Abstract
Objectives:
The acupuncture has been used in the therapy of Alzheimer disease (AD), however, its neural underpins are still unclear. The aim of this study is to examine the acupuncture effect on the default mode network (DMN) in AD by using resting state functional magnetic resonance imaging (RS-fMRI).
Methods:
Twenty-eight subjects (14 AD and 14 normal controls (NC)) participated in this study. RS-fMRI data were acquired before and after acupuncture, while during the acupuncture, the procession of acupuncture stimulation on the acupoints of Tai chong (Liv3) and Hegu (LI4) lasted for 3 minutes.
Results:
Region of interest analysis showed that the impaired DMN connectivity in AD (identified by comparing the pre-acupuncture RS-fMRI of AD and NC), specifically the left cingulate gyrus (CG) and right inferior parietal lobule (IPL), were significantly changed for the better. The whole-brain exploratory analysis further demonstrated these results and found some new regions respond to the acupuncture effect on AD, with a cluster in the left posterior cingulate cortex (PCC), the right middle temporal gyrus (MTG) together with right IPL showed increased within-DMN connectivity; and the bilateral CG and left PCu showed decreased within-DMN connectivity. Moreover, the acupuncture effect on the right MTG was significantly correlated with disease severity as measured by Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores.
Conclusion:
It was found that the acupuncture stimulation could modulate the DMN activity in AD. The current findings suggest that the acupuncture treatment on the relative earlier AD patients might have a better therapy effect.
Keywords: Alzheimer’s disease, acupuncture, functional MRI, resting state
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, characterized by progressive cognitive decline including impairment of memory and reasoning ability. The plaque of β-amyloid and the neurofibrillary tangle composed mainly of hyperphosphorylated τ protein are 2 major pathological hallmarks of AD. So far, there are over 24 million people have AD all over the world, and the population might be double to an estimated 42 million by 2020. 1 However, at present, there is still no effective therapy to slow the progression of AD and a growing worldwide health crisis are crying for effective treatment.
As a classic treatment in traditional Chinese medicine (TCM), acupuncture became a new method for treating neurodegenerative disease and has been widely used in the therapy of Parkinson’s disease (see a review by Lam et al 2 ) and vascular dementia. 3 In recent years, many researchers from different research groups demonstrated acupuncture as an effective treatment to AD4,5 (also see a recent review by Xia et al 6 ). The theoretical foundation of acupuncture is the TCM. According to the modern TCM theories, dementia is essentially a dysfunction of the brain along with other organs including heart, liver, spleen, and kidneys (note: these are concepts and terms used in TCM theory, and their interpretation is usually different from modern physiology and medicine). The factors involved in its pathogenesis are wind, fire, phlegm, stagnation, and weakness (note: TCM concepts). At the beginning, deficiency of the main organs leads to the phlegm stagnation and stagnant fire. It will decrease the nutritional (“Qi” and blood) supply to the brain and cause the cognitive impairment, thereby ending in development of dementia. 7 -9 Factors that influence the acupuncture efficacy in AD treatment include stage of the AD, selection of the acupoints, and methods of needle insertion.
Some mechanisms of the acupuncture effect on AD have been proposed, and most of them are based on the various “hypotheses” of the pathogenesis of AD. These mechanisms include amelioration of impaired cholinergic function by acupuncture, acupuncture relieving the amyloid neurotoxicity, acupuncture reducing levels of hyperphosphorylated τ protein, and acupuncture protecting cerebral neurons from oxidative stress (see a recent review by Zeng et al 10 ). However, all of them mainly got their evidences from animal studies but with a limited number of studies conducted on humans (see a summarization by Xia et al 6 ).
Some previous studies have used neuroimaging techniques to assess the effect of acupuncture treatment on AD and tried to find the potential mechanism of acupuncture therapy for AD. 11 -15 They found that effective acupuncture could activate some areas related to memory and learning. For example, Guo et al 13 reported that the stimulation of acupuncture points Jingming (BL-1) using electric acupuncture (EA) activated hippocampus and hypothalamus of senile dementia (mainly covers AD). Zhou and Jin 15 performed EA at Shenmen (HT-7), Zusanli (ST-36), Fenglong (ST-40), and Taixi (KI-3). Their results demonstrated many activations following EA, including hippocampus, insula, cerebellum, and some areas in temporal and parietal lobes. Wang and his colleague’s work 14 showed that stimulating Liv3 (Taichong) and LI4 (Hegu) increases the brain activation in some regions within frontal and temporal lobes. However, the underlying mechanism of these activations’ enhancement and pathways involved in these activated regions are still unclear.
