Abstract
Objectives:
The perioperative period is challenging and stressful for older adults. Those with depression and/or anxiety have an increased risk of adverse surgical outcomes. We assessed the feasibility of a perioperative mental health intervention composed of medication optimization and a wellness program following principles of behavioral activation and care coordination for older surgical patients.
Methods:
We included orthopedic, oncologic, and cardiac surgical patients aged 60 and older. Feasibility outcomes included study reach, the number of patients who agreed to participate out of the total eligible; and intervention reach, the number of patients who completed the intervention out of patients who agreed to participate. Intervention efficacy was assessed using the Patient Health Questionnaire for Anxiety and Depression (PHQ-ADS). Implementation potential and experiences were collected using patient surveys and qualitative interviews. Complementary caregiver feedback was also collected.
Results:
Twenty-three out of 28 eligible older adults participated in this study (mean age 68.0 years, 65% women), achieving study reach of 82% and intervention reach of 83%. In qualitative interviews, patients (n = 15) and caregivers (complementary data, n = 5) described overwhelmingly positive experiences with both the intervention components and the interventionist, and reported improvement in managing depression and/or anxiety. Preliminary efficacy analysis indicated improvement in PHQ-ADS scores (F = 12.13, p <0.001).
Conclusions:
The study procedures were reported by participants as feasible and the perioperative mental health intervention to reduce anxiety and depression in older surgical patients showed strong implementation potential. Preliminary data suggest its efficacy for improving depression and/or anxiety symptoms. A randomized controlled trial assessing the intervention and implementation effectiveness is currently ongoing.
Keywords: Mental health, older patients, surgeries, behavioral activation, medication optimization, implementation, anxiety, depression, early Phase evaluation
INTRODUCTION
Most older adults undergo one or more major surgical procedures in the later years of life. Across the United States, >14 million inpatient and >12 million ambulatory surgeries are performed yearly,1 with more than half of these in patients 65 years or older.2 Older adults with depression and/or anxiety are at higher risk for perioperative complications such as postoperative falls, venous thrombosis, delirium, short-term functional dependence, and postoperative nausea and vomiting.3,4
Despite the high-risk perioperative period for older adults with depression and/or anxiety, there are few perioperative-specific mental health interventions.5 Although psychotherapy may be beneficial, its delivery is challenging in inpatient settings; similarly, risk-benefit considerations of pharmacological interventions in inpatient settings are harder to assess. Moreover, older surgical patients have multimorbidities, which are associated with more medication-resistant depression,6 and are susceptible to adverse effects of central nervous system drugs due to physical and cognitive frailty and drug-drug interactions stemming from polypharmacy.7-10
Thus, perioperative mental health (PMH) is a challenging area requiring effective interventions. We conducted a study with older surgical patients and clinicians to gain perspective on patient and clinician management of anxiety and depression during the perioperative period and to assess PMH needs.11 Across 40 interviews, we identified key barriers to perioperative mental health management, including fear of surgery, limited understanding of what to expect during surgery and recovery, and complex medication management. Further informed by previous psychotherapeutic surgical studies, we identified the need for an intervention spanning the perioperative continuum, as interventions beginning in the preoperative period have been shown to improve mental health and alleviate symptoms of preoperative anxiety and depression—even resulting in earlier hospital discharges.12,13 Thus, we developed a multicomponent intervention composed of medication optimization (MO) to deprescribe brain-hazardous medications and escalate the dose of subtherapeutic dosed antidepressants,7 as well as a wellness program rooted in behavioral activation14 to address barriers to PMH management.
This paper reports on a feasibility study of the PMH intervention. Our study objectives were four-fold: 1) examine the feasibility of implementing a patient-centered PMH intervention for older surgical patients with clinically significant symptoms of depression and/or anxiety; 2) identify patient perspectives and experiences with the intervention, with specific emphasis on its implementation barriers, enablers, and strategies to ensure its reach, uptake, and sustainability in perioperative settings; 3) demonstrate acceptability and appropriateness of the interventions; and 4) assess the feasibility of study procedures including patient recruitment, screening, outcome assessments, and intervention materials. In addition, we also gathered feedback from patients’ caregivers on their perceptions of patient experiences and the impact of our intervention on patient wellbeing.
Conceptual Model
To guide treatment development and feasibility testing, we integrated two dissemination and implementation frameworks: the Consolidated Framework for Intervention Research (CFIR)15 and Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM)16 (Fig. 1).
FIGURE 1.
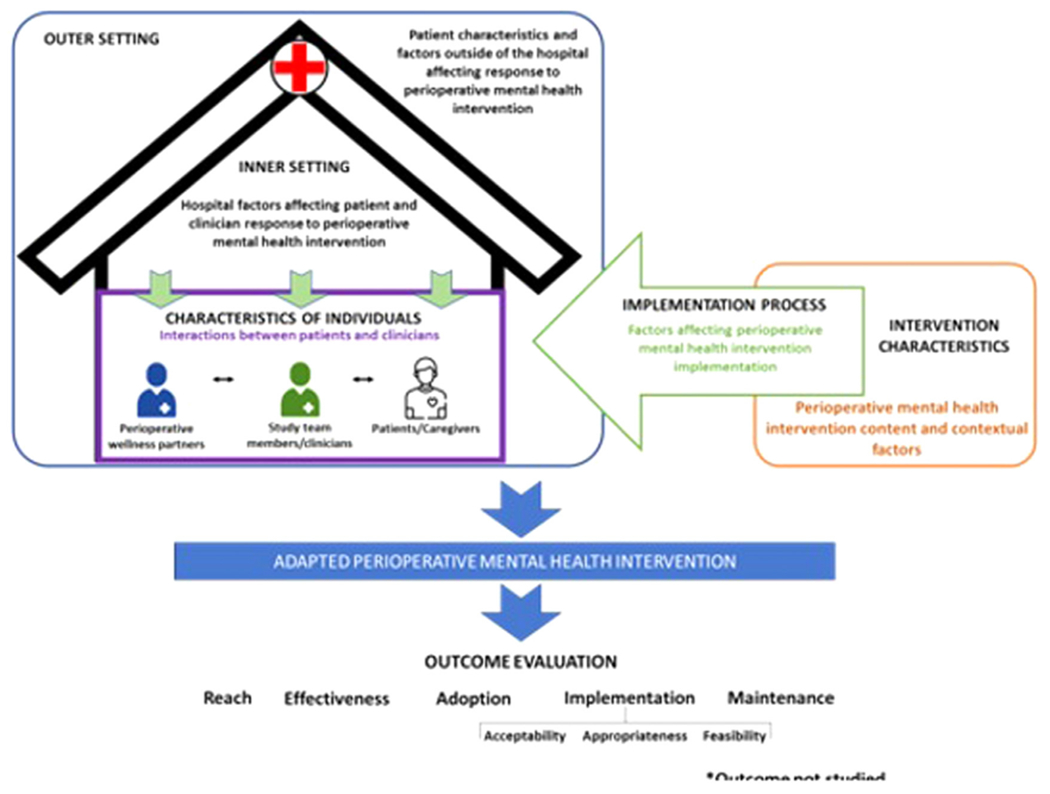
Integrated conceptual model: CFIR, consolidated framework for intervention research: RE-AIM, reach, effectiveness, adoption, implementation, and maintenance.
