Abstract
Background:
Many studies have highlighted the positive effects of dance in people with neurodegenerative diseases.
Objectives:
To explore the effects of International Ballroom Dancing on cognitive function in elders with amnestic mild cognitive impairment (aMCI).
Methods:
One-hundred twenty-nine elderly patients with aMCI diagnosis (mean age 66.8 ± 10.1 years) were randomly assigned into 2 groups: intervention group (IG, n = 66) and control group (CG, n = 63). The IG exercised systematically for 10 months, and both groups were submitted to extensive neuropsychological assessment prior and after the 10-month period.
Results:
According to the independent sample t test at the follow-up, significant differences between groups were found in benefit of the IG while the CG showed worse performance in the majority of neuropsychological tests. According to the Student t test, better performance is detected in IG in contrast with CG, which had worse performance almost in all scales.
Conclusion:
Dance may be an important nonpharmacological approach that can benefit cognitive functions.
Keywords: International Ballroom Dancing, elderly, mild cognitive impairment, Greek population, randomized controlled trial
Introduction
Background
It is widely known that Alzheimer’s disease (AD) is a major public health issue that will worsen over the next years, underlying the need for clinical research advances in prevention and treatment. 1 Such research is particularly important among elderly with mild cognitive impairment (MCI) who manifest the earliest symptoms of AD. 2 On the other hand, nonpharmaceutical interventions (eg, memory training, exercise, and dance) are considered as a promising solution for amelioration of cognitive deterioration. 3 The strong dancing tradition prevalent among the Greeks was likely inherited around 1500 BC while dancing has traditionally been one of the most ancient forms of community entertainment and expression. 4 This study comes to answer a major question: “What would be the outcome if this old Greek tradition from ancient years deployed as an intervention to enhance cognitively impaired people of a Greek community?”
The State of Mild Cognitive Impairment
The MCI is a syndrome defined as cognitive decline greater than expected for an individual’s age and education level but that does not interfere notably with activities of daily life. Prevalence in population-based epidemiological studies ranges from 3% to 19% in adults older than 65 years 5 -7 and is a condition that refers to an objective cognitive decline in nondemented patients as revealed by a standard neuropsychological evaluation. 8 Some people with MCI seem to remain stable or return to normal over time, but more than half progress to dementia within 5 years. The amnestic subtype of MCI has a high risk of progression to AD, and it could constitute a prodromal stage of this disorder. 5 Other definitions and subtypes of MCI need to be studied as potential prodromal stage of AD or other types of dementia. It is, thus, distinct from dementia, in which cognitive deficits are more severe and widespread and have a substantial effect on daily function. In general, MCI is an emerging term that encompasses the clinical state between elderly normal cognition and dementia. Controversy surrounds its characterization, implementation, and definition. MCI is now the focus of natural history studies, biomarker studies, along with AD prevention studies. The MCI stage, as predementia stage, may be the best stage for pharmacological and nonpharmacological interventions in order to forestall dementia progression.
The Added Value of Dance to Cognitive Impairment and Aging
In general, physical therapy interventions for elders who sustain frequent falls, often involve flexibility, strengthening, balance activities, and endurance training, are considered as a possible solution to address those difficulties. 9,10 Aerobic exercise has been proved as a possible solution for improvement of cognitive function in elderly people and as a contributing factor for prevention of neurodegeneration. 11 -13 Dance belongs to the category of aerobic exercise and is considered an ideal exercise to relieve tension and pressure because it is an enjoyable social activity that not only improves fitness levels but also promotes healthy activity in older adults and people with dementia. 14 -17 Moreover, dance is considered as a combination of endurance, strength, coordination and cognition, and social interaction. Also, dance as a form of physical activity can be performed in a range of environments while it is safe and more likely to be adopted by older adults as part of their lifestyle compared to other more structured and/or intensive exercises. It has been proved that it stimulates many cognitive functions within dementia spectrum, including both visual and auditory stimuli and the capacity to follow instructions. 18 Compared with other aerobic exercises, dance sport has the additional benefits of stimulating the emotions, promoting social interaction, and exposing patients to acoustic stimulation and music. 19 -21 Thus, dance might be a more effective modality to improve cognitive function than other aerobic exercises. It has been proved that deterioration of spatial memory is an early warning of cognitive impairment. 22 Neurophysiologically, there are specific cells, “hippocampal place cells, which are responsible for spatial recognition in response to spatial stimulations such as environmental landmarks and translational or directional movement inputs.” 23 In dancing with uncharacterized motion and pacing, a few hypotheses have come up: 21 (a) During slow motion, place cells would be activated in response to a unique, specific position in the environment 23,24 and (b) grid cells, within entorhinal networks, may be firing in response to a dance-induced rapid motion in multiple locations of the environment geometrically defined, 25 and these responses of grid cells are modulated by direction and speed of motion. 24 Therefore, dancing may provide a benefit by input stimulation following a simple exploration in motion of the surrounding environment. Depending on the importance of the virtual navigation paradigm in the stimulation process, a virtual training might enhance hippocampal and entorhinal volume and improve spatial cognition performance. 21 The long-term effect of auditory, multilingual, verbal, or musical workouts on the brain has been investigated underlying structural and functional adjustments. 26
Furthermore, dance interventions have been identified as measures that can significantly reduce anxiety and depression levels and improve quality of life in older adults. 27 There are many studies, which have highlighted the impact of dance on brain structure and function, cognition and balance abilities in healthy older adults. 28 -30 Additionally, the repetitive dance steps help older adults learn and retrieve a memorization technique, which may help by “telling neurons what to do.” 31 The therapeutic benefits therefore motivate individuals at all levels of fitness to adhere to the interventions. 32 Dance has been used successfully in motivating health promotion activity among elderly. 33 Physical therapists have expressed moderate to high agreement that dance could positively impact clients’ physiological and psychological states, thus, facilitating long-term participation and corresponding health benefits. Several studies have explored the effects of dance on mental health, balance, fall risk, and function. 34 -43 Individuals with serious mental illness showed significant improvements in Timed Up and Go scores and nonsignificant improvements in anxiety, depression, and balance confidence after regular 1-h dance lessons once a week for 10 weeks. In addition, music therapy may reduce therapeutic doses of some psychotropic medications, improve mood and self-expression, stimulate speech, organization, and mental processes. There are many studies that have highlighted the important contribution of dance as an intervention tool in people with Parkinson’s disease, 36,42,44 -48 stroke rehabilitation through music and dance, 49 and aging in general. 21,50,51 Moreover, a recent study identified dance as a motivator for the older adults to adhere to a physical activity program. 52 Moreover, a recent case report has described the clinically meaningful effects of Salsa dance therapy and its impact on functional recovery in a geriatric patient with multiple impairments. 32 Consequently, a dance program may reasonably be expected to produce measurable physical and cognitive improvements and subsequently forestall the risk of progress to dementia from MCI state by virtue of strengthening cognitive performance and promote a pleasurable experience.
