Abstract
The interest in poor magnesium (Mg) status as risk factor for Alzheimer’s disease (AD) is increasing due to its antioxidant and neuroprotective properties. A systematic PubMed literature search of studies investigating Mg status was undertaken comparing AD to healthy controls (HCs) or patients with medical illness (medical controls [MCs]). Standardized mean differences (SMDs) ± 95% confidence intervals (CIs) were calculated for all outcomes. Of 192 potentially eligible studies, 13 were included (559 patients with AD, 381 HCs, and 126 MCs). Compared to HCs, patients with AD had significantly lower Mg in cerebrospinal fluid (2 studies; SMD = −0.35; P = .02) and in hair (2 studies; SMD = −0.75; P = .0001). No differences between AD and controls were evident for serum Mg. In conclusion, AD seems to be associated with a lower Mg status when compared to HCs, while the scarcity of studies limited the findings about MCs.
Keywords: magnesium, Alzheimer’s disease, dementia, healthy controls, aging
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in older people. This condition is characterized by cognitive and memory deterioration, progressive impairment in the activities of daily living, and other disabling neuropsychiatric and nutritional problems. 1,2
Several studies have proposed a key role of dietary elements as risk factor in AD. While conditions due to an incorrect diet (such as obesity, metabolic syndrome, and diabetes) are well-known risk factors for the onset of AD, 3 -5 recent research studies have proposed the importance of some nutritional elements per se in the pathogenesis of AD. Patients with a diet rich in pro-oxidant (such as alcohol) and poor in antioxidant elements (such as mineral and vitamins) seem to have an increased risk of developing AD. 6 Among minerals, particular attention has been given to magnesium (Mg) as potential risk factor for AD. Data from human brains of autopsies of AD demonstrated that patients with AD had lower concentrations of Mg compared to healthy controls (HCs). 7,8 Factors related to lower Mg availability (malnutrition, inflammation, and sarcopenia) are particularly present in people with AD. 9
In vitro and experimental research have shown an active role of Mg in degenerative neurological disorders, Mg being involved in important pathways such as N-methyl-d-aspartate (NMDA)-receptor response to excitatory amino acids, cell membrane fluidity, and stability and regulation of intracellular calcium. 10 -13 Moreover, some studies also suggested a neuroprotective action of Mg in the synaptic function. 14
However, no systematic reviews have been conducted to explore whether persons with AD have or not a poorer Mg status compared to those without this condition. We thus conducted a systematic review comparing patients with AD to medical controls (MCs; ie, with similar diseases and/or clinical characteristics to those having a diagnosis of AD) and to HCs (ie, not affected by any disease), investigating whether AD was associated with a lower Mg status than controls.
Materials and Methods
Search Strategy
Two investigators (NV and MS) independently conducted a literature search using PubMed, without language restriction, from database inception until May 4, 2015, for studies comparing Mg parameters in AD versus MC or HC. Any inconsistencies were resolved by consensus. In PubMed, controlled vocabulary terms and the following key words were used: (“magnesium” [MeSH Terms] OR “magnesium”[All Fields] OR “magnesium deficiency” [MeSH Terms] OR “serum magnesium” [All Fields]) AND (“dementia” [Mesh Terms] OR “dementia” [All Fields] OR “Alzheimer Disease” [Mesh Terms] OR “Alzheimer Disease” [All Fields]). Conference abstracts as well as reference lists of included articles were also considered, and those relevant to the topic were hand-searched for identification of other additional, potentially relevant articles.
Study Selection
We included cross-sectional studies comparing data on Mg (in serum, plasma, ionized, blood cells, cerebrospinal fluid [CSF], or hair) between patients with a diagnosis of AD and controls. Controls were divided into MCs or HCs: MCs had similar diseases and/or clinical characteristics to those with AD, while HCs were not affected by any disease. In case of missing data, at least 4 attempts were made to contact study authors for additional information. Studies were excluded if no control group was present and if the data were not meta-analyzable (ie, data as median with range or interquartile range). Since poor renal function can significantly affect Mg levels (particularly in serum), studies including patients in dialysis or with important decline in renal function were also excluded.
