. Henderson EJ, Lord SR, Close JC, Lawrence AD, Whone A, Ben-Shlomo Y. The ReSPonD trial—rivastigmine to stabilise gait in Parkinson’s disease a phase II, randomised, double blind, placebo controlled trial to evaluate the effect of rivastigmine on gait in patients with Parkinson’s disease who have fallen. BMC Neurol. 2013;13:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gurevich T, Balash Y, Merims D, et al. Effect of rivastigmine on mobility of patients with higher-level gait disorder: a pilot exploratory study. Drugs R D. 2014;14(2):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beauchet O, Launauy CP, Allali G, Annweiler C. Changes in gait variability with dementia anti-dementia drugs: a systematic review and meta-analysis. CNS Drugs. 2014;28(6):513–518. [DOI] [PubMed] [Google Scholar]
- 36. Launay C, De Decker L, Annweiler C, Kabeshova A, Fantino B, Beauchet O. Association of depressive symptoms with recurrent falls: a cross-sectional elderly population based study and a systematic review. Nutr Health Aging. 2013;17(2):152–157. [DOI] [PubMed] [Google Scholar]
- 37. Kvelde T, McVeigh C, Toson B, et al. Depressive symptomatology as a risk factor for falls in older people: systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(5):694–706. [DOI] [PubMed] [Google Scholar]
- 38. Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54(2):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inzitari M, Gine-Garriga M, Martínez B, et al. Cerebrovascular disease and gait and balance impairment in mild to moderate Alzheimer’s disease. J Nutr Health Aging. 2013;17(1):45–48. [DOI] [PubMed] [Google Scholar]
- 40. Horikawa E, Matsui T, Arai H, Seki T, Iwasaki K, Sasaki H. Risk of falls in Alzheimer’s disease: a prospective study. Intern Med. 2005;44(7):717–721. [DOI] [PubMed] [Google Scholar]