CFIR is a framework to guide intervention implementation, comprised of 39 constructs across five domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and implementation process. RE-AIM is a -five-dimensional evaluation framework designed to assess public health interventions’ reach, effectiveness, adoption, implementation, and maintenance across multiple levels (e.g., individual, clinic). In this study, we used CFIR to identify the contextual determinants that affected the implementation and evaluation of our PMH intervention in the surgical setting and used RE-AIM to guide the outcomes that can inform the empirical evaluation of the effectiveness and implementation potential of our intervention.
METHODS
Study Setting and Design
This feasibility trial was conducted at a large academic hospital serving a catchment area, including urban and rural patients. We followed a mixed-methods approach informed by our conceptual framework and supported by a parallel-convergent study design, collecting and merging quantitative data from a prospective cohort, with qualitative data from our indepth interviews for comparison and interpretation. Further study and intervention details were reported in the protocol.17 The study was approved by the Washington University Human Research Protections Office (IRB # 202101103).
Study Participants
Inclusion criteria for patients were: 60 years of age or older; scheduled for major cardiac, orthopedic, or oncologic surgery; and clinically relevant depression and/or anxiety symptoms as indicated by a score of ≥10 on the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS).18 Exclusion criteria for surgical patients were: estimated life expectancy of fewer than 12 months; unable to read, speak, and understand English; severe cognitive impairment (assessed with Short Blessed Test19); or acutely suicidal (see17). To collect complementary feedback on patient experiences and their perspectives about the potential impact of our intervention on patients’ mood and emotional wellbeing during perioperative care, patients’ caregivers (e.g., spouse, partner, children, friend), as identified by the patient, were invited to participate in a semi-structured interview after the patien’s participation in the intervention was completed. Caregivers were not our target intervention users.
Patients were recruited by phone following clinician referral, self-referral, or screening through the electronic health record, whereas caregivers were recruited through patient referral. Patients consented via paper collected by mail, in person, or via an electronic REDCap link to e-consent; caregivers consented verbally.
Participant Incentives
Patients and caregivers were eligible for up to $125 and $25, respectively, as compensation.
PMH Intervention
Our PMH intervention aimed to improve patient preparedness for surgery and enhance recovery using an individualized approach with two components: MO and the wellness program incorporating principles of BA, and care coordination. Both components consisted of standardized core elements for all patients, and modifiable components, personalized according to patient preferences, needs, and constraints (Fig. 2). The intervention was administered by perioperative wellness partners (PWPs): Masters-trained social workers and counselors with experience in mental health treatment and training in psychological and pharmacological treatments including behavioral activation20 and MO.21 PWPs delivered the intervention with oversight from study team members, including practicing clinical pharmacists, a psychologist, and a geriatric psychiatrist.
FIGURE 2.
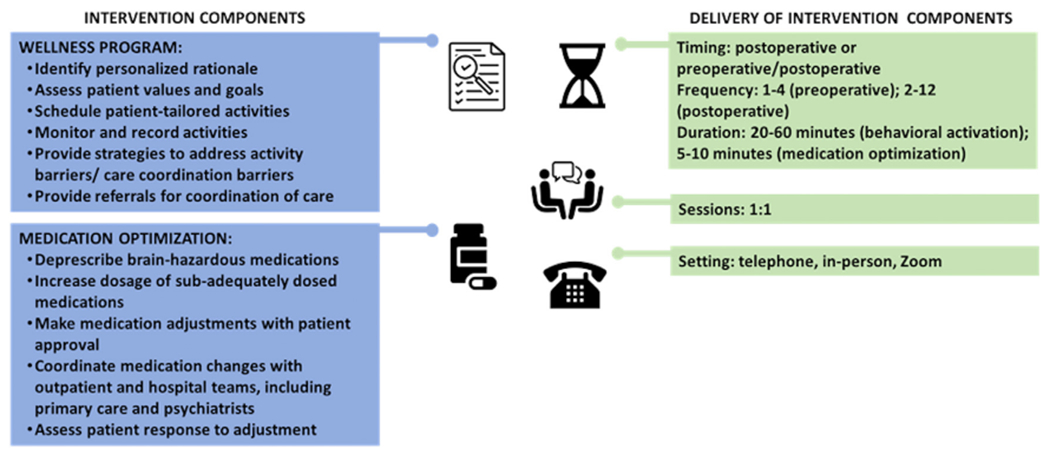
Multicomponent, patient-centered perioperative mental health intervention.
MO involves dose escalation or deprescription, as per pharmacist and psychiatrist recommendations. Following discussion with the patient, intervention team members involved with MO coordinated medication adjustments with outpatient and hospital teams. The sessions were conducted via telephone or Zoom per participant preference and PWPs completed a session documentation form (see Appendix S1).
Data Collection
Data were collected at several timepoints. At enrollment, baseline review and assessments included: demographics, history of comorbidities, the Brief Pain Inventory,22 the 3-minute diagnostic interview for confusion assessment method (3D-CAM)23), and patient medication lists. Table 1 presents the outcomes collected: 1) preoperatively at baseline, 2) postoperatively in-hospital, 3) postoperatively 1 month, 2 months, 3 months, and 4) at the end of study (following completion of the 3-month data collection).
TABLE 1.