This systematic review was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria for the quality assessment of included studies 15 and the indications of Preferred Reporting Items for Systematic reviews and Meta-Analyses statement. 16
Data Extraction
Two authors (NV and CL) independently extracted data from the selected studies into a standardized Microsoft Word spreadsheet. Any disagreement was resolved by consensus. The following information was extracted: (1) study population characteristics (eg, sample size, demographics, and inclusion and exclusion criteria), (2) criteria for the diagnosis of dementia; (3) severity of dementia, (4) Mini-Mental State Examination (MMSE) values, (5) matching variables between people with AD and controls, (6) method of ascertainment for Mg, and (7) quality indicators used for the STROBE assessment.
Statistical Analysis
When combining studies, the random effects model 17 was used to account for studies’ heterogeneity. For continuous data, standardized mean difference (SMD) with its 95% confidence interval (CI) was used. This analysis was made using RevMan 5.3. 18 Study heterogeneity was measured using the χ2 and I2 statistics, with χ2 P ≤ .05 and I2 ≥50% indicating the presence of significant heterogeneity. 19 For outcomes including at least 4 studies and with significant heterogeneity, we conducted a meta-regression analysis to explore whether some variables (year of publication, continent where the study was conducted, criteria for diagnosis of AD, severity of dementia, age, percentage of females between the 2 groups, sample size, or differences in mean MMSE values) were significant moderators. Meta-regression analyses were conducted using Comprehensive Meta-Analysis v.3. 20 We finally conducted stratified analyses exploring effects according to the moderators significant at meta-regression analysis. Funnel plots were visually inspected to assess the possibility of publication bias.
Results
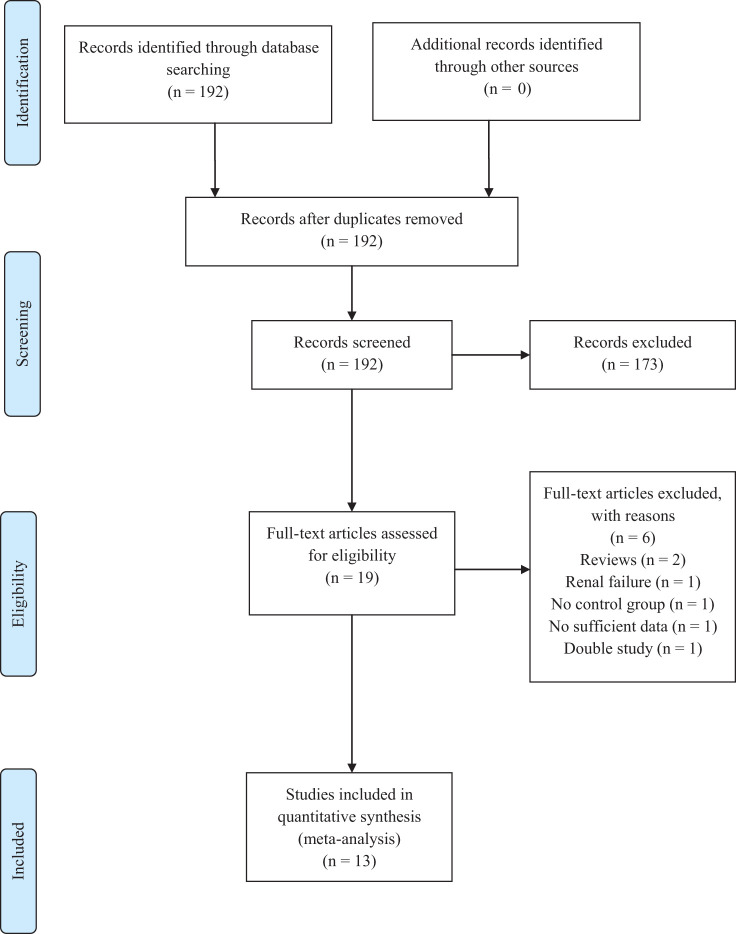
The search identified 192 potentially eligible studies. After excluding 173 manuscripts through title and abstract review, 19 full-text articles were examined with 13 studies included in the meta-analysis as illustrated in Figure 1. 21 -33
Figure 1.
PRISMA flow diagram of study selection process.