Default mode network (DMN) 16 is one of the earliest pathological site of AD. 17,18 The impairment of DMN is so far the most stable and reliable findings in functional neuroimaging studies of AD. 19 Given its crucial role in memory and cognitive processes of human brain, the abnormal activity of DMN can predict the progress of AD. 20,21 Moreover, the plasticity change in DMN activation has been widely confirmed by acupuncture. 22,23 It is thus of great interest to ask the question: Does acupuncture modulates the DMN activity of patients with AD at rest?
In this study, we tried to compare the differences in DMN during acupuncture stimulating the acupoints Liv3 and LI4 of AD with normal controls (NCs). According to TCM, both Liv3 and LI4 are known as source points. They are hubs for internal and external energies gathering and transforming. 24 The combination stimulus of Liv3 and LI4 is classically known as “The Four Gates,” which is usually applied to promote the circulation of “Qi” and blood throughout the entire body, with Liv3 handling the lower half while LI4 addressing the upper. 6 The effectiveness of acupuncture the Four Gates has been demonstrated in the treatment of pain and AD. 14 Using the blood oxygen level-dependent functional magnetic resonance imaging (fMRI), which relies on changes in the level of deoxygen in the human brain induced by alterations in blood flow, the DMN (including posterior cingulate cortex [PCC], medial prefrontal cortex, inferior parietal lobule [IPL], and inferior temporal cortex) has been reported in many studies. The activation of DMN can be found during rest, and it is highly related to self-awareness, memory retrieval 25 , and pain. 26,27 Specifically, PCC is one of the key hubs of the whole brain and has been shown to communicate with various brain networks simultaneously and regulate the balance between internally and externally directed cognition. 28 Combining the previous results from both TCM and neuroscience, it is of great interest for us to investigate whether the combination acupuncture of Liv3 and LI4 has an evident effect on the DMN activity.
Given the important functions of Liv3 and LI4, and their potential roles to the DMN, along with the plasticity of the DMN, we hypothesized that the DMN of AD would be modulated by acupuncture of Liv3 and LI4. An independent component analysis (ICA) method was applied to study the impairment within DMN of AD compared with NC before acupuncture. A region of interest (ROI) analysis was performed to test the acupuncture effect on the identified abnormal brain areas in AD. Then, the interaction effect of acupuncture by group was examined to show whether there were some regions specific for AD due to acupuncture of Liv3 and LI4. Additionally, the individual differences in the acupuncture effect were also been investigated to find which kind of patients have a relative better effect by acupuncture than others.
Materials and Methods
Patients
Twenty-eight right-handed (14 AD and 14 healthy controls) patients participated in this study after giving written informed consent. This study was approved by the Medical Research Ethics Committee of Xuanwu Hospital. Patients with AD were recruited from patients who had consulted the memory clinic at Xuanwu Hospital for memory complaints. The healthy elderly controls were recruited from the local community.
All patients with AD underwent a complete physical and neurological examination, standard laboratory tests, and an extensive battery of neuropsychological assessments. The diagnosis of AD fulfilled the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria for dementia 29 and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for possible or probable AD. 30 The patients were assessed with the clinical dementia rating (CDR) score, 31 CDR of 1 and 2 was assigned to the AD category.
Healthy controls met the following criteria: (1) no neurological or psychiatric disorders such as stroke, depression, and epilepsy; (2) no neurological deficiencies such as visual or hearing loss; (3) no abnormal findings such as infarction or focal lesion in conventional brain MR imaging; (4) no cognitive complaints; (5) Mini-Mental State Examination (MMSE) score of 28 or higher; and (6) CDR score of 0.
Participants with contraindications for MRI such as pacemaker, cardiac defibrillator, implanted material with electric or magnetic system, vascular clips or mechanical heart valve, cochlear implant, or claustrophobia were excluded. In addition, patients with a history of stroke, psychiatric diseases, drug abuse, severe hypertension, systematic diseases, and intellectual disability were excluded.
Data Acquisition
The MRI data acquisition was performed on a SIEMENS verio 3-T scanner (Siemens, Erlangen, Germany). Patients were instructed to hold still, keep eyes closed, and think nothing in particular. Functional MRI data were acquired axially using an echo-planar imaging (repetition time [TR]/echo time [TE]/flip angle [FA] = 2000 ms/40 ms/90° filed of view [FOV] = 24 cm, image matrix = 64 × 64, slice number = 33, thickness = 3 mm, gap = 1 mm, band width = 2232 Hz/pixel). Three-dimensional T1-weighted magnetization-prepared rapid gradient echo sagittal images were also obtained (TR/TE/inversion time/FA = 1900 ms/2.2 ms/900 ms/9°, image matrix = 256 × 256, slice number = 176, thickness = 1 mm.
