Abstract
Subjective cognitive complaints (SCCs) are being increasingly recognized as a preclinical phase of dementia. Thus, SCCs may represent a “promising” stage for planning and implementing preventive interventions aimed at reducing the incidence of cognitive disorders. The aim of the present study is to present and discuss the available evidence coming from clinical trials adopting cognitive interventions in individuals with SCCs. A systematic review of literature was conducted to evaluate the available trials testing nonpharmacological cognitive interventions for the prevention of dementia in subjects with SCCs. Six studies were included in the present study. Overall, most interventions showed to objectively improve cognitive performance in subjects with SCCs. A relevant heterogeneity was found concerning their characteristics and feasibility. Conversely, there is a current lack of evidence in the literature about the efficacy of nonpharmacological cognitive interventions for preventing dementia or cognitive impairment.
Keywords: subjective cognitive complaints, subjective cognitive impairment, dementia prevention, cognitive interventions
Introduction
Subjective cognitive complaints (SCCs) are defined as self- or informant-reported cognitive disturbances occurring in the absence of objective signs and known underlying pathological conditions. 1 Despite being heterogeneously defined and assessed, 2 the concept of SCCs has growingly attracted clinical and research interest in the field of dementias and cognitive disorders for several reasons. First, SCCs represent a common condition in the general population. Their prevalence has been estimated as ranging from approximately 25% to over 50% depending on the studied populations, settings, and adopted definitions. 3 Second, although with some conflicting results, SCCs are generally indicated as a potential risk factor for subsequent onset of cognitive impairment and dementia, including Alzheimer’s disease (AD). In fact, in a recent review of the literature performed on this topic, 10 of the considered 15 longitudinal studies showed SCCs as predictive of cognitive decline or dementia. 4 In this context, SCCs have been proposed as a possible preliminary stage of the natural course of AD, anticipating by approximately 15 years the subsequent onset of “mild cognitive impairment” (MCI). 5 Different from SCCs, MCI is characterized by the presence of objectively measurable cognitive deficits in the context of preserved functional independence. 6,7 More than 50% of the patients with MCI progresses to dementia within 5 years. 8 Thus, SCCs may represent the initial manifestation of a departure from normality in the cognitive domain of the older person’s health status. Consistently with this hypothesis, a neurobiological substratum has been attributed to SCCs that are shown to be associated with smaller hippocampal 9 and entorhinal cortex 10 volumes as well as with the apolipoprotein E ∊4 allele 11 (as typically observed in AD).
To date, interventions aimed at positively affecting the progression of cognitive decline to dementia largely failed, even when implemented in patients with MCI. 12 Moreover, taking into account that available treatments for dementia have only shown a moderate short-term symptomatic effect without any disease-modifying efficacy, 13 the need of developing alternative preventing strategies becomes urgent. Such priority is also related to the relevant burden that cognitive disorders impose to public health. 14,15 Thus, a growing consensus has been reached which indicates SCCs as a “promising” stage for planning and implementing preventive interventions with the aim of reducing the incidence of cognitive disorders. 1,5 In this regard, dementia preventive trials are increasingly enrolling individuals with subjective complaints as target populations. 16,17
The implementation of preventive strategies requires specific adaptations, mainly due to the larger population to target. Being applied to the general population (or, better, to subjects at higher risk of experiencing the disease), cost effectiveness and feasibility on a large scale becomes key factors to evaluate the possible interventions to test and/or implement. In this context, behavioral and nonpharmacological protocols become of special interest. Interestingly, these interventions have shown to attenuate the risk of cognitive impairment in healthy older persons 18 and improve global cognitive functioning in patients with MCI. 14,19 Moreover, several prospective studies have found that participating in mentally stimulating activities is associated with a reduced risk of developing AD. 20 The efficacy of such preventive interventions has been explained by a possible improvement of cognitive reserve 21 and plasticity. 22
A systematic review and meta-analysis aimed at examining available evidence on the efficacy of nonpharmacological interventions for individuals with SCCs was recently published. 1 Overall, the authors noted a generally low methodological quality of available trials in the field. Moreover, the tested interventions were found to be differently effective at improving both subjective and objective cognitive performance. Nevertheless, a possible limitation of the review was that it also included randomized controlled trials not explicitly or specifically targeting SCCs but recruiting healthy older persons and volunteers wishing to improve their cognitive performance. Such inclusion determined a heterogeneity of the included samples characteristics and partially limited the interpretation of findings. Individuals with SCCs are being increasingly considered at higher risk of cognitively declining than noncomplainers. Moreover, it is well established in the field of cognitive disorders and dementia preventive trials that the enrollment of healthy volunteers may produce a selection bias during recruitment leading to the selection of healthier and more educated participants. 17 This may limit the external validity of the study results.