Study and Patient Characteristics
Characteristics of studies and patients are summarized in Supplementary Table S1. The table shows that the 13 analyzed studies 21 -33 included a total of 1066 participants (AD = 559 and controls = 507). The majority of the studies was made in Europe (8 [61.5%] studies). Seven among the 13 studies used at least 1 variable for matching between treated and the control groups: 5 studies 21,25,28,29,32 used a matching for age and 2 studies 26,33 for age and sex (Table 1).
Table 1.
Standardized Mean Differences in Mg Parameters in Patients With AD Versus Healthy and Medical Controls (Cross-Sectional Studies).
| Parameter | Studies, N | AD Group, N | Control Group, N | SMD (95% CI) | P Value | Heterogeneity |
|---|---|---|---|---|---|---|
| Healthy Controls | ||||||
| Serum Mg | 4 | 190 | 189 | −0.07 (−0.98 to 0.85) | 0.89 | Tau2 = 0.79; χ2 = 45.12, df = 3 (P < .0001); I2 = 93% |
| Plasma Mg | 3 | 56 | 68 | −0.19 (−0.58 to 0.20) | 0.34 | Tau2 = 0.02; χ2 = 2.33, df = 2 (P = .31); I2 = 31% |
| CSF Mg | 2 | 171 | 56 | −0.35 (−0.65 to −0.04) | 0.02 | Tau2 = 0.00; χ2 = 0.05, df = 1 (P = .82); I2 = 0% |
| Ionized/blood cells Mg | 2 | 59 | 64 | −0.02 (−0.72 to 0.68) | 0.98 | Tau2 = 0.18; χ2 = 3.50, df = 1 (P = .06); I2 = 71% |
| Hair Mg | 2 | 44 | 80 | −0.75 (−1.13 to −0.36) | 0.0001 | Tau2 = 0.00; χ2 = 0.56, df = 1 (P = .46); I2 = 0% |
| Medical Controls | ||||||
| Serum Mg | 2 | 73 | 99 | −0.21 (−0.52 to 0.10) | 0.18 | Tau2 = 0.00; χ2 = 0.05, df = 1 (P = .82); I2 = 0% |
| Plasma Mg | 1 | 12 | 12 | −1.54 (−2.47 to −0.61) | 0.001 | Not applicable |
| CSF Mg | 1 | 21 | 15 | 0.42 (−0.25 to 1.09) | 0.22 | Not applicable |
| Ionized/blood cells Mg | 1 | 36 | 65 | −0.40 (−0.82 to 0.00) | 0.05 | Not applicable |
| Hair Mg | No studies available | |||||
Abbreviations: AD, Alzheimer’s disease; CI, confidence interval; CSF, cerebrospinal fluid; Mg, magnesium; SMD, standardized mean differences.
The mean age of patients with AD and controls was 70.3 ± 9.1 and 67.4 ± 11.0 years, while the percentage of females was 62.3% and 54.8%, respectively. For the diagnosis of AD, 5 studies used the criteria proposed by Diagnostic and Statistical Manual of Mental Disorders (Third edition; DSM-III), 24,25,26,30,31 3 followed a combination of Diagnostic and Statistical Manual of Mental Disorders (Fourth edition; DSM-IV) and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCS-ADRDA) criteria, 21,22,29 3 followed the criteria proposed by NINCS-ADRDA, 27,32,33 1 followed the criteria proposed by DSM-IV, 23 and 1 used a clinical identification of AD. 28 Among the 6 studies reporting the severity of dementia, 3 included mild to moderate stages of AD, 21,30,31 1 severe AD, 24 1 moderate, 29 and 1 all stages of severity of AD. 22 In 4 studies 21,24,27,29 reporting the mean values of MMSE, AD scored 19.1 ± 5.6 versus 27.6 ± 1.7 points in the controls (2 studies 21,24 ). Finally, 7 studies 21,22,25,27,29,31,32 investigated Mg in serum, 4 studies 24,26,28,33 in plasma, 3 studies 23,25,27 in CSF, 3 studies in blood cells or in ionized form, 21,26,28 and 2 studies in hair. 30,31
The STROBE quality indicators (Supplementary Table S2) indicated that 4 (30.7%) studies did not provide a clear description of participants 23,30,31,33 or a clear definition of the outcomes, 21,22,25,32 respectively. Moreover, in 5 (38.5%) studies 22,23,27,30,33 detailed information on the controls was missing as well as 1 (7.7%) study 28 for AD group (Supplemetary Table S2).