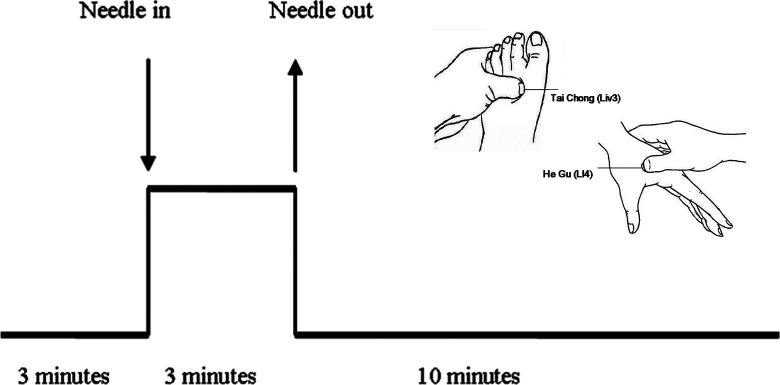
A single-block experimental design was adopted in the current study as shown in Figure 1. The baseline resting-state data were acquired in the initial 3 minutes and then followed by the procession of acupuncture stimulation during the following 3 minutes. A silver needle of 0.30 mm in diameter and 25 mm long was inserted and twirled at the 4 acupoints of the human body—Liv3 on the dorsum of the left and right foot and LI4 on the dorsum of the left and right hand. After the needle was withdrawn, another 10 minutes of resting-state fMRI scans were acquired.
Figure 1.
Protocols for acupuncture.
Imaging Preprocess
In this study, resting-state fMRI data of 3 minutes before acupuncture and 3 minutes directly following acupuncture were included in the following data analysis. The preprocessing of fMRI data was conducted using statistical parametric mapping software package (SPM5, Wellcome Dept. of Cognitive Neurology, London, UK). The first 10 volumes of the functional images were discarded to allow the signal to reach equilibrium and participants' adaptation to the scanning noise. The remaining fMRI images were first corrected for within-scan acquisition time differences between slices and then realigned to the first volume to correct for interscan head motion. The translation and rotation were checked, and the images with head movement greater than 2 mm in any direction or head rotation greater than 2° were excluded. The individual structural image was coregistered to the mean functional image after motion correction using a linear transformation. The transformed structural images were then segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid using a unified segmentation algorithm. 32 The motion corrected functional volumes were spatially normalized to the Montreal Neurological Institute space and resampled to 3 mm isotropic voxels using the normalization parameters estimated during unified segmentation. Subsequently, the functional images were spatially smoothed with a Gaussian kernel of 4 × 4 × 4 mm3 full width at half maximum (FWHM) to decrease spatial noise.
Independent Component Analysis
The group ICA (GICA) of the fMRI toolbox (Stable and Consistent Group ICA of the fMRI Toolbox, version 1.2) was used to decompose the preprocessed data of all patients into independent components (ICs; http://www.nitrc.org/projects/cogicat/). This toolbox supports a GICA approach that first concatenates the individual data across time and subsequently computes the patient specific components and time courses. The toolbox performed the analysis in the 3 stages: (1) data reduction using principal component analysis (PCA), (2) application of the ICA algorithm, and (3) back reconstruction for each patient. To determine the number of ICs, dimension estimation on each group of participants was first performed using minimum description length as implemented in the GIFT. 33 The data reduction step was achieved based on the selected number of components. Then, the infomax algorithm 34 was used to separate the data. Subsequently, the dual-regression approach was used to back reconstruct the individual patient components.
Different from GIFT 35 and Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC), 36 the Subject Order Independent Group ICA (SOI-GICA) algorithm launches multiple GICA, each time with randomized initial value and different patient order, and then the multiple results are integrated to form the final output. 37 The SOI-GICA algorithm is helpful to deal with the problem of participant concatenation orders-induced GICA instability, which might be present in GIFT and MELODIC in all likelihood. In the present study, the GICA had been performed 100 times, and the time courses of IC and its spatial distribution of each condition (including AD_before; AD_after, NC_before, and NC_after) and each participant were acquired for the following process. The subject-specific IC distribution maps were converted into z score, which reflect the degree to which the time series of a given voxel correlates with the mean time series of the component to which it belongs. The IC of DMN was selected via visual inspection.
Statistical Analysis
In the first stage, one-sample t test was used to achieve the DMN z-statistic maps for each condition (NC_before, NC_after, AD_before, and AD_after) under a combined threshold of P < .01 and a cluster size of 648 mm3. This yield a corrected threshold of P < .01, determined by Monte Carlo simulation using the AlphaSim program with parameters: FWHM = 4 mm, within the GM mask (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). Then 2 masks were made by combining the corresponding 2 DMN maps of 4 conditions (ie, NC_before and AD_before; NC_before, NC_after, AD_before, and AD_after), which were, respectively, used for analyzing the following group differences (NC-before vs AD_before) and the interaction effect of acupuncture by group ([AD_before > AD_after] > [NC_before > NC_after], [AD_after > AD_before] > [NC_after > NC_before]).