The aim of the present study is to present and discuss the available evidence coming from clinical trials adopting nonpharmacological cognitive interventions in persons with SCCs. In order to facilitate the translation of research results into a clinical friendly message, 3 main questions are specifically considered in the evaluation of available evidence.
Which is the design of the intervention aimed at improving cognition and/or preventing dementia in subjects with SCCs?
Was the adopted intervention effective at objectively improving cognitive performance and/or preventing dementia and cognitive impairment?
Is the adopted intervention feasible and apparently easy to implement?
Methods
Identification and Selection of Studies
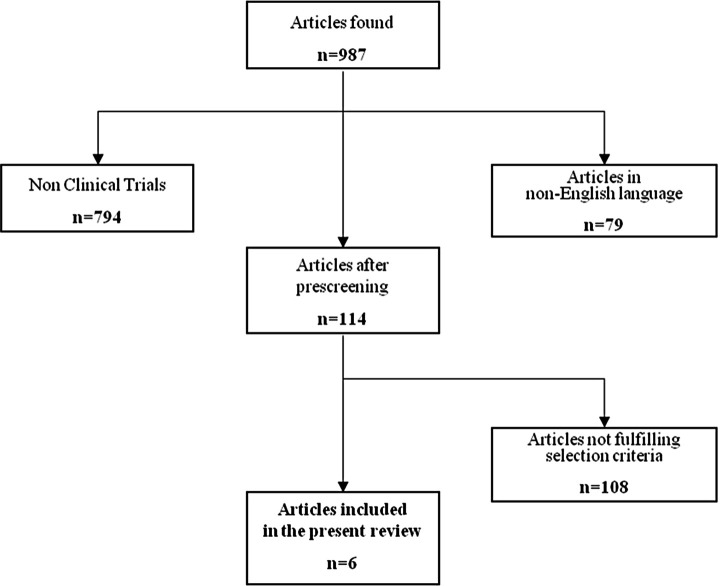
Figure 1 shows the flowchart of the process leading to the selection of the articles of interest for the present review. We performed a literature search using MEDLINE (updated to March 15, 2012) with the alternative key words “cognitive complaints,” “memory complaints,” and “subjective cognitive impairment.” First, only articles in English were retained. Second, only clinical trials were selected.
Figure 1.
Flowchart of the articles selection.
After prescreening, the remaining articles were singularly evaluated according to the following inclusion criteria: (1) recruitment of only subjects with SCCs, defined as cognitive disturbances occurring in the absence of known pathological conditions and/or objective cognitive impairment; (2) evaluation of the efficacy of nonpharmacological interventions; and (3) adoption of at least 1 outcome measure assessing cognitive performance and/or dementia incidence.
Exclusion criteria were (1) recruitment of patients with objective cognitive impairment (eg, dementia, MCI); (2) enrollment of healthy older persons without SCCs but simply wishing to improve cognitive performance and/or preventing the occurrence of cognitive impairment.
Data Extraction
For each study retained for the present review, authors abstracted the following data: number of participants, demographic characteristics of the samples, study design, characteristics of adopted interventions (including duration, organization, and time lines), outcome measures (in particular, cognitive function modifications and incidence of dementia), main results, and conclusions. Two authors (MCa and MCe) discussed the collected data and reached a consensus to resolve the existing discrepancies.