Cross-Sectional Studies Comparing AD Versus HCs
Pooling data from 2 studies, 25,27 including 171 patients with AD versus 56 HCs, patients with AD reported significantly lower Mg in CSF (SMD = −0.35; 95%CI: −0.65, −0.04, P = .02; I2 = 0%). Similar results were evident when considering Mg present in hair (2 studies 30,31 ; 44 patients with AD vs 80 HCs; SMD = −0.75; 95%CI: −1.13, −0.36, P = .0001; I 2 = 0%). Conversely, no significant differences were found for Mg in serum (4 studies 29,31,32,33 ; SMD = −0.07; 95%CI: −0.98, 0.85, P = .89; I 2 = 93%), plasma (3 studies 25,26,28 ; SMD = −0.19; 95%CI: −0.58, 0.20, P = .34; I 2 = 31%), or ionized/blood cells (2 studies 26,28 ; SMD = −0.02; 95%CI: −0.72, 0.68, P = .98; I 2 = 71%).
Cross-Sectional Studies Comparing AD Versus MCs
Pooling data from 2 cross-sectional studies 21,22 including 73 patients with AD versus 99 MCs, no significant differences were found for serum Mg (SMD = −0.21; 95%CI: −0.52, 0.10, P = .18; I 2 = 0%). One study 24 reported a significantly lower value for Mg in plasma (SMD = −1.54; 95%CI: −2.47, −0.61, P = .001) and in ionized/blood cells (studies =1 23 ; SMD = −0.40; 95%CI: −0.82, 0.00, P = .05) in patients with AD compared to MCs. Conversely, no differences were evident in the study reporting data about Mg in CSF (Table 1).
Meta-Regression Analysis
Altogether, we found 2 outcomes with high heterogeneity (defined as I 2 ≥ 50%) in the comparison between AD and HCs, that is, serum and ionized/blood cells Mg. Since the meta-regression analysis requires a minimum of 4 studies, only serum Mg was analyzed. As shown in Supplementary Table S3, the 2 studies made in Europe/America and that used NINCDS-ADRDA criteria for diagnosis of AD were significant moderators of our findings (slope: −1.77 ± 0.28, P < .0001).
After stratifying for these moderators, the studies made in Europe/America and using NINCDS-ADRDA criteria 29,31 showed that patients with AD had significantly higher serum Mg levels than HCs (SMD = 0.86; 95%CI: 0.10, 1.62, P = .03), while the other 2 studies 32,33 included in this analysis showed an opposite result (SMD = −0.82; 95%CI: −1.06, −0.59, P < .0001).
Publication Bias
Inspecting the funnel plots, there did not appear to be a publication bias for any of the outcomes.
Discussion
The major results from the study were that patients with AD showed significant lower Mg concentrations in CSF and in hair compared to HCs and significantly lower Mg values in plasma and in red blood cells compared to MCs. No differences in serum Mg were found between patients with AD and HCs or MCs, although in the comparison with HCs, this finding was significantly moderated by continent and criteria for the diagnosis of AD. The Mg levels were found to be decreased in the brain tissue of patients with AD, particularly in the hippocampus. 22 A poor Mg status seems to be deleterious for the function of this organ in which Mg-dependent tyrosine kinases are widely diffused. 21 Thus, a relationship between poor Mg status and decrease in hippocampus volume, a common finding in AD, was hypothesized. 34 Moreover, a pathophysiological role of Mg in AD is sustained by experimental evidence in which Mg seems to be fundamental for other neurological functions usually decreased in AD, like the function of NMDA receptor or the stability of neuronal membranes. 21 Finally, another relevant aspect is that recent research has shown the association between Mg deficiency and oxidative stress to be a potential risk factor for AD. Magnesium deficiency is associated with both increased oxidative stress and decreased antioxidant barriers, and Mg per se seems to have antioxidant properties, particularly at the mitochondrial level. 35