However, to the best of our knowledge, there is no other research which has explored the effects of International Ballroom Dancing on clinical and cognitive outcomes in the Greek adult population with MCI.
Conceptual Framework
The purpose of this single-blind randomized controlled trial was to evaluate the impact of age-appropriate dance class instruction on cognition and mood (specifically depression and anxiety) in patients with MCI, community-dwelling, Greek-speaking elderly ranging in age from 60 to 80. In 1 group, participants with amnestic mild cognitive impairment (aMCI) attended International Ballroom Dancing, performed in a group class with 1 instructor, performed with a partner. In the other, participants with aMCI attended no other program and were at waiting list. We chose International Ballroom Dancing, because it refers to many different rhythms, musical themes, body movements; requires partnership; and Greek elderly population is unfamiliar with it. Balance, postural control, dance and rhythm recognition, movement initiation and termination, turning, and moving in close proximity to another individual. We expected these movement and cognitive requirements to specifically target and ameliorate the memory deficits of our participants. We hypothesized that patients who participated in these dance classes will have improvement in cognitive function as detected in neuropsychological assessment after 1 year in contrast with the control group (CG).
Methods
Participants
This experimental study was carried out between September 2015 and June 2016 in Greek Alzheimer Association and Related disorders. Patients between 55 and 75 years who could walk independently and had MCI as categorized by both neuropsychological assessment and input from multidisciplinary team with expertise in dementia care. The recruited participants were selected based on Petersen criteria 53 for MCI and deemed to be fit to take part in the study by a neurologist specialized in the field of dementia and a neuropsychologist. We focused on stage 3 of the disease according to Global Deterioration Scale (GDS).
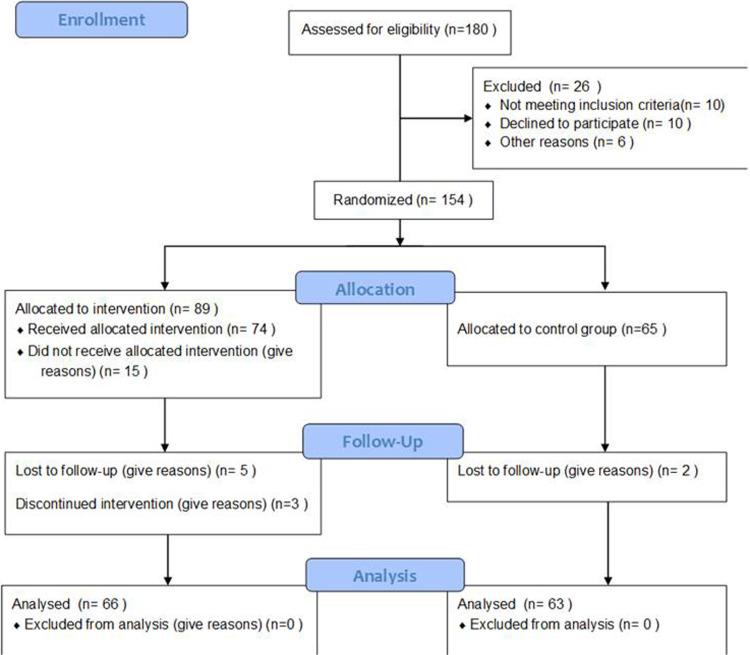
Of the 180 participants, 26 were excluded (10 withdrew participation; 10 because of uncontrolled hypertension, arrhythmia; and 3 could not be contacted), and 3 people dropped out within 4 weeks of the study initiation due to family problems. Thus, 154 randomized, while the primary analysis was conducted on 129 older adults (66 in the intervention group [IG] and 63 in the CG). Participants in the dance IG (n = 66, 53 women; age, 65.89 ± 10.7 years) and in the nondance CG (n = 63, 48 women; age, 67.92 ± 9.4 years) were randomly assigned (Figure 1). The age distribution was balanced, but there were slight differences in the education level (number of school years: IG, 10.55 ± 0.38; CG, 8.89 ± 0.56; Table 1). All the participants had equal performance in neuropsychological exam, and no statistically significant difference was found between CG and IG (Table 2). This study was approved by the Ethics Committee of Alzheimer Association, and all patients provided written informed consent.
Figure 1.
Consort flow diagram of participation.
Table 1.
Demographic Characteristics, M (SD) of the Participants in IG (n = 66) and CG (n = 63).
| Group | Mean | SD | P | |
|---|---|---|---|---|
| Age | CG | 67.92 | 9.47 | .25 |
| IG | 65.89 | 10.76 | ||
| Education | CG | 10.31 | 4.68 | .17 |
| IG | 11.40 | 4.47 |
Table 2.