Results
We examined a total of 987 articles retrieved from the literature to identify studies of potential interest for the present review (Figure 1). After excluding articles in language other than English and articles not reporting results of clinical trials (n = 873), we further excluded 108 studies that did not fulfill the predefined criteria of selection. In particular, many excluded articles focused on cognitive complaints occurring in association with objective cognitive impairment or enrolled healthy older persons or volunteers without SCCs. Finally, 6 articles were retained for the present evaluation. 23–28 Tables 1 and 2 provide an overview of the included studies for what concerns the characteristics of participants, experimental design, results of cognitive assessments, and main findings. Only 1 trial 26 of the selected 6 did not perform a randomization of the participants to the intervention groups. A total of 348 subjects were enrolled in the 6 selected studies (with sample sizes ranging from 17 to 139 participants). All the trials were conducted at single institutions and enrolled community-dwelling subjects. Recruitment of participants was largely based on media advertisement.
Table 1.
Clinical Trials Investigating the Cognitive Effects of Nonpharmacological Cognitive Interventions in Subjects with SCCs.
| Reference | Participants (n) | Age (years ± SD) | Randomization | Intervention | Duration of intervention | Conclusions |
|---|---|---|---|---|---|---|
| Hoogenhout et al 24 | EG: 30 CG: 30 | EG: 66.0 ± 4.2 CG: 66.1 ± 4.5 | Y | EG: eight 1.5-h group sessions of educational intervention focused at cognitive aging and contextual factors, skills, and compensatory behaviors. Homework (eg, cognitive diary). CG: waiting list. | 4 weeks | EG: no significant improvement in objective cognitive functioning; lower emotional reactions about cognitive functioning. |
| Tsai et al 26 | EG1: 14 EG2: 11 | EG1 + EG2: 69.4 | N | EG1: CT. Ten 2-h classes twice weekly with the training foci in mnemonics and problem-solving strategies. EG2: CS. Eight weekly 1.5-h sessions with reality orientation, quiz games, and situational problem-solving activities. | 5 weeks 8 weeks | EG1: improvement of global cognitive performance and results of verbal memory tests. EG2: improvement of global cognitive performance and executive functions. |
| van Hooren et al 28 | EG: 37 CG: 30 | EG: 62.35 ± 5.39 CG: 63.27 ± 5.95 | Y | EG: Goal management course. Twelve 1- or 1.5-h session, twice weekly. Psycho-education on cognitive functions (in particular, executive functions). CG: waiting list. | 6 weeks | EG: improvement in the ability of managing executive failures with diminished annoyance. No improvement of objective performance. |
| Small et al 25 | EG: 8 CG: 9 | EG: 54 ± 12 CG: 53 ± 10 | Y | EG: Healthy longevity lifestyle program. Individual program combining mental and physical exercise, stress reduction, and healthy diet. CG: usual lifestyle routine. | 2 weeks | EG: improvement in word fluency. |
| Valentijn et al 27 | EG1: 53 EG2: 43 CG: 43 | EG1: 69.32 ± 7.77 EG2: 68.07 ± 6.58 CG: 68.30 ± 8.03 | Y | EG1: collective training. Eight weekly 2-h sessions of memory training and education on cognitive functioning and decline. EG2: individual training. Book with the same topics of collective training (less practical). CG: no training. | 8 weeks | EG1: improvement in subjective and objective memory functioning, and recall of a previously learned word list. EG2: fewer feelings of anxiety in relation to memory functioning. |
| Andrewes et al 23 | EG: 20 CG: 20 | EG + CG: ranging from 60 to 70 | Y | EG: self-administered memory handbook. Two sections focused on remembering names and prospective memory. Individual training. CG: instructional pamphlet with a description of 3 list-learning mnemonics. | 4 weeks | EG: improvement in face-naming tasks and strategies knowledge. |
Abbreviations: EG, experimental group; CG, control group; CT, cognitive training; CS, cognitive stimulation; SD, standard deviation (if reported); Y, randomized; N, nonrandomized; SCCs, subjective cognitive complaints.