Patients with AD had significantly lower Mg levels in CSF than HCs, suggesting that the availability of Mg in brain could be reduced in AD, although concentrations in serum/plasma were not substantially different between patients with AD and HCs. Blood–brain barrier dysfunction increases with advancing age, likely due to vascular damages that are more evident in patients with AD. 27 It is thus possible that the blood–brain barrier in patients with AD has a different permeability compared to healthy individuals, leading to significantly lower Mg concentrations in CSF, despite the absence of significant differences in blood levels. It is known that the levels of Mg in CSF are fundamental for controlling peripheral and central vasomotor tone. Lower Mg levels in CSF may be an additional factor promoting neural degeneration and consequently cognitive decline in these patients through an arterial vasospasm that could lead to a chronic damage due to hypoxia. 36,37 The findings of lower Mg status in AD were confirmed by the results in hair. Hair could be considered as good proxy for intracellular Mg or tissue storage of Mg, since the collection of scalp hair includes hair papilla and hair matrix cells that are nourished by blood. 38 Some authors proposed that this tissue could represent the long-term storage of Mg better than other markers usually considered for Mg deficiency. 39,40
No differences were found between patients with AD and controls when considering the Mg in serum. Serum Mg is known to be a poor predictor of Mg homeostasis in general, 41 except for more severe conditions typical of older age such as sarcopenia, 42,43 osteoporosis, 44 or mortality. 45 However, since the meta-regression/sensitivity analysis showed that patients with AD had significantly higher serum Mg levels than HCs in studies made in Europe/America and using NINCDS-ADRDA criteria, but significantly lower in studies made in Asia and using DSM criteria, future studies are needed to verify whether serum Mg could also be used as marker of AD in selected populations, keeping in mind the poor reliability of serum Mg in the elderly individuals as marker of Mg status. 21
The findings of this study have several limitations. First, we analyzed only cross-sectional studies, and conclusions cannot be drawn regarding a casual association between Mg and AD. Second, these studies were limited in sample size and only 7 used a matching criteria. Thus, a bias for these studies cannot be excluded. Third, the majority of the studies used an older method for the diagnosis of AD, that is, criteria proposed by DSM-III. Finally, the studies included in our meta-analysis did not assess some confounding factors relevant for Mg homeostasis such as the use of drugs containing Mg (eg, laxatives) or affecting Mg metabolism (such as diuretics). Since patients with AD usually have other diseases as well, it is possible that these factors could have influenced our results. In future studies the influence of these confounders should be controlled for.
As the study was made on persons with an already existing disease, conclusions concerning causality cannot be drawn. The lower Mg values could equally well have been caused by dietary deficiencies in persons with existing disease as representing factors leading to the disease. Support for causality is, however, found in a study on 1450 persons who were followed for 8 years. 46 A high Mg intake was related to a lower risk to develop mild cognitive impairment. Several animal models also support a theory on causality. In a study on transgenic mice, the administration of Mg was found to reduce the deposition of Aβ-plaques that cause synapse dysfunction. 47 The formation of Aβ-plaques is also favored by acid conditions. 48 Acidity is common in elderly persons and will also lead to Mg deficiency due to a decreased reabsorption of Mg in the distal renal tubuli. 49 Furthermore, positive effects of Mg supplementation on cognitive function were reported in older mice. 50 Against this background, future trials with Mg supplementation or with diets rich in Mg for the treatment/prevention of AD are warranted.
In conclusion, AD seems to be associated with lower Mg status when compared to HCs, but not to medical ones, probably due to the scarcity of studies about these patients. Since both AD and poor Mg status are 2 common conditions in older people, further studies are needed to better understand the real pathophysiological role of Mg in AD.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online [appendices/data supplements/etc] are available at http://aja.sagepub.com/supplemental.