Mean and Standard Deviation of the First Neuropsychological Assessment Test for CG (n = 63) and IG (n = 66).a
| Domains | Tests | Groups | Mean | SD | P |
|---|---|---|---|---|---|
| Cognition | MMSE | CG | 26.88 | 2.1 | .07 |
| IG | 27.60 | 2.19 | |||
| MOCA | CG | 23.81 | 3.1 | .08 | |
| IG | 24.70 | 2.25 | |||
| Daily functionality | FUCAS | CG | 43.92 | 1.95 | .19 |
| IG | 44.47 | 2.71 | |||
| Attention | TEA 4 | CG | 6.32 | 3.29 | .22 |
| IG | 7.06 | 3.11 | |||
| Learning/verbal fluency | RAVLT total | CG | 36.17 | 11.02 | .09 |
| IG | 40.45 | 12.29 | |||
| FAS | CG | 9.63 | 3.31 | .61 | |
| IG | 9.30 | 3.51 | |||
| Memory/executive function | RBMT1 | CG | 10.70 | 3.26 | .24 |
| IG | 11.46 | 4.04 | |||
| RBMT2 | CG | 9.33 | 3.75 | .17 | |
| IG | 10.32 | 4.33 | |||
| ROCFT-copy | CG | 27.25 | 6.89 | .11 | |
| IG | 30.82 | 5.01 | |||
| ROCFT-delay | CG | 13.67 | 5.42 | .23 | |
| IG | 14.04 | 6.26 | |||
| Mood | GDS | CG | 2.34 | 2.03 | .42 |
| IG | 2.73 | 2.69 | |||
| NPI | CG | 2.97 | 4.04 | .84 | |
| IG | 3.18 | 4.91 |
Abbreviations: MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment Test; FUCAS, Functional and Cognitive Assessment Test; TEA, Test of Everyday Attention; NPI, Neuropsychiatric Inventory; RAVLT, Rey Auditory Verbal Learning Test; ROCFT, Rey Osterrieth Complex Figure Test; GDS, Global Deterioration Scale; CG, control group; IG, intervention group. aIndependent Sample t test.
Exclusion criteria included uncontrolled hypertension or terminal illness, severe cognitive and musculoskeletal impairments, cardiovascular disease, mental illness, stroke, administration of antipsychotic or anticholinergic drugs, hearing or vision deterioration, speech disturbances, difficulties in performing daily routines, and severe pain. Patients were divided into an IG and a CG; the IG undertook a combination of dance exercises while the CG received no intervention. Both groups had undergone neuropsychological examination 2 weeks before the beginning of dance lessons and after the end of the program—10 months later. Sessions conducted twice per week for 40 weeks resulting in a total of 80 sessions per person.
Randomization, Allocation Concealment, and Blinding
This study aims to minimize selection bias by using randomization and allocation concealment. Prior to inclusion of participants, a technician performed randomization using an algorithm. Until the completion of the last long-term follow-up, only the project leader and 2 persons responsible for the interviews and focus groups had access to the information on group allocation. Due to the nature of the therapy programs, blinding of the participants and instructor is not possible. However, all the independent evaluators are blinded with respect to group allocation, and the participants are not informed of primary outcome measure or the study hypothesis. To maintain group allocation confidential, participants are requested prior to each assessment phase to not reveal allocation or therapy content to the evaluators. Participants scheduled for qualitative studies are told that they must not talk to the evaluators about participation in interviews and neuropsychological assessment. Furthermore, the interviews and focus groups were performed in a way that does not reveal participants’ allocation. After the baseline assessment, eligible and consenting people with aMCI were randomized to the dance or CG.
Withdrawals and discontinuation
The study would stop if any of the following occurs:
Participant death or discharge.
Incident dizziness.
Medical problems related to exercises (fracture, heart problems, etc).
Participant experiences adverse event(s) that require discontinuation in the judgment of the principal investigator or instructor.
Participant has a need for additional intervention that would interfere with the trial.
The participant neglects to follow trial instructions.
The participant has lost more than 8 lessons and didn’t make additional lessons to cover this gap.
The participant or their legally authorized representative request consent withdrawal.
Assessment of Cognitive Function and Mood
Cognitive assessment was performed by means of a neuropsychological battery designed to comprehensively evaluate attention, working memory, memory, executive functioning, and language. The neuropsychological battery included scales for evaluation of (a) global cognition: the Greek version of Mini Mental State Examination (MMSE) 54 to assess the general cognitive function and Montreal Cognitive Assessment Test, 55 which has been proposed as a more appropriate tool for detection of MCI; (b) short-term and long-term memory: Rivermead Behavioral Memory Test (RBMT) story direct and delayed recall; 56 (c) the Verbal Fluency F-A-S test (FAS); 57 (d) mood: Neuropsychiatric Inventory (NPI), 58 GDS, 59 Beck Depression Inventory (BDI), 60 Hamilton Scale for Depression, 61 Perceived Stress Scale (PSS), 62 and Beck Anxiety Inventory; 63 (e) daily functionality: Functional Rating Scale for Dementia (FRSSD) and Functional and Cognitive Assessment Test (FUCAS); 64 (f) visuospatial ability and executive function: Trail Making Test part-B (TRAIL-B) 65 and Rey Osterrieth Complex Figure Test copy and delay recall (ROCFT-copy and delayed recall); 66 (g) learning: Rey Auditory Verbal Learning Test (RAVLT) in order to measure the ability of learning and long-term memory; (h) attention: Test of Everyday Attention (TEA) 67 subscales of map, visual elevator, and telephone to assess selective attention and attentional switching.
Dance Training Intervention
International Ballroom Dancing was selected as the exercise intervention. An experienced dance instructor supervised the dance class twice a week for 10 months. Each 60-minute dance class included a 5-minute warm-up revising previous dance sessions, 45 minutes of new material (figures/dances), and a 10-minute cool down period with a dance of participants’ preference. The participants familiarized with several international ballroom dances such as Tango, Waltz, Viennese Waltz, Fox trot, Rumba, Chachacha, Swing, Salsa, Merengue, Disco–Hustle, as well as with Greek traditional ballroom dancing. All dances were first introduced with their special music features and their origin. Each lesson included a combination of 2 or 3 dances, in rotation. The combination was based on movement similarities, so participants could remember step combination easier, and rhythm variation (slow and faster dances) in order to make lesson easier to attend by an elderly. Dance steps/figures presented, gradually became more complex. The intervention aims in Dance floor escalated from just recognize music, dance, and rhythm, to stay in the rhythm in basic simple steps, and finally remember step combinations, leading hints and following alertness.
Data Analysis
IBM SPSS Statistics for Windows (version 22, IBM Corp., Armonk, New York) was used for data analysis. Descriptive statistics were computed to describe the samples of participants using mean/medians for central tendency and standard deviations/ranges for dispersion for continuous type variables and frequency counts and percentages for categorical variables. Descriptive statistics were calculated. Independent samples t tests compared groups’ first and second assessment. The significance level was α = .05.