Data Collection Measures and Outcomes
| Outcome | Measurement | Timepoint | Source |
|---|---|---|---|
| Reach (Primary) | Reach of the study: patients who agreed to participate in the study out of the total eligible to participate | Baseline | Electronic health record/ surveys |
| Reach of the intervention: patients who completed the intervention out of patients who agreed to participate in the feasibility study | End of study | ||
| Completeness of planned RCT primary outcome data collection at specified timepoints (Secondary) | Percentage of instrument or data fields completed for Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS)18 | Baseline; 1 month; 3 months | Surveys |
| Completeness of planned RCT secondary outcomes data collection at specified time-points (Exploratory) | Quality of life (Veterans RAND 12-item health survey [VR-12]24) | Baseline; 1 month; 3 months | Surveys |
| Delirium incidence | Baseline; in-hospital | ||
| Postdischarge falls (and at baseline, participants are asked if they had any falls in the past 3 months) | Baseline; 1 month; 2 months; 3 months | ||
| Medications reviewed | Baseline; 1 month; 3 months | ||
| Persistent postsurgical pain (PPSP) | 1 month; 3 months | ||
| Length of stay (both hospital and ICU) | In-hospital | Research data warehouse | |
| All-cause rehospitalization | In-hospital; 1 month; 3 months | ||
| Implementation-potential (Exploratory) | Acceptability, appropriateness, and feasibility of the intervention: The Acceptability of Intervention Measure (AIM), the Intervention Appropriateness Measure (IAM), and the Feasibility of Intervention Measure (FIM)25 | End of study | Surveys |
| Shared decision-making (CollaboRATE survey26) | |||
| Patient experience (adapted from CAHPS surgical care survey27) | |||
| Patient and caregiver perceptions and attitudes about intervention use | Semistructured interviews |
We administered the Behavioral Activation for Depression Scale (BADS)28 as a measure of target engagement, assessing changes in behavioral activation levels during intervention use. We also simultaneously refined and recorded study and intervention adaptations to improve their fit in the surgery context for older patients (Table 1).
Data were collected in-person, over the phone, or over Zoom (depending on patient preference) and documented in REDCap by PWPs and the study coordinator, TC. Semi-structured interviews with patients and caregivers were conducted by trained researchers (A.M., F.L., J.A.) over the phone and recorded for analysis. Interviews with participants were conducted until we achieved data saturation (or no new data was found).
Data Analysis
Quantitative outcomes were summarized using descriptive statistics. Secondary and exploratory outcomes related to completion of data were described as a percentage of the instruments completed. Preliminary efficacy of the intervention based on PHQ-ADS scores for depression and anxiety were calculated using a mixed model repeated measures ANOVA.
We followed a hybrid inductive-deductive thematic analysis approach across qualitative data collected within session documentation forms and interviews. First, J.A. and A.M. read through interview transcripts multiple times for familiarity and open-ended answers within surveys and documentation forms on REDCap. Second, transcripts were openly coded using data-driven codes (e.g., positive experience, medication review). Third, transcripts were coded based on the CFIR.29 Fourth, similar and overlapping codes were organized into subthemes and compared within and between transcripts (e.g., medication review, participant motivation). Higher-level themes were generated based on subthemes (e.g., MO elements). J.A. and A.M. independently and iteratively coded data, creating a codebook and refining it through multiple rounds of team discussion to reach 100% consensus on themes and subthemes (see Appendix S2). To ensure the validity of our data coding and analysis approach, best practices were established and followed, including peer review and debriefing, as well as data triangulation.30 Data analyses were conducted independently by JA and AM – two study team members with over 15 and 4 years of experience with qualitative interviewing and analysis.
RESULTS
The study was conducted from November 2021 to December 2022. Twenty-four of 29 eligible patients were originally enrolled and allocated to the intervention (Fig. 3), but 1 patient was later found to be ineligible. Twenty-three patients were enrolled, and 19 of the 23 enrolled patients completed the intervention. Fifteen patients and 5 patient caregivers participated in end-of-study interviews. Table 2 presents patient demographics.
FIGURE 3.
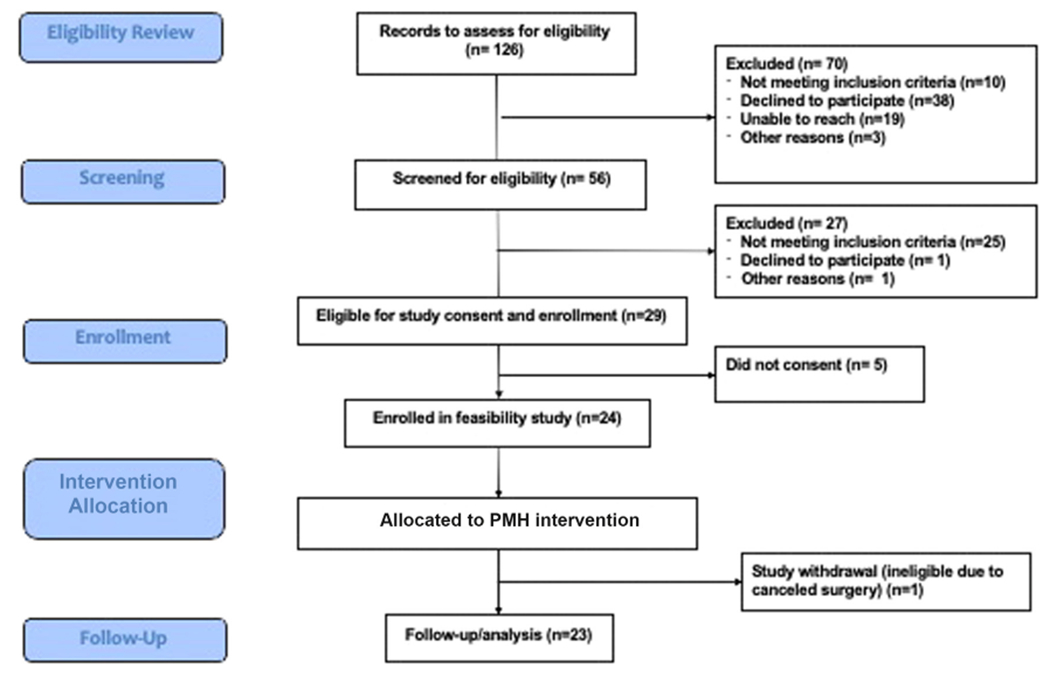
CONSORT figure.