References
- 1. Abbott A. Dementia: a problem of our age. Nature. 2011;475(7355):S2–S4. [DOI] [PubMed] [Google Scholar]
- 2. Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351(1):56–67. [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raffaitin C, Gin H, Empana JP, et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the three-city study. Diabetes Care. 2009;32(1):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes da Silva S, Vellas B, Elemans S, et al. Plasma nutrient status of patients with Alzheimer’s disease: systematic review and meta-analysis. Alzheimers Dement. 2014;10(4):485–502. [DOI] [PubMed] [Google Scholar]
- 7. Andrási E, Igaz S, Molnár Z, Makó S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes Res. 2000;13(3):189–196. [PubMed] [Google Scholar]
- 8. Glick JL. Dementias: the role of magnesium deficiency and an hypothesis concerning the pathogenesis of Alzheimer’s disease. Med Hypotheses. 1990;31(3):211–225. [DOI] [PubMed] [Google Scholar]
- 9. Sergi G, De Rui M, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer’s disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72(1):160–165. [DOI] [PubMed] [Google Scholar]
- 10. Mayer ML, Westbrook GL, Guthrie PB. Voltage dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. [DOI] [PubMed] [Google Scholar]
- 11. Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65(2):165–177. [DOI] [PubMed] [Google Scholar]
- 12. Ebel H, Günther T. Magnesium metabolism: a review. J Clin Chem Clin Biochem. 1980;18(5):257–270. [DOI] [PubMed] [Google Scholar]
- 13. Lee M, Jantaratnotai N, McGeer E, McLarnon JG, McGeer PL. Mg2+ ions reduce microglial and THP-1 cell neurotoxicity by inhibiting Ca2+ entry through purinergic channels. Brain Res. 2011;1369:21–35. [DOI] [PubMed] [Google Scholar]
- 14. Libien J, Sacktor TC, Kass IS. Magnesium blocks the loss of protein kinase C, leads to a transient translocation of PKC(alpha) and PKC(epsilon), and improves recovery after anoxia in rat hippocampal slices. Brain Res Mol Brain Res. 2005;136(1-2):104–111. [DOI] [PubMed] [Google Scholar]
- 15. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 18. RevMan 5.3. Informatics & knowledge management department. http://tech.cochrane.org/revman. Accessed May 4, 2015.
- 19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 20. Comprehensive Meta-Analysis (CMA) 3.0. http://www.meta-analysis.com/index.php. Accessed May 4, 2015.
- 21. Barbagallo M, Belvedere M, Di Bella G, Dominguez LJ. Altered ionized magnesium levels in mild-to-moderate Alzheimer’s disease. Magnes Res. 2011;24(3):S115–S121. [DOI] [PubMed] [Google Scholar]
- 22. Çilliler AE, Ozturk S, Ozbakir S. Serum magnesium level and clinical deterioration in Alzheimer’s disease. Gerontology. 2007;53(6):419–422. [DOI] [PubMed] [Google Scholar]
- 23. Hozumi I, Hasegawa T, Honda A, et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303(1-2):95–99. [DOI] [PubMed] [Google Scholar]
- 24. Lemke MR. Plasma magnesium decrease and altered calcium/magnesium ratio in severe dementia of the Alzheimer type. Biol Psychiatry. 1993;37(5):341–343. [DOI] [PubMed] [Google Scholar]
- 25. Basun H, Forssell LG, Wetterberg L, Winblad B. Metals and trace elements in plasma and cerebrospinal fluid in normal ageing and Alzheimer’s disease. J Neural Transm Park Dis Dement Sect. 1991;3(4): 231–258. [PubMed] [Google Scholar]
- 26. Borella P, Giardino A, Neri M, Andermarker E. Magnesium and potassium status in elderly subjects with and without dementia of Alzheimer type. Magnes Res. 1990;3(4):283–289. [PubMed] [Google Scholar]
- 27. Boström F, Hansson O, Blennow K, et al. Cerebrospinal fluid total tau is associated with shorter survival in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2009;28(4):314–319. [DOI] [PubMed] [Google Scholar]
- 28. Brackenridge JK, Mc Donald C. The concentrations of magnesium and potassium in erythrocytes and plasma of geriatric patients with psychiatric disorders. Med. J. Aust. 1969;2(8):390–394. [DOI] [PubMed] [Google Scholar]