Results
We performed a broad assessment of cognition, intelligence, attention, reaction times, and behavioral performance in order to explore the potential beneficial effects of a 10-month dance intervention in 2 groups of elderly participants having no regular record of dancing or sporting activities for at least the previous 3 years. Assessments were conducted before and after the 10-month period. Patients in the IG took part in a professional dance class for 1 h/twice a week, while patients in the CG continued their usual lifestyle without interventional measures. None of the participants in the CG reported changes in the physical or mental challenges during this period. After 10 months of dance intervention, we found significant improvements in most of the investigated parameters within the IG group, whereas no improvements were found for the CG group. In contrast, in many tasks, participants in the CG actually showed a decline in performance. In Tables 3 and 4, all the mean values are shown for both groups and for each task after the intervention or after the 10-month period, respectively. Initial neuropsychological performance of both groups revealed no statistical significant difference. The results of the initial and postural performance assessment of IG and CG are illustrated in Table 5 and Figure 2, respectively.
Table 3.
Mean and Standard Deviation of the Second Neuropsychological Assessment Test Between CG (n = 63) and IG (n = 66).a
| Mean | SD | P | ||
|---|---|---|---|---|
| MMSE | CG | 25.65 | 3.27 | .000b |
| IG | 28.00 | 2.39 | ||
| MOCA | CG | 19.82 | 5.18 | .03c |
| IG | 26.00 | 2.94 | ||
| FUCAS | CG | 45.88 | 3.71 | .057c |
| IG | 44.74 | 3.05 | ||
| TEA 4 | CG | 5.90 | 3.86 | .002b |
| IG | 7.89 | 3.02 | ||
| NPI | CG | 3.76 | 4.84 | .020c |
| IG | 1.78 | 2.28 | ||
| RAVLT total | CG | 38.86 | 10.88 | .003b |
| IG | 46.20 | 13.09 | ||
| FAS | CG | 9.14 | 3.06 | .005b |
| IG | 10.87 | 3.61 | ||
| RBMT1 | CG | 9.89 | 5.40 | .004b |
| IG | 12.53 | 4.78 | ||
| RBMT2 | CG | 8.38 | 4.28 | .001b |
| IG | 11.09 | 4.38 | ||
| ROCFT-copy | CG | 27.52 | 6.25 | .000b |
| IG | 31.15 | 4.81 | ||
| ROCFT-delay | CG | 11.10 | 6.50 | .000b |
| IG | 16.18 | 6.02 | ||
| GDS | CG | 2.22 | 2.03 | .022c |
| IG | 1.26 | 1.73 | ||
Abbreviations: MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment Test; FUCAS, Functional and Cognitive Assessment Test; TEA, Test of Everyday Attention; NPI, Neuropsychiatric Inventory; RAVLT, Rey Auditory Verbal Learning Test; ROCFT, Rey Osterrieth Complex Figure Test; GDS, Global Deterioration Scale; CG, control group; IG, intervention group.
aIndependent Sample t test.
b P < .01.
c P < .05.
Table 4.
Mean, Standard Deviation, and Results of the Student t Test.a
| Mean | SD | P | ||
|---|---|---|---|---|
| MMSE | First | 27.54 | 2.14 | .061 |
| Second | 28.00 | 2.39 | ||
| FAS | First | 9.31 | 3.53 | .000b |
| Second | 10.83 | 3.40 | ||
| RBMT-recall | First | 11.46 | 4.04 | .061 |
| Second | 12.53 | 4.78 | ||
| TRAIL-B | First | 192.79 | 107.03 | .052b |
| Second | 165.77 | 85.37 | ||
| ROCFT-delay recall | First | 14.04 | 6.26 | .004c |
| Second | 16.19 | 6.07 | ||
| RAVLT total | First | 40.45 | 12.29 | .001c |
| Second | 45.41 | 13.92 | ||
| NPI-total | First | 3.77 | 5.61 | .024b |
| Second | 1.77 | 2.41 | ||
| TEA 4 | First | 7.06 | 3.11 | .03b |
| Second | 7.72 | 3.17 | ||
| RAVLT1 | First | 4.78 | 1.89 | .002c |
| Second | 5.75 | 2.379 | ||
| GDS | First | 2.70 | 2.81 | .007c |
| Second | 1.32 | 1.77 | ||
| BDI | First | 10.68 | 5.89 | .04b |
| Second | 8.27 | 4.55 | ||
Abbreviations: MMSE, Mini Mental State Examination; ROCFT, Rey Osterrieth Complex Figure Test; RAVLT, Rey Auditory Verbal Learning Test; NPI, Neuropsychiatric Inventory; TEA, Test of Everyday Attention; GDS, Global Deterioration Scale; BDI, Beck Depression Inventory; CG, control group; IG, intervention group.
aIG During the First and Second Assessment (n = 66).
b P < .05.
c P < .01.
Table 5.
Mean, Standard Deviation, and Results of the Student t Test.a
| Mean | SD | P | ||
|---|---|---|---|---|
| MMSE | First | 26.92 | 2.01 | .01b |
| Second | 25.65 | 3.27 | ||
| FUCAS | First | 43.92 | 1.95 | .000b |
| Second | 45.88 | 3.71 | ||
| ROCFT-delay recall | First | 11.10 | 5.39 | .05c |
| Second | 9.77 | 6.50 | ||
| RBMT-delay recall | First | 9.39 | 3.71 | .03c |
| Second | 8.38 | 4.28 | ||
| FRSSD | First | 3.92 | 2.19 | .01b |
| Second | 4.85 | 2.71 | ||
| TEA map 1 | First | 34.69 | 17.56 | .01b |
| Second | 26.42 | 10.80 | ||
| TEA map 2 | First | 48.75 | 11.01 | .03c |
| Second | 43.61 | 13.10 | ||
| RAVLT 1 | First | 4.40 | 1.76 | .05c |
| Second | 5.06 | 1.97 | ||
| RAVLT 3 | First | −3.22 | 2.03 | .006b |
| Second | −2.1667 | 1.47 | ||
Abbreviations: MMSE, Mini Mental State Examination; FUCAS, Functional and Cognitive Assessment Test; ROCFT, Rey Osterrieth Complex Figure Test; FRSSD, Functional Rating Scale for Dementia; TEA, Test of Everyday Attention; RAVLT, Rey Auditory Verbal Learning Test; CG: control group.
aCG During the first and second assessment (n = 63).
b P < .01.
c P < .05.