TABLE 2.
Patient Demographics
| Characteristics | Cardiac, N = 8a | Oncologic, N = 8a | Orthopedic, N = 7a | Overall, N = 23a |
|---|---|---|---|---|
| Age at screening | 68.6 (4.2) | 69.3 (4.5) | 65.8 (4.6) | 68.0 (4.5) |
| Sex at birth | ||||
| Male | 2 (25%) | 4 (50%) | 2 (29%) | 8 (35%) |
| Female | 6 (75%) | 4 (50%) | 5 (71%) | 15 (65%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gender | ||||
| Male | 1 (17%) | 4 (50%) | 2 (29%) | 7 (33%) |
| Female | 5 (83%) | 4 (50%) | 5 (71%) | 14 (67%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 2 | 0 | 0 | 2 |
| Race | ||||
| African American | 0 (0%) | 1 (12%) | 1 (14%) | 2 (8.7%) |
| Caucasian | 7 (88%) | 6 (75%) | 6 (86%) | 19 (83%) |
| More than one race | 1 (12%) | 0 (0%) | 0 (0%) | 1 (4.3%) |
| Native American | 0 (0%) | 1 (12%) | 0 (0%) | 1 (4.3%) |
| Ethnicity | ||||
| Hispanic/Latino | 2 (25%) | 0 (0%) | 1 (14%) | 3 (13%) |
| Non-Hispanic/Non-Latino | 6 (75%) | 7 (88%) | 6(86%) | 19 (83%) |
| Prefer not to answer | 0 (0%) | 1 (12%) | 0 (0%) | 1 (4.3%) |
Mean (SD); n (%).
Wellness Program and MO Delivery
All patients were enrolled in the study prior to surgery. On average, participants completed 8.0 wellness program sessions with a PWP, lasting an average of 41.4 minutes per session (Table 3). We originally targeted 8–12 sessions per participant, but one of our goals was to determine how many sessions were feasible for individuals to engage in around the time of surgery. For some patients with a short period between time the surgery is scheduled and time of surgery, we were unable to complete many preoperative sessions. Some patients chose biweekly sessions and others chose weekly sessions. We prioritized patient preference when scheduling sessions. On average, participants engaged in 2.1 preoperative sessions and 5.9 postoperative sessions. Over 97% of sessions were conducted over telephone.
TABLE 3.
Perioperative Mental Health Intervention Session Details
| Cardiac | Orthopedic | Oncologic | Total | ||
|---|---|---|---|---|---|
| Mean number of total sessions | 7.8 | 8.5 | 7.7 | 8.0 | |
| Mean number of preoperative sessions | 1.4 | 2.9 | 1.9 | 2.1 | |
| Mean number of postoperative sessions | 6.4 | 5.6 | 5.8 | 5.9 | |
| Mean number of wellness program sessions | 7.3 | 7.9 | 7.3 | 7.5 | |
| Mean number of medication optimization sessions | 3.7 | 1.4 | 2.4 | 2.5 | |
| Mean session duration (minutes) | 39.0 | 44.8 | 40.8 | 41.4 | |
| Session modality (n) | Telephone | 66 | 59 | 53 | 178 |
| Zoom | 0 | 0 | 2 | 2 | |
| In person | 0 | 0 | 2 | 2 | |
| Mean number of medications at baseline | 20.6 | 15.8 | 15.6 | 17.3 | |
| Number of medications eligible for deprescribing Deprescribing outcomes |
12 | 4 | 7 | 23 | |
| Implemented | 3 | 2 | 2 | 7 | |
| Not Implemented | 6 | 2 | 4 | 12 | |
| Othera | 3 | 0 | 1 | 4 | |
| Number of antidepressants eligible for dose escalation Antidepressant dose escalation outcomes |
5 | 3 | 0 | 8 | |
| Implemented | 3 | 0 | 0 | 3 | |
| Not Implemented | 2 | 1 | 0 | 3 | |
| Otherb | 0 | 2 | 0 | 2 | |
For deprescribing: one participant taking two target medications declined MO, but it both medications were discontinued by the clinical team. one participant decreased their intake frequency but did not discontinue the medication completely and one participant stopped taking the medication voluntary without discussion with the Wellness Partner.
For dose escalation, other refers to one participant for whom it was determined that the dose should not be increased for clinical reasons and one participant was still in consultation with their medical team about increasing the dosage when the study ended.
The BADS target engagement measure was completed in 96% of participants at baseline; 74% at month 1; and 87% at month 3. Across surgical populations, cardiac patients had the highest mean number of medications at baseline and highest mean number of medications eligible for de-prescribing. Across 23 patients, 16 patients were eligible for MO: there were 14 patients with medications eligible for deprescription and 5 patients with medications eligible for dose escalation, 3 of these participants had medications eligible for both deprescription and dose escalation (Table 3).
Primary, Secondary, and Exploratory Outcomes: Reach and Completeness of Data Collection
Among 29 potential patients, we recruited 24 patients. However, one patient became ineligible later in the study (due to canceled surgery) and hence we achieved a study reach of 82% (i.e., 23 out of 28 patients). Among the 23 who participated in our intervention, one patient was lost to follow-up, one patient passed away, and two withdrew early, with intervention reach of 83%.
Patients completed their PHQ-ADS relatively consistently: 100% at baseline, 66% at 1 month, and 87% at 3 months. Reasons for incomplete 3D-CAM assessments are provided in Appendix S3. Completeness of data collection ranged from 65% to 100% on secondary outcomes (Table 4). Additional details on changes in outcomes across timepoints are provided in Appendix S4. Results of the mixed model repeated measures ANOVA indicated a significant reduction in PHQ-ADS score over time (F = 12.13, p for time effect <0.001) (Table 4).
TABLE 4.
Exploratory Outcomes: Completeness of Secondary Outcome Data Collection Across 3 Months
| Outcomes | Baseline | 1 Month | 2 Months | 3 Months | Total |
|---|---|---|---|---|---|
| VR-12 | 91% | 70% | N/A | 87% | 83% |
| PPSP | N/A | 74% | N/A | 87% | 81% |
| Falls | 100% | 74% | 74% | 87% | 84% |
| Medication review | 100% | 30% | N/A | 65% | 65% |
| Readmissions | 100% | 100% | N/A | 100% | 100% |
| Length of stay | 100% | N/A | N/A | N/A | 100% |
| 3D-CAM | Baseline | POD 1 | POD 2 | POD 3 | |
| AM | 95%a | 71% | 75% | 80% | 80% |
| PM | 85% | 80% | 89% |
POD: postoperative day.