- 29. Gustaw-Rothenberg K, Kowalczuk K, Stryjecka-Zimmer M. Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr Gerontol Int. 2010;10(2):161–166. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi S, Fujiwara S, Arimoto S, et al. Hair aluminium in normal aged and senile dementia of Alzheimer type. Prog Clin Biol Res. 1989;317:1095–1109. [PubMed] [Google Scholar]
- 31. Shore D, Henkin RI, Nelson NR, Agarwal RP, Wyatt RJ. Hair and serum copper, zinc, calcium, and magnesium concentrations in Alzheimer-type dementia. J. Am Geriatr Soc. 1984;32(4):892–895. [DOI] [PubMed] [Google Scholar]
- 32. Singh NK, Banerjee BD, Bala K, Basu M, Chhillar N. Polymorphism in cytochrome p450 2d6, glutathione s-transferases pi 1 genes, and organochlorine pesticides in Alzheimer disease: a case–control study in North Indian Population. J Geriatr Psychiatry Neurol. 2014;27(2):119–127. [DOI] [PubMed] [Google Scholar]
- 33. Vural H, Demirin H, Kara Y, Eren I, Delibas N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J Trace Elem Med Biol. 2010;24(3):169–173. [DOI] [PubMed] [Google Scholar]
- 34. Francis PT. Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18( suppl 1):15–21. [DOI] [PubMed] [Google Scholar]
- 35. Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res. 2009;22(4):235–246. [DOI] [PubMed] [Google Scholar]
- 36. Turlapaty PD, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208(4440):198–200. [DOI] [PubMed] [Google Scholar]
- 37. Lemke MR. Plasma magnesium decrease and altered calcium/magnesium ratio in severe dementia of the Alzheimer type. Biol Psychiatry. 1995;37(5):341–343. [DOI] [PubMed] [Google Scholar]
- 38. Ochi A, Ishimura E, Tsujimoto Y, et al. Hair magnesium, but not serum magnesium, is associated with left ventricular wall thickness in hemodialysis patients. Circ J. 2013;77(12):3029–3036. [DOI] [PubMed] [Google Scholar]
- 39. Kazi TG, Afridi HI, Kazi GH, Jamali MK, Arain MB, Jalbani N. Evaluation of essential and toxic metals by ultrasound-assisted acid leaching from scalp hair samples of children with macular degeneration patients. Clin Chim Acta. 2006;369(1):52–60. [DOI] [PubMed] [Google Scholar]
- 40. Hac E, Czarnowski W, Gos T, Krechniak J. Lead and fluoride content in human bone and hair in the Gdansk region. Sci Total Environ. 1997;206(2-3):249–254. [PubMed] [Google Scholar]
- 41. Ranade VV, Somberg JC. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am J Ther. 2001;8(5):345–357. [DOI] [PubMed] [Google Scholar]
- 42. Dominguez LJ, Barbagallo M, Lauretani F, et al. Magnesium and muscle performance in older persons: the InCHIANTI study. Am J Clin Nutr. 2006;84(2):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veronese N, Berton L, Carraro S, et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am J Clin Nutr. 2014;100(3):974–981. [DOI] [PubMed] [Google Scholar]
- 44. Zheng J, Mao X, Ling J, He Q, Quan J, Jiang H. Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol Trace Elem Res. 2014;159(1-3):8–14. [DOI] [PubMed] [Google Scholar]
- 45. Reffelmann T, Ittermann T, Dörr M, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219(1):280–284. [DOI] [PubMed] [Google Scholar]
- 46. Cherbuin N, Kumar R, Sachdev PS, Anstey KJ. Dietary mineral intake and risk of mild cognitive impairment: the PATH through life project. Front Aging Neurosci. 2014;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li W, Yu J, Liu Y, et al. Elevation of brain magnesium prevents synaptic loss and reverse cognitive deficits in Alzheimer’s disease mouse model. Mol Brain. 2014;7:65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physical acidosis. J Biol Chem. 1998;273(21):12817–12826. [DOI] [PubMed] [Google Scholar]
- 49. Rylander R, Remer T, Berkemeyer S, Vormann J. Acid-base status affects renal magnesium losses in healthy, elderly persons. J Nutr. 2006;136(9):2374–2377. [DOI] [PubMed] [Google Scholar]
- 50. Landfield PW, Morgan GA. Chronically elevating plasma Mg2+ improves hippocampal frequency potentiation and reversal learning in aged and young rats. Brain Res. 1984;322(1):167–171. [DOI] [PubMed] [Google Scholar]