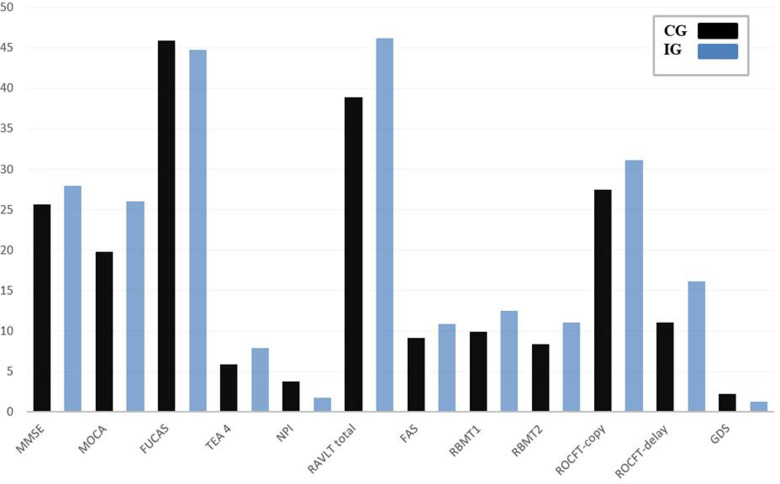
Figure 2.
Longitudinal mean changes in neuropsychological performance (total MMSE, MoCA, FUCAS, TEA4, NPI, RAVLT, FAS, RBMT immediate (1) and delay recall (2), ROCFT copy delay, GDS) in the CG (black) and IG (blue). MMSE indicates Mini Mental State Examination; MoCA, Montreal Cognitive Assessment Test; FUCAS, Functional and Cognitive Assessment Test; TEA, Test of Everyday Attention; NPI, Neuropsychiatric Inventory; RAVLT, Rey Auditory Verbal Learning Test; ROCFT, Rey Osterrieth Complex Figure Test; GDS, Global Deterioration Scale; CG, control group; IG, intervention group.
Discussion
The present study is the first to demonstrate the effect of dance exercise on cognitive function in Greek older adults with MCI. Our study indicated that dancing might be an ideal option for intervention in age-related degradations, especially with people with limited social life and several cognitive limitations. To provide direct evidence for a beneficial role of dancing in ameliorating age-related performance cognitive decline in elderly individuals, we investigated the effect of a 10-month long professional senior dance class with a workload of 1 hour twice a week in a pre–postdesign study on a group of MCI elderly patients (IG). This group, which had no record of regular dancing or sporting activities within at least the last 3 years, was compared to a matched CG lacking any intervention over the same period. The interpretation of the results shows a positive impact of physical activity of a dancing program on the maintenance of cognitive functions, mood, and behavior, without increasing the risk of cognitive deterioration during a year. While the elderly participants with MCI who did not take part in the dance program, there was a significant decline in all variables (Figure 2).
Following our hypothesis about the effect of dancing activities in elderly dwelling MCI participants, we investigated the elderly participants of the IG who had a significant improvement in the performance of the MMSE, FAS, RBMT, TRAIL-B, ROCFT, RAVLT, NPI, ΤΕα4, GDS, and BDI, while in the rest of the neuropsychological tests, they maintained the same time or noticed improvement but not statistically significant. In contrast, the CG worsened significantly in the MMSE, FUCAS, ROCFT delay recall, RBMT delay recall, FRSSD, RAVLT, and TEA map. Overall, after the dance intervention, the patients in the IG group showed improvements in almost all investigated domains such as cognition, reaction time, visuospatial skills, selective attention, attentional switching, and mood and behavior. No improvements were found after a period of 10 months within patients in the CG group; instead, the participants showed degradation of performance in many tasks.
To our knowledge, there are a number of studies examining improvements in cognitive function by dancing interventions in elderly, demonstrating conflicting results. 9,20,33,68 -70 However, most studies assumed weak methodology (ie, pre–postevaluation small samples <50) to test the efficacy of dance as a cognitive intervention. Nevertheless, there is a plenty of studies reported there is a plenty of studies reported not only statistical significant results in improving cognitive functions which are in compliance to our study, but also amelioration in not only significant improvement in cognitive functions, as well as it was stressed also in our study, 33,68,69,71 -73 but also amelioration in dance-related parameters such as posture balance, sensory functions, reaction time, and motor performance. 19,20 More specifically, elders with metabolic syndrome participated in Latin dancing (the Cha–Cha) twice weekly over 6 months, improved some domains of cognitive function such as verbal fluency, word list delayed recall, and recognition of participants but did not improve the MMSE and TRAIL-B scores as in our study. 33 Methodology and group differences may explain the discrepancies with our results. However, different findings by McKee Hackney 73 indicated no improvements in elder’s cognitive functions after a 3-month Tango program, 70 may be because of the small duration of the intervention. A recent randomized controlled trial conducted between healthy older adults (older than 60) participating either in the ballroom dancing group (representing a complex multidimensional physical activity) or in the walking group (representing the simplest, functional, and most accessible physical activity in old age) suggested that participation in dance may improve visuospatial learning and memory (delayed recall) to a greater extent than walking. 74 These findings are also according to our results in visuospatial functions (ROCFT and TRAIL-B) as well as verbal memory (RAVLT). Another randomized control trial comparing mild physical exercise in patients with early dementia on the physical and psychological well-being of elderly Chinese individuals stressed a substantial evidence of the efficacy of a dance movement–based intervention in slowing down dementia progression. 75
In a slightly different concept, other studies tested the impact of virtual reality training on mental abilities stressed interesting findings. Aerobic training such as virtual reality tours and competitions while cycling indicated that can increase mental abilities. 76 In a similar study, walking on treadmill while practicing in verbal memory or virtual video-dance game also demonstrated an amelioration in cognitive function. 72 Combined mental and physical load seemed to have a positive impact on cognitive function but only in specific domains such as shifting attention and working memory, 72 probably because these domains were specifically trained while playing the video games.