3D-CAM is Assessed Only Once at Baseline.
Exploratory Outcomes: Implementation-Potential
Acceptability, appropriateness, and feasibility of the intervention
All surveyed patients somewhat agreed or completely agreed that the wellness program was implementable, and over 90% somewhat agreed or completely agreed that the wellness program was acceptable and appropriate. In comparison, more patients neither agreed nor disagreed that MO was acceptable, appropriate, and feasible. Only 70% of patients felt that MO was fitting and applicable (see Appendix S5 for details).
Shared decision-making and patient experiences with PWPs
Patient perceptions of shared decision-making were largely positive, with almost all patients agreeing that their PWPs put in significant effort to help them understand the intervention components and choose how to use the PMH intervention according to patient priorities and needs (see Appendix S6). Additionally, when asked about patient experiences with their PWPs, patients were largely positive, describing them as caring, encouraging, and informative. On average, from a scale of 0 to 10, with 0 being the worst PWP possible and 10 being the best PWP possible, patients rated their PWPs at 9.3 (see Appendix S7).
Patient and Caregiver Perceptions and Attitudes Towards Intervention and Study
Interviews with participants uncovered several themes related to PMH intervention experiences, PWP experiences, sustainability considerations and study experiences (see Appendix S8 for additional details).
PMH intervention experiences
Most patients both enjoyed and learned from the wellness program, listing several benefits: personalized rationale, setting goals and values, scheduling activities, and tracking activities. In tailoring sessions based on individual priorities, patients could address what mattered most to them, engaging in activities such as gardening, walking, and practicing sleep hygiene. In doing this and talking to PWPs about what concerned them or exacerbated their depression and/or anxiety, patients reported substantial symptom reduction. One patient noted that wellness program sessions provided a “reduction in anxiety and that scared feeling because you are going into something that [you] have never done before… Having someone… that understood what I was talking about and had empathy and then could help get me to doing things to deal with that [was great]. (Orthopedic-90).” Across interviews, patients noted that their PWPs and intervention team were kind, caring, and helpful in providing resources both related and unrelated to mental health.
However, two patients reported that their anxiety caused some stress, hindering phone-based sessions. Four patients described the tediousness associated with the documentation forms; whereas six others noted that the forms facilitated accountability. Many found other ways to keep track of their wellness program progress (e.g., entering activities in their calendars or memorizing their activities). Other challenges included external factors such as work, other responsibilities, pain, and weakness due to recent surgery.
Although only a few patients spoke about their MO experiences, considerable benefits, including medication awareness and tailored medication review, were noted. Seven patients noted no changes to their medications throughout the study. Patients who engaged in MO sessions reported satisfaction with these sessions. However, although MO helped some patients better understand what medications they took and why, a few patients admitted that they had never reviewed their medication list previously with their clinicians. Two patients reported that while medication changes were suggested, no changes were made as per each patien’s wishes, as they were skeptical about medication dose escalations and its impact on their mood.
Suggestions for improvement across intervention components were primarily related to timing, format, and patient buy-in. Every patient who was asked noted that they preferred one-on-one sessions over group sessions. One patient commented, “It would have been harder [as] a group thing, and you [would] have to try to get your schedule to go with other people’s schedule… I thought that [the one-on-one modality] was well set up for older people in that way (Orthopedic-60).” All interviewed patients stated that they enjoyed telephone sessions due to the more casual atmosphere and convenience – although three noted that they would have also enjoyed in-person sessions. All patients responded positively to weekly and biweekly session frequency. While most stated they would want at least one preoperative session, five felt that postoperative sessions were most helpful.
Regarding reach and buy-in for the PMH intervention, patients provided positive feedback and several reasons for their perceptions, noting that their PWPs made each step of MO and the wellness program easy to understand and feasible to implement. Over half were explicitly confident that the PMH intervention would be helpful to future patients – even patients who were not experiencing depressive or anxious symptoms. One such patient heavily endorsed the PMH intervention: “A program like that would be nothing but good for [patients]… If you have anxiety issues, or anything like that, I think it’d be excellent (Orthopedic-21).” Suggestions for promoting the intervention were discussed, including emphasizing intervention flexibility to meet patient needs, presenting evidence of intervention success, and engaging with caregivers.
Experiences with PWP
Patient experiences with their PWPs were almost universally positive, with most themes related to compassion, easy conversational sessions, resource support, and skill building. Eight patients applauded their PWPs for their compassionate listening and consideration. Five also stated that talking to their PWPs sometimes felt easier than talking to close friends or family. In addition, three noted that their PWPs referred them to other resources and supported them with other concerns. For example, one patient reported that when they were feeling overwhelmed with the financial burden of hospitalization and surgery, their PWP “went to administration and social workers, and they managed to clear [the patient’s] … medical debt, so [the patient] wouldn’ have to pay anything (Oncologic-6).”
Most importantly, many felt that PWPs gave patients the necessary tools and taught them how to help themselves. One patient was adamant that “[Our PWPs are] giving us the tools [to overcome our own anxious or depressive symptoms], (so) we have to use them… We want to take the time to do [our tasks], because it’s all good information that [our PWPs are] giving out, and good suggestions… It’s up to us… to take the time and make the time (Orthopedic-21).”
Long-term intervention adoption
Most patients felt that the effects of intervention use were sustainable, continuing with their wellness program activities after the study and checking in regularly with outpatient clinicians regarding medication review. Positive buy-in from patients extended to recommending aspects of the wellness program (e.g., behavioral activation) to friends and family members undergoing surgery. For example, a patient recalled using what they learned from the PMH intervention to support their friend undergoing major surgery (Cardiac-1). When asked, most patients cited that integrating activities from their wellness program sessions into a daily routine was crucial to sustainment.
However, when discussing challenges to sustainability, two patients elucidated that the primary barrier to sustained PMH intervention use was the prioritization of other activities of daily living. One patient explained that it was challenging to maintain wellness activities while he worked and attended physical therapy (Cardiac-1). To improve sustainability, patients suggested increasing motivation. A couple of patients mentioned apps to help them monitor their mental health. Some others suggested that their PWPs add “a periodic check-in… not every week, but like every few months… to reinforce [behavioral activation and to remind us of how to manage our mental health] (Orthopedic-21).”