Table 2.
Outcome Measures of Objective Cognitive Performance and Results of Selected Clinical Trials.
| References | Measures | At the end of the intervention | Reevaluation at distance after the end of intervention |
|---|---|---|---|
| Hoogenhout et al 24 | VVLT SCWT, CST, and LDST | ns ns | No follow-up |
| Tsai et al 26 | ADAS-Cog MMSE SRT CDT | P < .05 in EG1 and EG2 P < .05 in EG1 and EG2 P < .05 in EG1, ns in EG2 ns in EG1, P < .05 in EG2 | After 6 weeks ns in EG1, P < .05 in EG2 ns in EG1 and EG2 P < .05 in EG1, ns in EG2 ns in EG1 and EG2 |
| van Hooren et al 28 | SCWT | ns | After 24 weeks ns |
| Small et al 25 | SRT COWAT | ns P = .015 | No follow-up |
| Valentijn et al 27 | VVLT total VVLT recall SST immediate recall SST delayed recall | ns P = .001 in EG1 vs CG; P = .002 in EG1 vs EG2 ns ns | No follow-up |
| Andrewes et al 23 | Face-naming test Prospective memory | P < .01 ns | No follow-up |
Abbreviations: VVLT, visual-verbal learning test; SCWT, Stroop color-word test; CST, concept shifting test; LDST, letter digit substitution test; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive subscale; MMSE, Mini-Mental State Examination; SRT, selective reminding test; CDT, clock-drawing test; COWAT, controlled oral word association test; SST, short story test; EG, experimental group; CG, control group; ns, nonsignificant.
Two-third of the trials explored the efficacy of the experimental interventions in comparison with a placebo condition (eg, waiting list, usual lifestyle routine, or no training). One study compared 2 different cognitive interventions, 26 while another compared 2 different cognitive interventions with a control group. 27
Which Is the Design of the Interventions Aimed at Improving Cognition and/or Preventing Dementia in Subjects With SCCs?
A total of 8 different interventions were tested in the selected studies (Table 1). In all, 5 interventions were conducted in groups of participants (maximum 14 participants), whereas 3 individually. One collective intervention 28 also included an individual session.
Overall, 65% of the interventions 24–28 included (or were entirely based on) a psychological educational component consisting of the formation about cognitive and memory functioning, cognitive aging and contextual factors (eg, negative age stereotypes and beliefs), compensatory strategies and behaviors, and dementia and cognitive impairment. Six interventions 23,25–28 were structured as cognitive training, an approach involving guided practices of standardized tasks, strategies, and skills in order to directly optimize or maintain specific cognitive functions. 29,30 The cognitive training programs were mainly targeted on episodic memory training, but attention, executive functions, and visuospatial abilities were also considered.
One intervention 26 consisted of a generic cognitive stimulation, widely engaging the subject in a range of activities and discussions aimed at the general enhancement of cognitive and social functioning. 29,30 Finally, 1 study 25 adopted a multidomain intervention combining cognitive training, physical exercise, nutrition recommendations, and techniques for stress reduction.
Were the Adopted Interventions Effective at Objectively Improving Objective Cognitive Performance and/or Preventing Dementia and Cognitive Impairment?