Next, between-group difference in NC and AD before acupuncture was examined by a 2-sample t test with age, gender, and structural atrophy as covariates. The structural atrophy was calculated and used as in the previous studies. 38 -40 The threshold was set to a combined threshold of P < .01 and a cluster size of 459 mm3, which yield a corrected threshold of P < .01, determined by the AlphaSim program with parameters: FWHM = 4 mm, with mask. Based on the group differences before acunpuncture (NC_before vs AD_before), ROIs can be defined according to the activated clusters. To examine the ameliorate of the impaired DMN connectivity, we would first extract the z values for the DMN of 4 conditions. Then, a paired t test was run for each ROI to compare the acupuncture effect on NC and AD.
Third, a 2 × 2 analysis of covariance was run based on the flexible factorial modeling procedure in SPM5 to examine the interaction effect of acupuncture (pre vs post) by group (AD vs NC). Voxels survived a corrected threshold of P < .01 (single voxel threshold of P < .01 and a cluster size of 513 mm3, using the AlphaSim program with parameters: FWHM = 4 mm, with mask) and were considered to show significant difference. In case of significant interactions, we additionally calculated the respective simple main effects (ie, AD_before vs AD_after and NC_before vs NC_after).
Correlation Between the Acupuncture Effect and Individual Differences as Reflected by Neuropsychological Measures
We are interested to explore the individual differences in the acupuncture effect, which patients had the stronger effect of acupuncture on which regions. Based on the identified clusters in the whole-brain analysis of the acupuncture effect in AD (ie, the clusters in the interaction effect), Pearson’s correlation was performed to examine the correlation between the mean connectivity change in each cluster by acupuncture and 2 clinical variables (MMSE and Montreal Cognitive Assessment [MoCA]) in AD and NC groups separately. The P values were corrected for multiple comparisons using the Bonferroni method.
Results
Clinical Data and Neuropsychological Test
In all, 8 participants (3 NC and 5 AD) who exhibited large amounts of head motions (>3 mm) during scanning after acupuncture were excluded. The demographic and neuropsychological details for the remaining 20 patients are shown in Table 1. There were no significant differences between the 2 groups in gender, age, and years of education, but the neuropsychological test such as MMSE, CDR, and auditory verbal learning test scores was significantly different (P < .01) between the 2 groups.
Table 1.
Characteristics of the Patients With AD and Normal Controls.a
| AD (N = 9) | Controls (N = 11) | P value | |
|---|---|---|---|
| Sex, female/male | 6/3 | 8/3 | .769b |
| Age, year | 65.11 ± 9.84 | 66.45 ± 5.55 | .736c |
| Education, year | 9.78 ± 3.26 | 11.18 ± 4.39 | .459c |
| MMSEa | 16.00 ± 4.45 | 27.91 ± 1.44 | <.01c |
| MoCA | 13.22 ± 1.20 | 26.73 ± 0.63 | <.01c |
| CDR | 1.17 ± 0.47 | 0.00 ± 0.00 | <.01c |
| AVLT (immediate) | 11.44 ± 4.22 | 25.55 ± 4.05 | <.01c |
| AVLT (short time) | 2.44 ± 1.34 | 10.73 ± 2.77 | <.01c |
| AVLT (long time) | 3.00 ± 1.41 | 12.82 ± 1.59 | <.01c |
Abbreviations: MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; CDR, clinical dementia rate; AD, Alzheimer's disease; SD, standard deviation; AVLT, auditory verbal learning test; immediate, immediate recall of learning verbal; delayed; delayed recall of learning verbal; recognition, recognition of learning verbal.
a Plus-minus values are means ± SDs.
b The P value for gender distribution in the 4 groups was obtained by chi-square test.
c The P values were obtained by one-way analysis of variance tests.
Connectivity Differences in DMN Between AD and NC Before Acupuncture
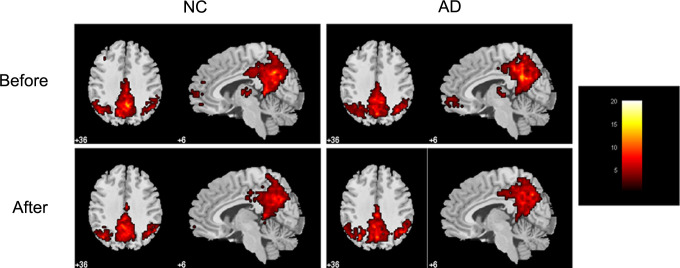
Figure 2 shows the DMN maps for 4 conditions (AD_before, AD_after, NC_before, and NC_after). Figure 3 shows the group differences before acupuncture. As compared to NC, a large cluster mainly located at PCC, extending into precuneus (PCu), right IPL (especially the supramarginal gyrus), and right PCu showed significantly decreased activity in AD, while a cluster in the left cingulate gyrus (CG) showed increased activity in AD. Based on these results, 4 functional ROIs were defined, as shown in Table 2 and Figure 3.