Study participation experiences
Most patients felt that participation in the study resulted in a positive and valuable experience; eleven explicitly recommended the intervention and study for future patients. One patient stated, “I was really glad that… I got to talk to [my PWP] and [the study team] set this deal up. It made me feel so much better, so much relief I’d guess you’d call it (Cardiac-1).” Several patients also remarked that the intervention did relieve them of depressive and anxious symptoms. Four stated that, while the intervention was helpful, they felt they did not need it.
When asked about challenges to study involvement, a few patients targeted study language. One patient remarked that questionnaires did not provide accurate choices: “Well it would be some things like… ‘how often did you feel depressed?’ And then it would be on a scale, let’s say from 1 to 5, and then, the wording was like ‘1, not at all, 2, some days, 3, several days.’ It was by number of days and I felt that was inappropriate, and it kept coming up… Because as a mood thing, you’re not gonna have the same mood over the course of a whole day (Orthopedic-60).” Other patients also noted that due to mental health stigma, they did not want to talk about depression and/or anxiety during the study or with their outpatient clinicians, preferring to focus more on stress, discomfort, and worry.
When asked for suggestions to improve the study, some suggested reaching out to future patients to introduce the intervention in more detail before surgery. For example, a patient insisted that study team members explain the PMH intervention to patients “before the surgery. [Patients] need to know what benefits (of the intervention) are, what to do, and who to talk to for help (Cardiac-2).” Additionally, while a few noted that the monetary incentive motivated them to join the study, many were inspired to help others and ultimately learned how to improve their mental health and physical recovery following surgery.
With feedback from patients and caregivers regarding the study and PMH intervention implementation, we compiled a list of considerations and suggestions for successful study implementation (Table 5). To address study challenges, we mapped specific recommendations to strategies from the Expert Recommendations for Implementing Change (ERIC), a list of suggested strategies to support intervention implementation.31 ERIC consists of 73 discrete implementation strategies can target address contextual determinants related to effective and sustainable PMH intervention bundle implementation and uptake32 (Table 5).
TABLE 5.
Implementation Strategies for Future Trials
| Barriers | Potential Strategies | ||
|---|---|---|---|
| Barriers to Reach of Study | Limited pool of participants eligible for the study | Strategies to Improve Reach of Study | • Expand the inclusion criteria to other surgical populations • Extend to additional hospital sites |
| Limited pool of participants to approach due to participation in competing trials | • Conduct local consensus discussions with other clinical research trial teams • Identify and prepare clinician champions. • Use advisory boards and workgroups to support institutional readiness and participant engagement • Increase awareness of trial across the institution |
||
| Limited representation of diverse and historically marginalized participant groups | • Tailor recruitment script to welcome historically marginalized groups • Train research team on cultural norms and sensitivity • Modify study incentive/allowance structures based on the number of surveys/interviews |
||
| Single recruitment method using telephone contact | • Distribute educational materials about the study and the intervention process and content details • Incorporate additional recruitment methods (e.g., text, in-person clinic visits, mass media, surgeon referrals, patient portal) |
||
| Increasing number of participant declines at the screening and consent stage | • Tailor recruitment language (easy to understand and concise) • Emphasize the added value and benefits to participants with intervention and its impact on surgical outcomes • Highlight the patient-centered aspect of intervention and its flexibility • Highlight the no-cost structure of the intervention • Provide study incentives to all participants (e.g., in an RCT, incentives to both groups) • Provide all patients with wellness resources (for e.g., in an RCT, resources should be provided to usual care) • Invite patient champions to help with recruitment efforts |
||
| Barriers to Reach of Intervention | Missed intervention sessions Intervention drop-outs | Strategies to Improve Reach of Intervention | • Involve patients/caregivers in finding ways to enhance reach, uptake, and adherence • Interact with patients with attention to compassion • Promote intervention flexibility based on patient needs and comfort (e.g., simple-to-use activity schedules and tracking forms) • Reduce time and effort commitment for intervention sessions to accommodate patients’ schedules (e.g., increasing or reducing session frequency) |
DISCUSSION
We report on a feasibility evaluation of a PMH intervention among 23 older patients undergoing the cardiac, orthopedic, and oncologic surgical settings. We were able to demonstrate and achieve a study reach of 82% of eligible participants, and an intervention reach of 83%. Our PMH intervention tested in this study will be the first-ever attempted solution to support cognitive and mental health and wellbeing of older surgical patients -through a personalized approach supported by a combination of wellness program components and MO, driven by each individual patien’s needs and preferences. All patients felt strongly that the intervention flexibility in our multi-component intervention catered to their needs and preferences. Overall, patients reported overwhelmingly positive experiences with both intervention components in terms of intervention feasibility, acceptability and appropriateness. However, a few patients and caregivers raised some concerns/limitations including the time required for these intervention sessions with their partners, and outside-session patient work and independent efforts to keep up with the wellness program’s core elements (for instance, activity tracking in BA).
In particular, all patients found their interactions with their perioperative wellness partners to be invaluable in managing distress and anxiety during their perioperative journey. This is also observed in the intial efficacy results from this study suggesting significant improvements in patients’ depression and/or anxiety scores over time. Lastly, the study demonstrated that baseline and 3-month timepoints had recorded higher data completion rates, compared to completion rates at 1-month and 2-month time-points.
Informed by results from this feasibility study, we adapted both the PMH intervention and the study processes. These adaptations were systematically tracked during the study through patient case review meetings, intervention session audio-recordings and documentation forms, and patient and caregiver semistructured interviews. Additional feedback from interventionists, researchers, and our internal patient and caregiver advisory board members was obtained through workshop studios, intervention refinement, and periodic reflection meetings. In addition to behavioral activation and care coordination, our updated wellness program incoroporates an exclusive emphasis on following principles of compassion. Our data found the importance of the PWP establishing trust with their patient and assessing patient needs and preferences while approaching their situation with empathy and compassion. Other examples of adaptations to the PMH intervention included incorporating a distinct MO team with pharmacy students and a supervising pharmacist and psychiatrist—to help with both managing the volume of MO sessions and also to have in-training or trained professionals with appropriate pharmacy credentials to lead this component; explicitly incorporating the core principles underlying compassionate care and empathetic listening as part of our intervention; key adaptations to the study included minimizing the number of outcome surveys required at 1-month and 2-months, given the poor completion rates; replacing in-hospital delirium assessments with a chart review method (i.e., Chart-Del33) given the limited feasibility in collecting in-person delirium data as per the in-person delirium protocol.