None of the selected studies evaluated the efficacy of adopted cognitive interventions for primary prevention of dementia or cognitive impairment. A relevant heterogeneity can be noted about the instruments used to objectively assess cognitive performance (Table 2). Of the 6 studies, 523–27 adopted instruments measuring specific memory functions; the remaining 1 study 28 exclusively assessed executive functions. Verbal memory was evaluated in 4 studies 24–27 by the visual–verbal learning test 31 and the selective reminding test. 32 One intervention 27 additionally targeted logical memory, assessed by the short story test, 33 while, in another, 23 face-name associative memory and prospective memory were investigated. Three studies 24,26,28 explored executive function modifications using the Stroop color-word test (SCWT), 34 the clock-drawing test, 35 and an “executive functioning and speed quotient” (composed by the SCWT, the concept shifting test, 36 and the letter digit substitution test 37 ). One study 25 targeted verbal fluency as outcome of interest using the controlled oral word association test. 38 Finally, 1 study 26 evaluated global cognitive functioning assessed by the Mini-Mental State Examination (MMSE) 39 and the Alzheimer’s Disease Assessment Scale-Cognitive subscale. 40
The main results of the trials are summarized in Table 2. All the studies measured the outcomes of interest at the end of the cognitive training intervention. Only 2 studies 26,28 additionally performed a subsequent assessment (one at 7 weeks and the other at 6 months) to verify the maintenance of effects over time after the end of the cognitive training. Contrasting results were obtained overall. Of the 8 interventions, 5 were significantly associated with objective improvements in cognitive functions. The heterogeneity of the design of the interventions and adopted outcomes does not allow direct comparisons among protocols to clearly elucidate the superiority of one over the others.
Are the Adopted Interventions Feasible and Apparently Easy to Implement?
The mean duration of the interventions was 5.6 weeks (ranging from 2 to 8 weeks). Interventions conducted in groups of participants consisted of a mean of 9.2 sessions (ranging from 8 to 12 with weekly or biweekly meetings). Each session lasted for 1 to 2 hours. All the group interventions were conducted in outpatient clinical centers. For this reason, 2 studies 27,28 specifically considered the subjects’ capacity to independently travel as additional inclusion criterion. One group intervention 27 was conducted by experienced teachers. Another intervention 28 involved the participation of 4 different professionals (2 trainers, a health care psychologist with extensive experience in interventions for people with cognitive problems, and a research psychologist). In the other 2 studies based on the group interventions, 24,26 the involvement of specific professionals was not described. Most of the group interventions also included the assignment of homework to participants.
Individual programs were conducted adopting external aids that participants were asked to use by their own. These supports were represented by books 23,27 or notebooks 25 containing detailed information and practice exercises concerning cognitive strategies easily applicable to everyday situations. The adherence to the intervention and the established time lines was regularly monitored by telephone call or interview.
Discussion
To our knowledge, this is the first systematic review of trials testing cognitive interventions in subjects with SCCs. Only 6 studies were found. Overall, the limited available evidence is characterized by heterogeneous profiles of the tested populations, adopted interventions, and study designs.
Most interventions were shown to objectively improve cognitive performance in participants with SCCs. However, their efficacy was mainly confined to the specifically targeted cognitive domains (consistently with trials applying cognitive programs in healthy older persons without SCCs 41,42 ). The scarce number of available studies and the heterogeneity of the adopted methodologies make the evaluation of a possible superiority of specific interventions over others difficult. Moreover, the available evidence in this field is based on the study of small sample populations. Only 1 of the 2 studies performing a reevaluation after the end of the program suggested a long-lasting benefit (ie, 6 months) of the cognitive interventions.
The cognitive interventions tested in subjects with SCCs were generally previously tested in large samples of healthy older individuals not expressly complaining about cognitive issues, resulting in a long-term cognitive benefits 41,43 and reduced incidence of dementia. 18 From these promising preliminary results, such nonpharmacological interventions for cognition were started to be considered as potentially applicable in the prevention of cognitive disorders. 44 Moreover, a growing body of evidence has recently attributed a biological plausibility to these interventions. 44
In the last decades, the worldwide epidemiological increase in dementia 13 (in the absence of effective treatments) has risen the interest for the preclinical condition of SCCs. Such phase may represent the ideal target for implementing successful preventive interventions. 5 An intervention needs to be effective, feasible on a large scale, and inexpensive to be adopted for preventive purposes. In the present study, the feasibility of adopted interventions has been evaluated taking into account several factors such as duration, setting, number and length of sessions, health professionals involved, and typology of external aids. None of the selected studies performed a cost-effective analysis in order to discuss the possibility of adopting the proposed interventions in larger populations. Similarly, only 1 study 27 objectively described the feasibility of the intervention (ie, reporting the proportion of participants completing the program). Overall, the group interventions were found to be difficult to reproduce outside the experimental conditions (ie, clinical trials). Indeed, the increased numbers of sessions, the need for subjects to reach the outpatient units weekly (or biweekly), and the involvement of highly specialized figures are likely to be associated with high costs (consequently limiting a wide diffusion in the general population). On the other hand, findings from individual interventions suggest the possibility of implementing effective and easy-to-implement strategies. In fact, the use of specific external aids containing cognitive instructions and exercises reduced the number of professionals involved without apparently affecting the resulting cognitive benefits.