Figure 2.
Within-condition default mode network (DMN) connectivity patterns identified by independent component analysis (ICA), including patterns for the 2 conditions before acupuncture (ie, NC_before and AD_before) and 2 conditions after acupuncture (ie, NC_after and AD_after).
Figure 3.
Between-group differences in NC and AD before acupuncture, based on a 2-sample t test with age, sex, and structural atrophy as covariates. The threshold was set to a corrected threshold of P < .01, determined by AlphaSim. Left in picture is left side of the brain. The color scale represents t values. Warm color represents decreased connectivity in AD as compared to NC. AD indicates Alzheimer’s disease; NC, normal control.
Table 2.
The DMN Group Differences Before Acupuncture (ie, NC_Before vs AD_Before).a
| Brain regions | MNI coordinate | BA | Cluster size | t score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| NC_before > AD_before | ||||||
| Posterior cingulate | −6 | −39 | 18 | 29 | 50 | 4.384 |
| Posterior cingulate | 6 | −45 | 18 | 30 | 2.858 | |
| Cingulate gyrus | 3 | −42 | 27 | 31 | 2.654 | |
| Inferior parietal lobule | 60 | −39 | 39 | 40 | 52 | 3.586 |
| Inferior parietal lobule | 54 | −42 | 27 | 40 | 3.251 | |
| Supramarginal gyrus | 48 | −42 | 33 | 40 | 3.243 | |
| Precuneus | 24 | −60 | 27 | 7 | 73 | 3.571 |
| Cuneus | 0 | −78 | 33 | 7 | 3.455 | |
| AD_before > NC_before | ||||||
| Cingulate gyrus | −9 | −48 | 39 | 31 | 49 | 3.325 |
| Cingulate gyrus | −12 | −30 | 39 | 31 | 3.289 | |
Abbreviations: DMN, default mode network; BA, Broadman area; MNI, Montreal Neurological Institute; x, y, and z, coordinates of primary peak locations in the MNI space; AD, Alzheimer's disease; NC, normal control.
a The threshold was set to a corrected P < .01 using AlphaSim.
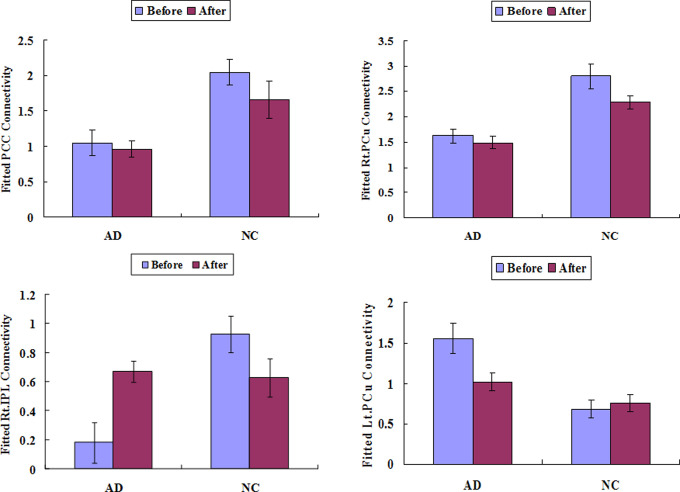
Based on these 4 ROIs, we examined the relative patterns of the acupuncture effect on NC and AD, as shown in Figure 4. For the identified PCC ROIs, there was no significant acupuncture on both patients with AD and NC participants. For the right PCu, there was no significant acupuncture effect on AD but had an effect on NC participants (t = 2.45, P = .04). For the right IPL, there was a significant increased activity following acupuncture for AD (t = 4.513, P = .002) but there was a decreased trend for NC (t = 2.44, P = .04). For the left CG, there was a significant decreased activity for AD (t = 4.036, P = .004) but not for NC (t = 0.008, P = .994).
Figure 4.
Region of interest analysis of the regions identified in the between-group comparison of normal control (NC) and Alzheimer’s disease (AD) before acupuncture, in order to look at theirs patterns to make sure whether they were affected by acupuncture.