With preliminary evidence of improvements in patient depression and/or anxiety, we have developed a promising intervention to treat and manage mood symptoms in older surgical adults. We are currently conducting randomized clinical trials to evaluate the effectiveness of our updated PMH intervention in the oncologic, cardiac and orthopedic surgery cohorts (NCT05575128, NCT05685511, NCT05697835). The adapted PMH intervention will be administered as a remote intervention, beginning prior to surgery and ending 2–3 months postsurgery (depending on the number of sessions required by the patient), with optional in-person visits if needed. The adapted interventionist team, consisting of PWP and MO members, will focus on instilling compassion, empathy, and validation of emotions along with a tailored wellness program and medication review and optimization during intervention sessions to meet individual patients’ wishes, needs, and treatment goals. Approximately 40-minute sessions will occur weekly and will be reduced to a biweekly basis.
We acknowledge our study limitations. First, the study was conducted at a single site, with a small participant sample size. Hence, outcomes and feedback may not fully represent the target population’s experiences or needs. Nevertheless, given that this is only a feasibility study, we plan to conduct larger multisite evaluations to gather more evidence on the large-scale effectiveness of our PMH intervention. Additionally, the mixed-methods approach allowed us to integrate and triangulate findings on the efficacy and feasibility of PMH intervention, based on patients’ and caregivers experiences with the intervention and the study execution—which informed our adaptations to the intervention and the study procedures. Second, this study was carried out at an academic center and may require further adaptations to both the intervention, interventionists, and the study procedures to address potential challenges and limitations for implementation in other settings, including small community hospitals or more rural settings. These areas serve different populations and have varying degrees of access to resources necessary to execute the study procedures and intervention as designed. However, given that this is the first study attempting to develop and test the feasibility of a patient-centered PMH intervention for older surgical patients, our findings on adaptations and implementation strategies can be a useful guide for other hospitals who wish to embark on similar design and adaptation of the intervention to meet their surgical population and setting needs and constraints. Third, data collection was found to be comparatively poor at month 1 postsurgery, which can be attributed to a number of reasons: 1) all outcomes were collected by a single method of data collection (i.e., follow-up telephone calls) by the research coordinator; 2) given that the first 30 days after surgery were critical for recovery, many patients were hard to reach for a telephone follow-up. These are important lessons we learned from this feasibility study and hence, we used this to adapt our study procedures for the current RCT to include additional methods of outcome assessment (REDCap surveys, telephone). Fourth, the study sample had limited diverse patient representation (for example, historically underserved and racially minoritized patients), therefore, the adapted intervention may not accurately incorporate the needs and priorities of these populations. To address this, we are conducting focus groups to examine the perioperative mental health needs of historically underserved and racially minoritized patients with depression and/or anxiety and also gather insights on how to better engage this population in mental health research. Furthermore to address general challenges for recruiting older patients with mental health concerns,34 we examined the stigma attached to mental health in older patients and modified our screening and consenting language, rewording intervention elements to emphasize holistic surgical recovery, wellness, and stress relief, rather than using clinical terminology.
Our study provides preliminary evidence for our PMH intervention implementation and evaluation, with minor adjustments. With preliminary evidence of significant improvements to patient anxiety and depression, we have identified a promising adaptation of perioperative mental health treatments for older adults, tackling previously identified issues such as patients’ limited understanding of medications or insufficient access to mental health resources during perioperative care, and limited holistic treatment to address older patients’ multimorbidity.
Supplementary Material
Highlights.
What is the primary question addressed by this study?
This study examines the feasibility of testing and implementing a perioperative mental health intervention bundle composed of psychological and pharmacological components, for older surgical patients.
What is the main finding of this study?
Twenty-three older adults (mean age 68.0 years, 65% women) participated in this study, achieving a study reach of 82% and intervention reach of 83%. Preliminary efficacy analysis indicated improvement in PHQ-ADS scores (F = 12.13, p <0.001).
What is the meaning of the finding?
Patients described overwhelmingly positive experiences with both the intervention activities and the interventionist, and reported improvement in managing depression and/or anxiety.
ACKNOWLEDGMENTS
We would like to offer special thanks to Dr. Kenneth Freedland for his significant contributions in developing and adapting the intervention and guidance on the fidelity assessment of the intervention; and to Ms. Flora Laurent (FL), a medical student who helped us with conducting interviews, their transcription, and initial coding; We would also like to acknowledge all team members from the Center for Perioperative Mental Health, including Dr. Rebecca Aslakson, Laura Carpenter, Jennifer Carron, Marci Damiano, Dr. Renée El-Gabalawy, Margie Grant, Dr. Charles Hannon, Dr. Sharon Inouye, Avi Klein, Dr. Kunal Kotkar, Alex Kronzer, Matthew Lauer, Dr. Muhammad Faraz Masood, Dr. Jay Piccirillo, Dr. Charles Reynolds III, Marissa Rhea, Dr. Mayola Rowser, Dr. Marilyn Schallom, Dr. Elizabeth Stuart, Kyle Stumbaugh, Terri Swider, Wilberforce Tumwesige, and Lei Yang. We would like to thank the members of our Center’s Internal Advisory Board for their continuous review of our intervention and their constructive feedback.
DISCLOSURES
This research was supported in part by a grant from the National Institute of Mental Health (P50MH122351). No disclosures to report.
Abbreviations:
- PMH
perioperative mental health
- MO
medication optimization
- PWP
perioperative wellness partner
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.jagp.2023.09.003.
DATA STATEMENT
The data has not been previously presented orally or by poster at scientific meetings.