Given the fact that dancing is related to rhythm and balance, several studies have explored its effects on Parkinson’s disease. Particularly, Tango dancing improved measures of balance, falls, gait, and confidence, in healthy individuals and the older adults with Parkinson’s disease when compared with traditional exercises. Dance provides sensory cueing for Parkinson’s patient, which appears to be a powerful means of improving gait in these individuals. But all of the above-mentioned studies have reported the beneficial effects of dance in cognition of these patients as well. However, in the literature, there is still no consensus on nonpharmacological approaches, such as physical activity, for the treatment of cognitive impairment. 3
Furthermore, studies including dance interventions have demonstrated mood and behavior changes. More specific dance multimodal training and progressive muscle relaxation therapy have been identified as measures that can significantly reduce anxiety and depression levels and improve quality of life in older adults. 77 -79 Also, social dancing seems to be a nursing intervention that supports patients’ positive feelings, communication, and behavior. 80 These findings are compatible with our results in which anxiety and depressive symptoms were significantly decreased.
The present study aimed to contribute to the development of the methodology and the effectiveness of these approaches and to promote a deeper understanding of this ancient Greek tradition. Such nonpharmaceutical approaches may be identified as adjunct treatments to drug treatment and will contribute to the study of physical therapy and rehabilitation sciences. Given these findings, we conclude that patients with MCI who participated in the program of systematic dance lessons improved maintenance of cognitive functions and performance in daily functionality, mood, and behavior. In contrast, patients with MCI who did not take part in the physical activity program showed a big decline in cognitive function. Bearing in mind that the age-related decline in cognitive performance is a major factor negatively affecting life quality, many attempts have been made at utilizing training strategies to delay and ameliorate cognitive decline. Here, we found a widespread improvement of cognition and attention, as assessed by TEA, MMSE, and MoCA after the dance intervention, while no differences were found in the CG group. Significant improvement was noticed also in GDS, FUCAS, BDI, and PSS regarding participants’ performance in the second assessment.
This study adds support to the benefits of dance as a therapeutic intervention in the elders with aMCI. It would be beneficial to design future studies that evaluate the effects of dance therapy on individuals with specific impairments and compare functional outcomes to conventional therapy. The identification of reliable predictors of exercise adherence will allow health-care providers to effectively intervene and change patterns of physical activity in sedentary aging adults. Dance is promising as an efficient, effective, and exhilarating physical therapy intervention, with inherent sources of motivation for long-term neurological patients in a hospital or community settings. 32 It could be beneficial to develop programs that promote dancing as a form of exercise in aging adults to increase activity level, exercise compliance, and possibly increase function. Our research documents clinically significant improvements across a variety of functional measures in a sedentary patient with multiple comorbidities after receiving salsa dance as a therapeutic intervention. Further studies are needed to investigate the effects of this form of therapy on different conditions. In addition, culturally sensitive dance forms could increase activity levels and exercise compliance in sedentary aging adults.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the project “Augmentation of the Support of Patients suffering from Alzheimer’s Disease and their caregivers (ASPAD/2875),” which is materialized by the Special Account of the Research Committee at Aristotle University of Thessaloniki. The project is funded by the European Union (European Social Fund) and the Ministry of Education, Lifelong Learning and Religious Affairs in the context of the National Strategic Reference Framework (NSRF, 2007-2013).
References
- 1. Prince M, Wimo A, Guerchet M, Gemma-Claire A, Wu YT, Prina M. World Alzheimer report 2015: The global impact of dementia—an analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int. 2015:84. doi:10.1111/j.0963-7214.2004.00293.x. [Google Scholar]
- 2. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–246. doi:10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3. Tsolaki M, Kounti F, Agogiatou C, et al. Effectiveness of nonpharmacological approaches in patients with mild cognitive impairment. Neurodegener Dis. 2011;8(3):138–145. doi:10.1159/000320575. [DOI] [PubMed] [Google Scholar]
- 4. Tsatsou-simeonidi A, Stratou D, Syrtos T, Greeks S, Kalamatianos T. Traditional Dance in Greece yvonne hunt centre for asia minor studies, athens 139. The Anthropology of East Europe Review, 2004;22(1). [Google Scholar]
- 5. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet (London, England). 2006;367(9518):1262–1270. doi:10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 6. Tsolaki M, Gkioka M, Verykouki E, Galoutzi N, Kavalou E, Pattakou-Parasyri V. Prevalence of dementia, depression, and mild cognitive impairment in a rural area of the Island of crete, Greece. Am J Alzheimers Dis Other Demen. 2017;32(5):252–264. doi:10.1177/1533317517698789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsolaki M, Kakoudaki T, Tsolaki A, Verykouki E, Pattakou V. Prevalence of mild cognitive impairment in individuals aged over 65 in a rural area in North Greece. Adv Alzheimers Dis. 2014;3:11–19. doi:10.4236/aad.2014.31002. [Google Scholar]
- 8. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi:10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 9. Alpert PT, Miller SK, Wallmann H, et al. The effect of modified jazz dance on balance, cognition, and mood in older adults. J Am Acad Nurse Pract. 2009;21(2):108–115. doi:10.1111/j.1745-7599.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 10. Iliffe S, Wilcock J, Drennan V, et al. Changing practice in dementia care in the community: developing and testing evidence-based interventions, from timely diagnosis to end of life (EVIDEM). Program Grants Appl Res. 2015;3(3):1–596. doi:10.3310/pgfar03030. [PubMed] [Google Scholar]
- 11. Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. http://www.ncbi.nlm.nih.gov/pubmed/12586857. [DOI] [PubMed] [Google Scholar]