The Whole-Brain Explorations of the Acupuncture Effect on AD
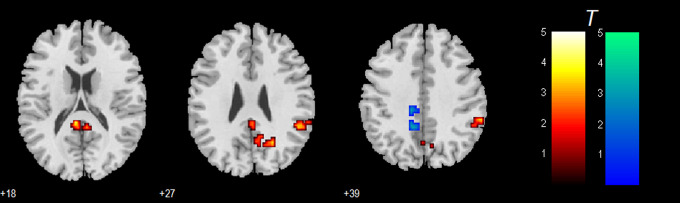
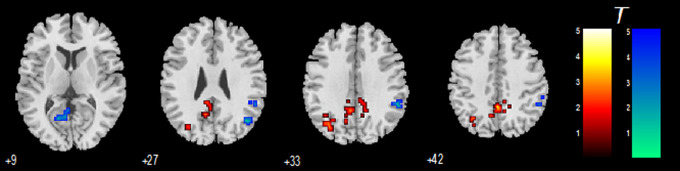
Figure 5 shows the interaction effect of acupuncture by group, and the details of the related activations are shown in Table 3. It was found that, after controlling for the effect on NC, patients with AD showed decreased connectivity in the bilateral CG and left PCu, while showed increased connectivity in the right IPL, right middle temporal gyrus (MTG), and a cluster in left PCC.
Figure 5.
Regions identified by the interaction effect of acupuncture by group, which show the relative specific acupuncture effect on Alzheimer’s disease (AD). The threshold was set to a corrected threshold of P < .01, determined by AlphaSim. Warm color represents decreased connectivity by acupuncture.
Table 3.
Regions Showing Decreased and Increased DMN Connectivity Specific for AD Identified by the Interaction Effect of Acupuncture by Group.a
| Brain regions | MNI coordinate | BA | Cluster size | t score | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| (AD_before > AD_after) > (NC_before > NC_after) | |||||||
| Cingulate gyrus | 9 | −42 | 39 | 31 | 144 | 4.406 | |
| Cingulate gyrus | −9 | −39 | 30 | 31 | 3.841 | ||
| Cingulate gyrus | 0 | −48 | 42 | 31 | 3.767 | ||
| Precuneus | −33 | −69 | 33 | 39 | 50 | 3.049 | |
| (AD_after > AD_before) > (NC_after > NC_before) | |||||||
| Inferior parietal lobule | 51 | −45 | 36 | 40 | 44 | 4.777 | |
| Inferior parietal lobule | 54 | −42 | 48 | 40 | 4.084 | ||
| Middle temporal gyrus | 45 | −66 | 27 | 39 | 27 | 4.228 | |
| Middle temporal gyrus | 51 | −69 | 18 | 39 | 3.215 | ||
| Posterior cingulate | −15 | −63 | 9 | 30 | 38 | 3.305 | |
| Posterior cingulate | −6 | −60 | 9 | 30 | 3.116 | ||
Abbreviations: AD, Alzheimer's disease; DMN, default mode network; BA, Broadman area; MNI, Montreal Neurological Institute; x, y, and z, coordinates of primary peak locations in the MNI space; AD, Alzheimer's disease; NC, normal control.
a The threshold report here is a corrected P < .01 using AlphaSim.
Correlation Between the Acupuncture Effect and Neuropsychological Measures
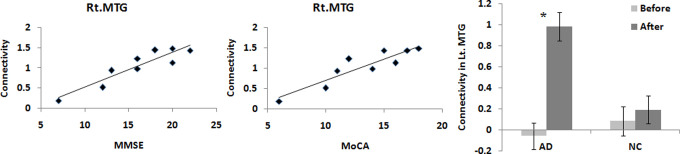
Only 1 significant positive correlation was found in the right MTG between the connectivity change and MMSE (R = .919, P = .0005) and MoCA (R = .910, P = .0007) for patients with AD (as shown in Figure 6), which were corrected for multiple comparisons using Bonferroni (P < .05/4 = .0125). These effects were specifically for AD, as the corresponding correlations for NC (MMSE: R = −.221, P = .514; MoCA: R = −.203, P = .549) were slightly negative and not significant.
Figure 6.
The significant correlation in the right middle temporal gyrus (MTG) between the connectivity change and Mini-Mental State Examination (MMSE; R = .919, P = .0005) and Montreal Cognitive Assessment (MoCA; R = .910, P = .0007) for patients with Alzheimer's disease (AD).
Discussions
Our study concentrated on the sustained effect of acupuncture on the DMN of patients with AD. There were 2 main findings. First, the abnormal regions in AD, which were impaired as compared to NC, were ameliorated by acupuncture of Liv3 and LI4. This result was demonstrated by the ROI analysis and then confirmed by an exploratory analysis. Second, the acupuncture effect at the left MTG was positively correlated with individual differences in patients with AD measured by MMSE and MoCA. To the best of our knowledge, this is the first study to report the modulated effect of the DMN by acupuncture in AD. These findings may have new implications to the therapy for patients with AD.