References
- 1.Weiss AJ, Jiang HJ: Differences in Hospital Stays With Operating Room Procedures by Patient Race and Ethnicity, 2019. HCUP Statistical Brief #297. September 2022. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/reports/statbriefs/sb297-OR-procedures-racial-disparities-2019.pdf. Accessed May 6, 2023. [PubMed] [Google Scholar]
- 2.Yang R, Wolfson M, Lewis MC: Unique aspects of the elderly surgical population: an anesthesiologist’s perspective. Geriatr Orthop Surg Rehabil 2011; 2(2):56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorel JC, Veltman ES, Honig A, Poolman RW: The influence of preoperative psychological distress on pain and function after total knee arthroplasty: a systematic review and meta-analysis. Bone Joint J 2019; 101(1):7–14 [DOI] [PubMed] [Google Scholar]
- 4.Strøm J, Bjerrum MB, Nielsen CV, et al. : Anxiety and depression in spine surgery: a systematic integrative review. Spine J 2018; 18(7):1272–1285 [DOI] [PubMed] [Google Scholar]
- 5.Dao TK, Youssef NA, Armsworth M, et al. : Randomized controlled trial of brief cognitive behavioral intervention for depression and anxiety symptoms preoperatively in patients undergoing coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2011; 142(3):e109–e115 [DOI] [PubMed] [Google Scholar]
- 6.Reynolds CF 3rd, Dew MA, Pollock BG, et al. : Maintenance treatment of major depression in old age. N Engl J Med 2006; 354 (11):1130–1138 [DOI] [PubMed] [Google Scholar]
- 7.Cristancho P, Lenard E, Lenze EJ, et al. : Optimizing outcomes of treatment-resistant depression in older adults (OPTIMUM): study design and treatment characteristics of the first 396 participants randomized. Am J Geriatr Psychiatry 2019; 27(10):1138–1152 [DOI] [PubMed] [Google Scholar]
- 8.Gilron I: Antidepressant drugs for postsurgical pain: current status and future directions. Drugs 2016; 76(2):159–167 [DOI] [PubMed] [Google Scholar]
- 9.Makary MA, Segev DL, Pronovost PJ, et al. : Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210(6):901–908 [DOI] [PubMed] [Google Scholar]
- 10.Salive ME: Multimorbidity in older adults. Epidemiol Rev 2013; 35(1):75–83 [DOI] [PubMed] [Google Scholar]
- 11.Abraham J, Meng A, Siraco S, et al. : A qualitative study of perioperative depression and anxiety in older adults. Am J Geriatr Psychiatry 2020; 28(10):1107–1118 [DOI] [PubMed] [Google Scholar]
- 12.Dao TK, Youssef NA, Armsworth M, et al. : Randomized controlled trial of brief cognitive behavioral intervention for depression and anxiety symptoms preoperatively in patients undergoing coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2011; 142(3):e109–e115 [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Ma L, Chen X, et al. : Psychological nursing intervention improve the mental health status of young patients with lung cancer surgery during the perioperative period. Medicine (Baltimore) 2021; 100(31):e26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanter JW, Bowe WM, Baruch DE, et al. : Behavioral activation for depression. In: Springer DW, Beevers C, Rubin A, eds. Treatment of depression in youth and adults, Wiley, 2011:113–182 [Google Scholar]
- 15.Damschroder LJ, Aron DC, Keith RE, et al. : Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow RE, Vogt TM, Boles SM: Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999; 89(9):1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham J, Holzer KJ, Lenard EM, et al. : Perioperative mental health intervention bundle for older surgical patients: protocol for an intervention development and feasibility study. BMJ Open 2022; 12(8):e062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke K, Wu J, Yu Z, et al. : The patient health questionnaire anxiety and depression scale (PHQ-ADS): initial validation in three clinical trials. Psychosom Med 2016; 78(6):716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball LJ, Bisher GB, Birge SJ: A simple test of central processing speed: an extension of the short blessed test. J Am Geriatr Soc 1999; 47(11):1359–1363 [DOI] [PubMed] [Google Scholar]
- 20.Puspitasari AJ, Kanter JW, Busch AM, et al. : A randomized controlled trial of an online, modular, active learning training program for behavioral activation for depression. J Consult Clin Psychol 2017; 85(8):814. [DOI] [PubMed] [Google Scholar]
- 21.Forman DE, Racette SB, Toto PE, et al. : Modified application of cardiac rehabilitation in older adults (MACRO) trial: protocol changes in a pragmatic multi-site randomized controlled trial in response to the COVID-19 pandemic. Contemp Clin Trials 2022; 112:106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YW, HajGhanbari B, Road JD, et al. : Reliability and validity of the brief pain inventory in individuals with chronic obstructive pulmonary disease. Eur J Pain 2018; 22(10):1718–1726 [DOI] [PubMed] [Google Scholar]
- 23.Marcantonio ER, Ngo LH, O’Connor M, et al. : 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014; 161(8):554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selim AJ, Rogers W, Fleishman JA, et al. : Updated US population standard for the veterans RAND 12-item health survey (VR-12). Qual Life Res 2009; 18:43–52 [DOI] [PubMed] [Google Scholar]
- 25.Weiner BJ, Lewis CC, Stanick C, et al. : Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017; 12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forcino RC, Barr PJ, O’Malley AJ, et al. : Using collabo RATE, a brief patient-reported measure of shared decision making: results from three clinical settings in the United States. Health Expect 2018; 21(1):82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmocker RK, Stafford LMC, Siy AB, et al. : Understanding the determinants of patient satisfaction with surgical care using the consumer assessment of healthcare providers and systems surgical care survey (S-CAHPS). Surgery 2015; 158 (6):1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanter PS Mulick, AM Busch JW, et al. : The behavioral activation for depression scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess 2007; 29:191–202 [Google Scholar]
- 29.Damschroder LJ, Lowery JC: Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci 2013; 8:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cypress BSJDoccn: Rigor or reliability and validity in qualitative research: perspectives, strategies, reconceptualization, and recommendations. Dimens Crit Care Nurs 2017; 36(4):253–263 [DOI] [PubMed] [Google Scholar]
- 31.Powell BJ, Waltz TJ, Chinman MJ, et al. : A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci 2015; 10(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waltz TJ, Powell BJ, Fernández ME, et al. : Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci 2019; 14(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krewulak KD, Hiploylee C, Ely EW, et al. : Adaptation and validation of a chart-based delirium detection tool for the ICU (CHART-DEL-ICU). J Am Geriatr Soc 2021; 69(4):1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown G, Marshall M, Bower P, et al. : Barriers to recruiting ethnic minorities to mental health research: a systematic review. Int J Methods Psychiatr Res 2014; 23(1):36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data has not been previously presented orally or by poster at scientific meetings.