- 12. Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi:10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 13. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi:noc00302 [pii]. [DOI] [PubMed] [Google Scholar]
- 14. Hokkanen L, Rantala L, Remes AM, Härkönen B, Viramo P, Winblad I. Dance and movement therapeutic methods in management of dementia: A randomized, controlled study. J Am Geriatr Soc. 2008;56(4):771–772. doi:10.1111/j.1532-5415.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 15. Jeon MY, Bark ES, Lee EG, Im JS, Jeong BS, Choe ES. The effects of a Korean traditional dance movement program in elderly women. Taehan Kanho Hakhoe Chi. 2005;35(7):1268–1276. [DOI] [PubMed] [Google Scholar]
- 16. Kim CG, June KJ, Song R. Effects of a health-promotion program on cardiovascular risk factors, health behaviors, and life satisfaction in institutionalized elderly women. Int J Nurs Stud. 2003;40(4):375–381. [DOI] [PubMed] [Google Scholar]
- 17. Palo-Bengtsson L, Ekman SL. Emotional response to social dancing and walks in persons with dementia. Am J Alzheimers Dis Other Demen. 2002;17(3):149–153. doi:10.1177/153331750201700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown S, Martinez MJ, Parsons LM. The neural basis of human dance. Cereb Cortex. 2006;16(8):1157–1167. doi:10.1093/cercor/bhj057. [DOI] [PubMed] [Google Scholar]
- 19. Kattenstroth JC, Kolankowska I, Kalisch T, Dinse HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosci. 2010;2. doi:10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kattenstroth JC, Kalisch T, Holt S, Tegenthoff M, Dinse HR. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front Aging Neurosci. 2013;5:5. doi:10.3389/fnagi.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foster PP. How does dancing promote brain reconditioning in the elderly? Front Aging Neurosci. 2013;5:4. doi:10.3389/fnagi.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun X, Beglopoulos V, Mattson MP, Shen J. Hippocampal spatial memory impairments caused by the familial Alzheimer’s disease-linked presenilin 1 M146 V mutation. Neurodegener Dis. 2005;2(1):6–15. doi:10.1159/000086426. [DOI] [PubMed] [Google Scholar]
- 23. O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381(6581):425–428. doi:10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 24. Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463(7281):657–661. doi:10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hafting T, Fyhn M, Molden S, Moser M, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801–806. doi:10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 26. Oechslin MS, Meyer M, Jäncke L. Absolute pitch-functional evidence of speech-relevant auditory acuity. Cereb Cortex. 2010;20(2):447–455. doi:10.1093/cercor/bhp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGough EL, Kirk-Sanchez N, Moore JG, Nelson M. Exercise interventions targeting neuroplasticity and neuroprotection in adults with neurodegenerative diseases. Not to be copied without permission. 2013. APTA Combined Sections Meeting, 21–24 January 2013, San Diego, CA. [Google Scholar]
- 28. Rehfeld K, Müller P, Aye N, et al. Dancing or fitness sport? The effects of two training programs on hippocampal plasticity and balance abilities in healthy seniors. Front Hum Neurosci. 2017;11:305. http://journal.frontiersin.org/article/10.3389/fnhum.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Müller P, Rehfeld K, Schmicker M, et al. Evolution of neuroplasticity in response to physical activity in old age: the case for dancing. Front Aging Neurosci. 2017;9:56. http://journal.frontiersin.org/article/10.3389/fnagi.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burzynska AZ, Jiao Y, Knecht AM, et al. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci. 2017;9:59. http://journal.frontiersin.org/article/10.3389/fnagi.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13(3):406–415. [DOI] [PubMed] [Google Scholar]
- 32. Abreu M, Hartley G. The effects of salsa dance on balance, gait, and fall risk in a sedentary patient with Alzheimer's dementia, multiple comorbidities, and recurrent falls. J Geriatr Phys Ther. 2013;36(2):100–108. doi:10.1519/JPT.0b013e318267aa54. [DOI] [PubMed] [Google Scholar]
- 33. Kim SH, Kim M, Ahn YB, et al. Effect of dance exercise on cognitive function in elderly patients with metabolic syndrome: a pilot study. J Sports Sci Med. 2011;10(4):671–678. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3761497&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 34. Mangeri F, Montesi L, Forlani G, Dalle Grave R, Marchesini G. A standard ballroom and Latin dance program to improve fitness and adherence to physical activity in individuals with type 2 diabetes and in obesity. Diabetol Metab Syndr. 2014;6(1):74. doi:10.1186/1758-5996-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melhuish R, Beuzeboc C, Guzmán A. Developing relationships between care staff and people with dementia through music therapy and dance movement therapy: A preliminary phenomenological study. Dementia. (London). 2017;16(3):282–296. doi:10.1177/1471301215588030. [DOI] [PubMed] [Google Scholar]
- 36. de Dreu MJ, van der Wilk ASD, Poppe E, Kwakkel G, van Wegen EEH. Rehabilitation, exercise therapy and music in patients with Parkinson’s disease: a meta-analysis of the effects of music-based movement therapy on walking ability, balance and quality of life. Parkinsonism Relat Disord. 2012;18(suppl 1):S114–S119. doi:10.1016/S1353-8020(11)70036-0. [DOI] [PubMed] [Google Scholar]
- 37. Hackney ME, Bennett CG. Dance therapy for individuals with Parkinson’s disease: improving quality of life. Journal of Parkinsonism and Restless Legs Syndrome, DovePress. 2014:17–25. [Google Scholar]
- 38. Rowe N, Zeitner-Smith D. Teaching creative dexterity to dancers: critical reflections on conservatory dance education in the UK, Denmark and New Zealand. Res Danc Educ. 2011;12(1):41–52. doi:10.1080/14647893.2011.556716. [Google Scholar]
- 39. Murray DK, Sacheli MA, Eng JJ, Stoessl A. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener. 2014;3(1):5. doi:10.1186/2047-9158-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hackney ME, Earhart GM. Comparison of Argentine Tango and American Ballroom. Clin Rehabil. 2010;41(6):475–481. doi:10.2340/16501977-0362.Effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giguere M. Dancing thoughts: an examination of children’s cognition and creative process in dance. Res Danc Educ. 2011;12:5–28. doi:10.1080/14647893.2011.554975. [Google Scholar]
- 42. Borrione P. Effects of physical activity in Parkinson’s disease: a new tool for rehabilitation. World J Methodol. 2014;4(3):133–143. doi:10.5662/wjm.v4.i3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vukadinovic M, Sad N. Aesthetic experience and emotional identification in the performances of different types of artistic dance. Art, Emotion and Value. 5th Mediterranean Congress of Aesthetics. 2011:393–408. [Google Scholar]