The Significant Acupuncture Effect on the Abnormal DMN Regions in AD
By analyzing the resting-state fMRI data collected before acupuncture, we found abnormal DMN connectivity in the patients with AD relative to controls. Compared to NC, patients with AD showed significant decreased activations in PCC extending into PCu, right IPL, and IPL, while increased activations in bilateral CG. These results were totally consistent with previous reports using ICA 19,21 or seed-based functional connectivity. 9,37 It was found that these abnormal regions were changed to be better to the different extent by acupuncture, with right IPL showed increased activity and left CG showed decreased activity in AD (Figure 4), as compared to the condition before acupuncture.
Whole-Brain Localization of the Modulation Effect on DMN by Acupuncture
The whole-brain analysis has confirmed the acupuncture effect on the DMN in AD. The regions already having abnormal connectivity, identified by comparing the conditions of AD and NC before acupuncture (Figure 4), were almost located within the regions with the acupuncture effect in AD, identified by the interaction effect of acupuncture by group (Table 3 and Figure 5). Additionally, some regions located in the left PCu showed decreased connectivity by acupuncture, while the left MTG showed increased connectivity after acupuncture therapy.
The activation of the right IPL, MTG, along with a cluster in the left PCC was significantly increased in AD after acupuncture. As the important parts of the multiple interacting subsystems in DMN, these regions may play important but different roles in resting-state brain activities, such as MTG for providing information, IPL for spatial attention, and PCC for information integration. 25,41 Therefore, our current findings may suggest that acupuncture of Liv3 and LI4 may promote the memory functions of AD.
Specificity and Individual Difference in the Acupuncture Effect on AD
We found that all the brain activations modulated by acupuncture in AD were specifically for AD but not for NC, such as right SPL and CG (Figure 4). It was suggested that acupuncture per se had an effect on all kinds of patients. However, the baseline for evaluating the extent of the acupuncture therapy was different for AD and NC. Since AD is a pathological state, so the patients with AD possibly have a lower baseline level than that of NC. This may explain the (relative) specificity of the acupuncture effect on AD or why AD may benefit more from acupuncture. This explanation was consistent with previous reports that NC can also benefit from acupuncture. 22,23,42
We also found that the acupuncture effect as measured by the activation in a region of right MTG was significantly positively correlated with the disease severity of AD (Figure 6). This implicates that there may be a better therapy effect for AD with relatively mild symptoms. However, this should be further demonstrated in the future, as there are many factors that might affect the acupuncture effect.
Potential Mechanism of Acupuncture Therapy for AD
By using fMRI, it was found that effective acupuncture could activate several brain areas controlling the memory and learning functions. 6,11 -14 This could be a potential mechanism of acupuncture on AD, albeit this explanation is at the relative higher concept hierarchies. The current study has added new evidences to this explanation that acupuncture can activate cerebral memory and learning areas. Although still on investigating, the DMN, which is found to be modulated by acupuncture in the current study, is convergent on its key role to human cognition at rest and during tasks, especially in memory. 25
A further question is how acupuncture can help to activate the regions related to memory and learning. According to TCM, acupuncture of Liv3 and LI4 can promote the “Qi” and blood cycle in all the organs and thus can boost the nutrition and blood supply of the brain. 6 These effects can be first detected in the DMN regions, which are also the hubs of the whole brain system. This explanation is worthy of respect, however, it explains the facts at a very higher level and does not explain the underlying mechanism at the molecular level. We will hope for the explanation from the contemporary neuroscience. It is still a long way to go to fully uncover the intricacies of acupuncture use in AD. Many studies should be done in the future to test these hypotheses.
Limitations and Further Considerations
There are still some issues to be addressed. First, it may benefit the current study to include a control state on the real-needle acupuncture by using sham acupuncture. Second, a longitudinal design will be necessary to elucidate the impaction of acupuncture on the DMN. In the future, we will trace these patients at different time points and explore the DMN connectivity changes and its influence on cognitive function in patients with AD after acupuncture. Third, many more imaging studies with larger population should be done to test the findings in the current study. Additionally, it is still unknown whether the current findings are only specifically for the Liv3 and LI4 acupuncture or also apply to the other acupoints. The future studies should cover all these considerations.
Conclusion
In conclusion, our study found that the DMN activity of patients with AD could be modulated by the acupuncture stimulation on the acupoints of Liv3 and LI4. It may provide deep understanding of the therapeutic effect of acupuncture and demonstrate a new avenue for the treatment of AD in the future.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of China (Grant Nos. 61105118 and 81000606), Beijing Nova Program (Nos. Z12111000250000 and Z131107000413120), and the Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (CNLZD1302).