Different from the other reviews performed on the topic, 1 we decided to include only studies explicitly targeting SCCs (thus excluding studies testing interventions on healthy volunteers without SCCs). Nevertheless, it is noteworthy that only 1 study 28 from those included in our review specified how SCCs were defined or evaluated. This may have concurred to the relevant heterogeneity in the participants’ selection. Thus, it may be considered as a major limitation of available evidence on the topic. A great debate is currently ongoing about the clinical definition of SCCs, 2 which is mainly hampered by their subjective nature. Recently, a set of criteria specifically enhancing the predictive value of these symptoms for the onset of dementia have been proposed. 2 However, its validity has not yet been well established. Moreover, several clinical trials are increasingly adopting standardized tools for the screening of SCCs, aiming to make the recruitment of participants more homogeneous. 45 Interestingly, none of the retained studies adopted an extensive neuropsychological test battery in order to rule out the presence of preexisting objective signs of subtle cognitive decline at baseline. The presence of dementia or cognitive impairment was mainly excluded by adopting single cognitive measures and/or screening instruments assessing global cognitive functioning (eg, MMSE). This may have led to the recruitment of subjects already exhibiting impaired cognitive abilities and, thus, influenced the study findings.
In conclusion, our review represents the first attempt to systematically organize the available evidence concerning the efficacy and feasibility of cognitive training interventions in the specific context of SCCs. To date, the available evidence is still too limited to retrieve definitive conclusions about the role that cognitive interventions may play in the prevention of dementia and cognitive disorders. In particular, larger studies specifically designed to explore the preventive value of such interventions against clinically meaningful outcomes (eg, incidence of MCI or dementia) are needed. Given the relevance of the topic, appropriate objective measures of SCCs should be necessarily developed, validated, and adopted.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Metternich B, Kosch D, Kriston L, Härter M, Hüll M. The effects of nonpharmacological interventions on subjective memory complaints: a systematic review and meta-analysis. Psychother Psychosom. 2010;79(1):6–19. [DOI] [PubMed] [Google Scholar]
- 2. Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23(5):321–330. [DOI] [PubMed] [Google Scholar]
- 3. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15(11):983–991. [DOI] [PubMed] [Google Scholar]
- 4. Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5-6):471–485. [DOI] [PubMed] [Google Scholar]
- 5. Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4(1 suppl 1):S98–S108. [DOI] [PubMed] [Google Scholar]
- 6. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 7. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. [DOI] [PubMed] [Google Scholar]
- 9. Van der Flier WM, Van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251(6):671–675. [DOI] [PubMed] [Google Scholar]
- 10. Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–1756. [DOI] [PubMed] [Google Scholar]
- 11. Small GW, Chen ST, Komo S, et al. Memory self-appraisal in middle-aged and older adults with the apolipoprotein E-4 allele. Am J Psychiatry. 1999;156(7):1035–1038. [DOI] [PubMed] [Google Scholar]
- 12. Aisen PS. Treatment for MCI: is the evidence sufficient? Neurology. 2008;70(22):2020–2021. [DOI] [PubMed] [Google Scholar]
- 13. Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. [DOI] [PubMed] [Google Scholar]
- 14. Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr. 2011;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Houles M, Canevelli M, Abellan van Kan G, Ousset PJ, Cesari M, Vellas B. Frailty and cognition. J Frailty Aging. 2012;1(2):56–63. [DOI] [PubMed] [Google Scholar]
- 16. Carrié I, Van Kan GA, Gillette-Guyonnet S, et al. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial). J Nutr Health Aging. 2012;16(4):355–359. [DOI] [PubMed] [Google Scholar]