- 44. Bolte B, Franco S, Xu T. NBB190: Brain Injury & Innovation Dr. Shreckengost. 1st May, 2014. [Google Scholar]
- 45. Bongioanni P, Vannini C, Girò ME, Canova A, Cianci V, Sherkat S. Biodanza ® effects on Parkinsonian patients’ functionality. 2007;36. [Google Scholar]
- 46. Cameron IGM, Brien DC, Links K, et al. Changes to saccade behaviors in Parkinson’s disease following dancing and observation of dancing. Front Neurol. 2013;4:22. doi:10.3389/fneur.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Canning CG. Rehabilitation in Parkinson’s disease the challenge to provide early and ongoing, evidence-based, patient-centred care. Arq Neuropsiquiatr. 2013;71(12):917–919. doi:10.1590/0004-282X20130228. [DOI] [PubMed] [Google Scholar]
- 48. Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's Disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33(1):14–26. doi:10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- 49. Bunketorp Käll L, Lundgren-Nilsson Å, Blomstrand C, Pekna M, Pekny M, Nilsson M. The effects of a rhythm and music-based therapy program and therapeutic riding in late recovery phase following stroke: a study protocol for a three-armed randomized controlled trial. BMC Neurol. 2012;12:141. doi:10.1186/1471-2377-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coubard OA, Ferrufino L, Nonaka T, Zelada O, Bril B, Dietrich G. One month of contemporary dance modulates fractal posture in aging. Front Aging Neurosci. 2014;6:17. doi:10.3389/fnagi.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrufino L, Bril B, Dietrich G, Nonaka T, Coubard OA. Practice of contemporary dance promotes stochastic postural control in aging. Front Hum Neurosci. 2011;5:169. doi:10.3389/fnhum.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song R, June KJ, Kim CG, Jeon MY. Comparisons of motivation, health behaviors, and functional status among elders in residential homes in Korea. Public Health Nurs. 2004;21(4):361–371. doi:10.1111/j.0737-1209.2004.21410.x. [DOI] [PubMed] [Google Scholar]
- 53. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi:10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 54. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 55. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 56. Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol. 1989;11(6):855–870. doi:10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 57. Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. doi:10.1016/0028-3932(67)90015-2. [Google Scholar]
- 58. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 59. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS). Clin Gerontol. 1986;5(1-2):165–173. doi:10.1300/J018v05n01_09. [Google Scholar]
- 60. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 61. Hamilton M. A rating scale for depression. J Neurol. 1960;23:56–62. http:/Users/Kayleigh/Desktop/Papers/ReadCube/Hamilton-1960-Journalofneurology.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 63. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 64. Kounti F, Tsolaki M, Kiosseoglou G. Functional cognitive assessment scale (FUCAS): a new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Hum Psychopharmacol. 2006;21(5):305–311. [DOI] [PubMed] [Google Scholar]
- 65. Tombaugh T. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi:10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 66. Osterrieth PA. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.]. Arch Psychol (Geneve). Edition commerciale de: Thése de doctorat: Philosophie. Genéve: Neuchâtel : Delachaux & Niestlé. 1944;30:206–356. [Google Scholar]
- 67. Robertson IH, Ward T, Ridgeway V, Nimmo-smith I. Test reviews the Test of Everyday Attention (TEA). Appl Occup Psychol to Employ Disabil. 2001;4(1):51–55. [Google Scholar]
- 68. Coubard OA, Duretz S, Lefebvre V, Lapalus P, Ferrufino L. Practice of contemporary dance improves cognitive flexibility in aging. Front Aging Neurosci. 2011;3:13. doi:10.3389/fnagi.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kimura K, Hozumi N. Investigating the acute effect of an aerobic dance exercise program on neuro-cognitive function in the elderly. Psychol Sport Exerc. 2012;13:623–629. doi:10.1016/j.psychsport.2012.04.001. [Google Scholar]
- 70. Hackney ME, Byers C, Butler G, Sweeney M, Rossbach L, Bozzorg A. Adapted tango improves mobility, motor-cognitive function, and gait but not cognition in older adults in independent living. J Am Geriatr Soc. 2015;63(10):2105–2113. doi:10.1111/jgs.13650. [DOI] [PubMed] [Google Scholar]
- 71. Alves HVD. Dancing and the aging brain: the effect is of a 4-month ballroom dance intervention on the cognition of healthy older adults. 2013.
- 72. Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin ED. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin Interv Aging. 2015;10:1335–1349. doi:10.2147/CIA.S87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav. 2013;45(6):519–529. doi:10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Merom D, Grunseit A, Eramudugolla R, Jefferis B, Mcneill J, Anstey KJ. Cognitive benefits of social dancing and walking in old age: the dancing mind randomized controlled trial. Front Aging Neurosci. 2016;8:26. doi:10.3389/fnagi.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ho RT, Cheung JK, Chan WC, Cheung IK, Lam LC. A 3-arm randomized controlled trial on the effects of dance movement intervention and exercises on elderly with early dementia. BMC Geriatr. 2015;15:127. doi:10.1186/s12877-015-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Anderson-Hanley C, Arciero PJ, Brickman AM, et al. Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med. 2012;42(2):109–119. doi:10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 77. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Adv Psychiatr Treat. 2004;10:171–177. doi: 10.1192/apt.10.3.171. [Google Scholar]
- 78. Adam D, Ramli A, Shahar S. Effectiveness of a combined dance and relaxation intervention on reducing anxiety and depression and improving quality of life among the cognitively impaired elderly. Sultan Qaboos Univ Med J. 2016;16(1):e47–e53. doi:10.18295/squmj.2016.16.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. da Silva Borges EG, de Souza Vale RG, Cader SA, et al. Postural balance and falls in elderly nursing home residents enrolled in a ballroom dancing program. Arch Gerontol Geriatr. 2014;59(2):312–316. doi:10.1016/j.archger.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 80. Palo-Bengtsson L, Winblad B, Ekman SL. Social dancing: a way to support intellectual, emotional and motor functions in persons with dementia. J Psychiatr Ment Health Nurs. 1998;5(6):545–554. [DOI] [PubMed] [Google Scholar]