References
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366 (9503):2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam YC, Kum WF, Durairajan SS, et al. Efficacy and safety of acupuncture for idiopathic Parkinson's disease: a systematic review. J Altern Complement Med. 2008;14 (6):663–671. [DOI] [PubMed] [Google Scholar]
- 3. Chen YS, Chen FQ, Zhao JX, Tian YX. Progression of theory and application of acupuncture on treatment of vascular dementia. Chin J Clin Rehabil. 2004;8 (22):4664–4666. [Google Scholar]
- 4. Kao H. Acupuncture Enhancement in Clinical Symptoms and Cognitive-Motor Abilities of the Alzheimer’s Disease Patients. Washington, DC: World Alzheimer’s Congress; 2000. [Google Scholar]
- 5. Emerson Lombardo N. Acupuncture to Treat Anxiety and Depression in Alzheimer’s Disease and Vascular Dementia: A Pilot Feasibility and Effectiveness Trial. Washington DC: Alzheimer's Association, World Alzheimer Congress; 2000. [Google Scholar]
- 6. Xia Y, Ding GH, Wu GC, eds. Current Research in Acupuncture. New York: Springer; 2011. [Google Scholar]
- 7. Deng ZM, Yuan YJ. Stroke and dementia. Chin J Tradit Chin Med Pharm. [in Chinese] 1991;6 (3):13–15. [Google Scholar]
- 8. Zhu WM, Hu HY. A survey of TCM treatment for Alzheimer’s disease. J Traditi Chin Med. 2007;27 (3):226–232. [PubMed] [Google Scholar]
- 9. Zhang HY, Wang SJ, Xing J, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res. 2009;197 (1):103–108. [DOI] [PubMed] [Google Scholar]
- 10. Zeng BY, Zhao KC, Liang FR. Neurobiology of Acupuncture. 1st ed. Waltham, MA: Academic Press; 2013. [Google Scholar]
- 11. Fu P, Jia JP, Min BQ. Acupuncture at Neiguan acupoint for brain functional MRI of patients with Alzheimer disease. Chin J Neurol. [in Chinese] 2005;38 (2):118–119. [Google Scholar]
- 12. Fu P, Jia JP, Wang M. Acupuncture at shenmen acupoint for brain functional MRI of patients with Alzheimer disease. Chin J Clin Rehabil. [in Chinese] 2005;9 (1):120–121. [Google Scholar]
- 13. Guo Y, Shi XM, Utiyama N, Hasegawa AMT, Fukumoto Y. Study of a new rehabilitation method of dementia with electrostimulation on the JingMing-point. J Jpn E Med Assoc. [in Japanese] 2000;16 (4):17–30. [Google Scholar]
- 14. Wang Z, Nie B, Li D, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One. 2012;7 (8):e42730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Jin J. Effect of acupuncture given at the HT 7, ST 36, ST 40 and KI 3 acupoints on various parts of the brains of Alzheimer's disease patients. Acupunct Electrother Res. 2008;33 (1-2):9–17. [PubMed] [Google Scholar]
- 16. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98 (2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16 (3):271–278. [DOI] [PubMed] [Google Scholar]
- 18. Chetelat G, Baron JC. Early diagnosis of Alzheimer's disease: contribution of structural neuroimaging. Neuroimage. 2003;18 (2):525–541. [DOI] [PubMed] [Google Scholar]
- 19. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101 (13):4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2007;104 (47):18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chhatwal JP, Schultz AP, Johnson K, et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology. 2013;81 (8):736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136 (3):407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang J, Jin Z, Wang Y, et al. The salient characteristics of the central effects of acupuncture needling: limbic–paralimbic–neocortical network modulation. Hum Brain Mapp. 2009;30 (4):1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pirog JE. The Practical Application of Meridian Style Acupuncture. Pacific View Press, 1996. [Google Scholar]
- 25. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 26. Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28 (6):1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154 (1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2013;32 (1):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association Press; 1994. [Google Scholar]
- 30. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34 (7):939–944. [DOI] [PubMed] [Google Scholar]
- 31. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43 (11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 32. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26 (3):839–851. [DOI] [PubMed] [Google Scholar]
- 33. Li YO, Adal T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28 (11):1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7 (6):1129–1159. [DOI] [PubMed] [Google Scholar]
- 35. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14 (3):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 37. Zhang HY, Wang SJ, Liu B, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256 (2):598–606. [DOI] [PubMed] [Google Scholar]
- 38. He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35 (2):488–500. [DOI] [PubMed] [Google Scholar]
- 39. Liang P, Wang Z, Yang Y, Jia X, Li K. Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011;6 (7):e22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang P, Wang Z, Yang Y, Li K. Three subsystems of the lateral parietal cortex are differently affected in amnesia mild cognitive impairment. J Alzheimers Dis. 2012;30 (3):475–487. [DOI] [PubMed] [Google Scholar]
- 41. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev Neurosci. 2002;3 (3):201–215. [DOI] [PubMed] [Google Scholar]
- 42. Bai L, Qin W, Tian J, et al. Acupuncture modulates spontaneous activities in the anticorrelated resting brain networks. Brain Res. 2009;1279:37–49. [DOI] [PubMed] [Google Scholar]