- 17. Vellas B, Coley N, Ousset PJ, et al. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851–859. [DOI] [PubMed] [Google Scholar]
- 18. Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17(3):179–187. [DOI] [PubMed] [Google Scholar]
- 19. Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic mild cognitive impairment: a systematic review. Neurosci Biobehav Rev. 2012;36(4):1163–1178. [DOI] [PubMed] [Google Scholar]
- 20. Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. [DOI] [PubMed] [Google Scholar]
- 21. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 22. Greenwood PM, Parasuraman R. Neuronal and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Front Aging Neurosci. 2010;2:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrewes DG, Kinsella G, Murphy M. Using a memory handbook to improve everyday memory in community-dwelling older adults with memory complaints. Exp Aging Res. 1996;22(3):305–322. [DOI] [PubMed] [Google Scholar]
- 24. Hoogenhout EM, De Groot RHM, Van der Elst W, Jolles J. Effects of a comprehensive educational group intervention in older women with cognitive complaints: a randomized controlled trial. Aging Ment Health. 2012;16(2):135–144. [DOI] [PubMed] [Google Scholar]
- 25. Small GW, Silverman DH, Siddarth P, et al. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14(6):538–545. [DOI] [PubMed] [Google Scholar]
- 26. Tsai AY, Yang MJ, Lan CF, Chen CS. Evaluation of effect of cognitive intervention programs for the community-dwelling elderly with subjective memory complaints. Int J Geriatr Psychiatry. 2008;23(11):1172–1174. [DOI] [PubMed] [Google Scholar]
- 27. Valentijn SA, Van Hooren SA, Bosma H, et al. The effect of two types of memory training on subjective and objective memory performance in healthy individuals aged 55 years and older: a randomized controlled trial. Patient Educ Couns. 2005;57(1):106–114. [DOI] [PubMed] [Google Scholar]
- 28. Van Hooren SA, Valentijn SA, Bosma H, et al. Effect of a structured course involving goal management training in older adults: a randomised controlled trial. Patient Educ Couns. 2007;65(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clare L, Woods R. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil. 2004;14(4):385–401. [Google Scholar]
- 30. Buschert V, Bokde AL, Hampel H. Cognitive intervention in Alzheimer disease. Nat Rev Neurol. 2010;6(9):508–517. [DOI] [PubMed] [Google Scholar]
- 31. Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol. 1985;112(2):201–210. [DOI] [PubMed] [Google Scholar]
- 32. Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. [DOI] [PubMed] [Google Scholar]
- 33. Niehof J. Validering van een zestal logical memory taken [Validity of Six Logical Memory Tasks]. Groningen, the Netherlands: University of Groningen; 1995. [Google Scholar]
- 34. Houx PJ, Jolles J, Vreeling FW. Stroop interference: aging effects assessed with the Stroop Color-Word Test. Exp Aging Res. 1993;19(3):209–224. [DOI] [PubMed] [Google Scholar]
- 35. Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc. 1989;37(8):730–734. [DOI] [PubMed] [Google Scholar]
- 36. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The concept shifting test: adult normative data. Psychol Assess. 2006;18(4):424–232. [DOI] [PubMed] [Google Scholar]
- 37. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The letter digit substitution test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. [DOI] [PubMed] [Google Scholar]
- 38. Benton A, Hamsher K, Silvan A. Multilingual Aphasia Examination. 3rd ed. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 39. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 40. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. [DOI] [PubMed] [Google Scholar]
- 41. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oswald WD, Rupprecht R, Gunzelmann T, Tritt K. The SIMA-project: effects of 1 year cognitive and psychomotor training on cognitive abilities of the elderly. Behav Brain Res. 1996;78(1):67–72. [DOI] [PubMed] [Google Scholar]
- 44. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. [DOI] [PubMed] [Google Scholar]
- 45. Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H. The GuidAge study: methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761 for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology. 2006;67(9 suppl 3):S6–S11. [DOI] [PubMed] [Google Scholar]